Abstract
RhoB, a member of the Rho subfamily of small GTPases, mediates diverse cellular functions, including cytoskeletal organization, cell transformation and vesicle trafficking. The thymus undergoes progressive decline in its structure and function after puberty. We found that RhoB was expressed in thymic medullary epithelium. To investigate a role of RhoB in the regulation of thymic epithelial organization or thymocyte development, we analyzed the thymi of RhoB-deficient mice. RhoB-deficient mice were found to display earlier thymic atrophy. RhoB deficiency showed significant reductions in thymus weight and cellularity, beginning as early as 5 weeks of age. The enhanced expression of TGF-β receptor type II (TGFβRII) in thymic medullary epithelium was observed in RhoB-null mice. In addition, the expression of fibronectin, which is shown to be regulated by TGF-β signaling, was accordingly increased in the mutant thymic medulla. Since there is no age-related change of RhoB expression in the thymus, it is unlikely that RhoB in thymic epithelium directly contributes to age-related thymic involution. Nevertheless, our findings strongly support a physiological role of RhoB in regulation of thymus development and maintenance through the inhibition of TGF-β signaling in thymic medullary epithelium.
Keywords: atrophy, RhoB, thymus, TGF-β
Introduction
Rho GTPases belong to the Ras superfamily of small GTP-binding proteins and are involved in the regulation of cell shape, polarity and migration through their effects on cytoskeletal dynamics and cell adhesion (1, 2). Among three closely related mammalian Rho proteins, RhoA, RhoB and RhoC, a number of studies have demonstrated a unique function for RhoB (3, 4). Whereas RhoA and RhoC promote tumorigenesis and invasion, RhoB rather acts as a tumor suppressor (5–8). RhoB expression levels are inversely related to malignancy of various tumors (9–11), antagonizing cell migration, tumor growth and metastasis (12). Furthermore, RhoB inhibits UVB-induced apoptosis (13) and radiation-induced mitotic cell death (14). RhoB is predominantly localized in the plasma membrane (15, 16) and endosomes (17). RhoB regulates the trafficking of EGF receptor from the late endosomal compartment to the lysosome (17, 18). The importance of RhoB in the endocytic pathway includes the trafficking of important cellular factors, such as Akt, Src and CXCR2 (19). RhoB-null mice have increased susceptibility to Ras-induced tumorigenesis (20). In addition, RhoB-null mice exhibit retarded vascularization of the retina (19) and altered cell adhesion in macrophages (21). It was thus concluded that RhoB plays important roles in the subcellular targeting of signaling molecules.
The thymus is critical for the development, selection and maintenance of the peripheral T-cell pool of a diverse repertoire. The thymus is capable of generating T cells throughout the life span. However, the thymus undergoes age-related involution (22, 23). This process becomes most prominent after puberty, largely due to elevated steroid hormonal levels (24, 25). The importance of both androgens and estrogens in the process of thymic involution is demonstrated and surgical or chemical gonadectomy reverses thymus atrophy (26, 27). Physiologic doses of androgens induce increased levels of TGF-β1 in the thymus (28). The levels of several thymic cytokines including TGF-β1 are increased in the aged human thymus (29). TGF-β2 is related with age-associated thymic involution in mice (30). IL-7, a crucial cytokine involved in both T- and B-cell development in mice, is associated with thymic involution (31). Age-related defects in mice have been observed among the bone marrow hemopoietic stem cells (32, 33), the early T-lineage precursors (34) and the transition of CD4−CD8−CD44+CD25− thymocytes to CD4−CD8−CD44+CD25+ thymocytes (35). Thymic epithelial cells represent a predominant stromal cell component and have a paramount role in T-cell development and selection by providing essential thymopoietic signals. The thymic epithelium is classically divided into two specialized and spatially distinct subsets, cortical and medullary epithelial cells that manifest different functional properties (36). Age-related changes in the epithelial component of the thymus are important in thymic involution (37–39). Nevertheless, the precise mechanisms responsible for the physiologic senescence of the thymus, presumably involving complex multisystem interactions, have yet to be unequivocally identified.
Here, we showed the expression of RhoB in thymic medullary epithelium. To better understand the physiological relevance of RhoB on thymus development and maintenance, we examined RhoB-knockout mice for any alterations in the thymi. It was found that RhoB deficiency displayed early thymic atrophy. A novel link between TGF-β signaling in thymic medullary epithelium and thymic atrophy was proposed.
Materials and methods
Mice
SV129 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The RhoB-null mice were generated as previously reported (20). C57BL/6 mice were obtained from Jackson Laboratories or Japan SLC (Hamamatsu, Japan). Animal protocols were approved by our Institutional Animal Care and Use Committee.
RNA isolation and quantitative real-time reverse transcription–PCR
Total RNA was isolated from each tissue using the Trizol reagent (Invitrogen). The cDNA was synthesized by TaqMan Reverse Transcription Reagents with random primers (PE Applied Biosystems, Foster, CA, USA). For quantification of mRNA expression, real-time PCR was carried out in triplicates on an ABI-PRISM 7700 sequence detection system (PE Applied Biosystems) using the following parameters: 2 min at 50°C; 10 min at 95°C; 40 cycles of 15 s at 95°C, 30 s at 50°C and 1 min at 60°C. The primers used were TGF-β1, forward 5′-CTCGGGGGCTGCGGCTACTG-3′, reverse 5′-GGCGTATCAGTGGGGGTCA-3′; TGF-β2, forward 5′-CGAGCGGAGCGAGCAGGAG-3′, reverse 5′-TAGGAGGGCAACAACATTA-3′; TGF-β3, forward 5′-GGTCCTGGCACTTTACAAC-3′, reverse 5′-GGCGTACACAGCAGTTCTC-3′; IL-7, forward 5′-GACAGGAACTGATAGTAATTGCCCG-3′, reverse 5′-TCACCAGTGTTTGTGTGCCTTGTG-3′; and GAPDH, forward 5′-AACTTTGGCATTGTGGAAGGGCTC-3′, reverse 5′-TGGAAGAGTGGGAGTTGCTGTTGA-3′. Acquired data were analyzed by Sequence Detector software (PE Applied Biosystems). Gene expression level of each sample was normalized using the expression of GAPDH gene. To compare relative levels of mRNAs, the equation ΔFC = 2 − (dCT), with delta CT (dCT) calculated as the difference in CT values between the gene of interest and GAPDH.
Thymus weight and thymocyte cellularity
Thymus was dissected from humanely killed mouse, weighed and minced through a nylon mesh. Mononuclear cells were counted and >95% of the cells were Thy-1-positive.
Flow cytometry
Fluorescence-conjugated antibodies used in a flow cytometry, such as anti-CD3 (145-2C11), CD4 (GK1.5), CD8 (53–6.7), CD44 (IM7), CD25 (PC61.5) and CD117 (2B8), were all purchased from eBioscience (San Diego, CA, USA). Thymocyte cell suspensions were prepared by pressing the tissue through a sieve into ice-cold Dulbecco’s PBS supplemented with 3% FCS. RBCs were removed by the lysis in ammonium chloride buffer. After washing, cells were blocked with anti-CD16/32 and then stained with directly labeled antibodies against CD3, CD4, CD8, CD44, CD25 and CD117, and 30 000 thymocytes were analyzed on a FacsCanto II flow cytometer using FacsDiva software (BD Biosciences, San Jose, CA, USA).
Immunofluorescence staining and confocal microscopy
Thymus tissue sections were prepared and immunohistology was performed on cryosections as previously reported (40) using rabbit anti-RhoB (sc-180; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), rabbit anti-keratin 5 (Covance, Berkeley, CA, USA), rabbit anti-TGF-β receptor type I (AnaSpec Inc., San Jose, CA, USA), rabbit anti-TGF-β receptor type II (41), mouse anti-fibronectin (clone FBN11; Thermo Fisher Scientific, Fremont, CA, USA) and rat ER-TR7 (BMA Biomedicals, August, Switzerland). Alexa Fluor-labeled donkey secondary antibodies (Molecular Probes, Eugene, OR, USA) were also used. The binding to biotinylated Ulex europaeus agglutinin-1 (UEA-1; Vector Laboratories, Burlingame, CA, USA) was followed by PE-conjugated streptavidin (eBioscience). Confocal laser-scanning microscopy analysis was performed on a Zeiss LSM 510 (Carl Zeiss, Oberhochen, Germany). Negative controls were performed by replacement of first-step antibodies by isotype-matched monoclonal antibodies or species-matched antibodies. Representative images were chosen from each experiment for figure editing.
Statistics
Statistical analysis was performed with the non-parametric unpaired Mann–Whitney test using Prism software. Probability values <0.05 were considered statistically significant.
Results
RhoB expression in thymic medullary epithelium
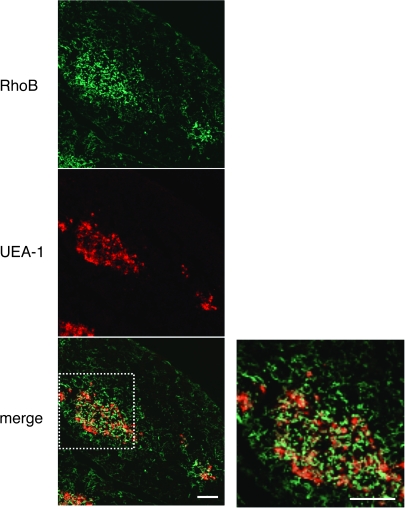
Our initial studies focused on examining the expression of RhoB in mouse thymus. The frozen thymus sections from 6-week-old C57BL/6 mice were labeled with anti-RhoB antibody and analyzed by a confocal microscopy. The sites of RhoB expression were scattered throughout the thymic medulla (Fig. 1). Thymus sections were stained together with the lectin UAE-1, which specifically recognizes thymic medullary epithelium. The UEA-1-positive epithelial cells were present in the thymic medullary region, while RhoB was expressed in thymic medullary epithelium as expected (Fig. 1). RhoB expression was undetectable in other subsets such as thymic vascular smooth muscle cells (data not shown).
Fig. 1.
Expression of RhoB in mouse thymus. Immunofluorescence staining of thymus sections of 6-week-old C57BL/6 mice was performed to detect RhoB (green) and the binding to thymic medullary epithelium marker UEA-1 (red). Right panel: high magnification from white box in the left panel. Double labeling for RhoB and UAE-1 shows their co-localization, indicating that thymic medullary epithelium expresses RhoB. Data are representative of at least three independent experiments with more than five mice. Scale bars = 100 μm.
RhoB-deficient mice exhibit earlier thymic atrophy
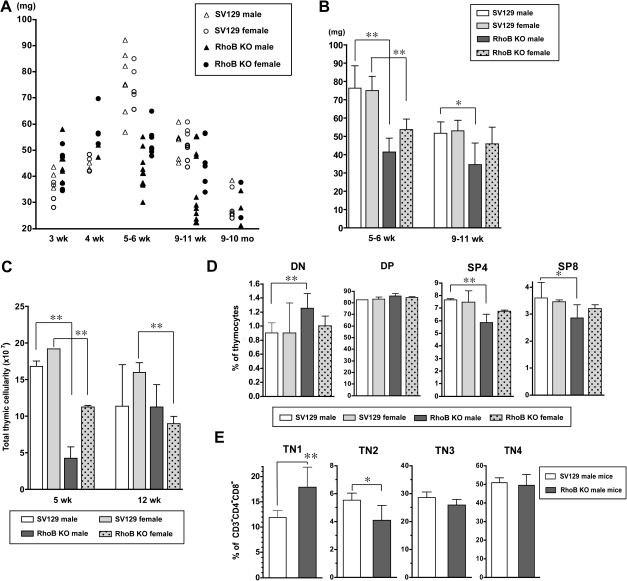
To better understand the physiological role of RhoB in thymic epithelial organization or thymocyte development, we examined the thymi of RhoB-null mice (20). Interestingly, a significant decrease in thymus size of the young mice was notable when compared with that of wild-type SV129. The development of thymus continues until puberty (∼4 to 6 weeks of age in mice) at which time it reaches to its maximum size (38, 42). Thymus involution in mice is profound by 9 months of age and thereafter proceeded slowly (35). Thymus weight and thymocyte cellularity of SV129 and RhoB-null mice at different ages from age 3 weeks through 10 months were analyzed. RhoB deficiency did not lead to abnormal thymus development by the age of 4 weeks, but a significant decrease in the thymus weight was noticed at 5–6 weeks of age (Figs. 2A and B). At this stage, the number of thymocytes was significantly lower in the mutant mice than in age-matched wild-type mice (Fig. 2C). The results unambiguously demonstrate that RhoB-deficient mice exhibited a marked thymic atrophy at 5–6 weeks of age. However, thymic weight and cellularity in RhoB-null mice were comparable to those in control mice at 9–10 months of age.
Fig. 2.
RhoB-null mice exhibit earlier thymus atrophy. (A and B) Thymus weight in SV129 and RhoB-null mice. Thymus weight in SV129 and RhoB-null mice at various ages was measured. (A) Each point indicates the thymus weight of a single animal. (B) Data are represented as mean ± SD of each group. RhoB deficiency results in the significant decrease in thymus weight as early as 5 weeks of age. (C) Thymocyte counts in SV129 and RhoB-null mice at 5 and 12 weeks of age. Data are represented as mean ± SD of each group (n = 4 per group). RhoB deficiency induces a marked decrease in total thymocyte numbers. (D) The profiles of CD4/CD8 in thymocytes of SV129 and RhoB-null mice at 5 weeks of age. RhoB-null mice exhibit an increase in DP thymocytes and a decrease in SP mature thymocytes, when compared with wild-type. (E) Block of thymocyte development in RhoB-null mice. CD3, CD4 and CD8-triple-negative (TN) thymocytes in each mouse at 5 weeks of age were analyzed for the expression of CD44 and CD25. Proportions of CD44+CD25− TN cells in total thymocytes of RhoB-null mice are increased compared with age-matched SV129 mice. (D and E) Data from at least three independent experiments are present as means ± SD (n = 4 per group (D) and n = 3–4 per group (E)). *P < 0.05; **P < 0.01.
We next analyzed thymocytes for the cell surface expression of CD4 and CD8 in SV129 and RhoB-null mice at 5 weeks of age (Fig. 2D). It was observed that the ratios of CD4−CD8− double-negative (DN) thymocyte were higher in RhoB-knockout male mice than age-matched controls. Mature phenotype (CD3+CD4+ and CD3+CD8+ single-positive) thymocytes were decreased in the mutant mice. In addition, the populations of CD3−CD4−CD8− triple-negative (TN) thymocyte were monitored for the expression of CD44 and CD25 (Fig. 2E). The fraction of CD44+CD25− TN1 population was elevated in 5-week-old mutant mice in comparison with controls. Early T-lineage progenitors within the DN1 population, identified as Lin−CD25−CD44+cKit+, were previously shown to decline with age (34). However, we could not detect any significant reduction of early thymocyte precursors under RhoB deficiency (data not shown).
Analysis of TGF-β and IL-7 mRNA in RhoB-null thymus
Three TGF-β isoforms encoded by distinct loci have been identified, sharing a high level of sequence and structural homology (43). The individual TGF-β isoform mediates unique set of physiological functions due to its different tissue distribution and temporal expression pattern (43).
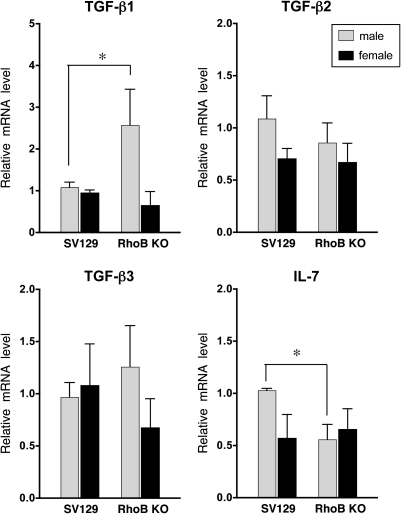
To identify the influence of RhoB in thymic expression of TGF-β and IL-7, we analyzed the levels of TGF-β and IL-7 in individual thymus tissue by real-time quantitative RT–PCR. As shown in Fig. 3, the levels of TGF-β1 mRNA in RhoB-knockout male thymus were higher at the age of 5 weeks than in the wild-type. The levels of TGF-β2 and TGF-β3 were not remarkably different between both mice. IL-7 was expressed at decreased levels in the thymus of the mutant male at 5 weeks of age.
Fig. 3.
Expression of TGF-β and IL-7 in RhoB-null thymus. TGF-β and IL-7 mRNA levels in each thymus tissue of 5-week-old SV129 and RhoB-null mice (n = 4–6) were analyzed by quantitative real-time RT–PCR. The each gene level of each thymus was normalized with GAPDH. A SV129 male mouse was used as a control. The relative mRNA level was calculated as described in Materials and methods. The values in graphs are the average fold-differences with SD. Data are presented as means ± SD from three independent experiments. *P < 0.05.
Changes in the composition of thymic epithelium in RhoB-null mice
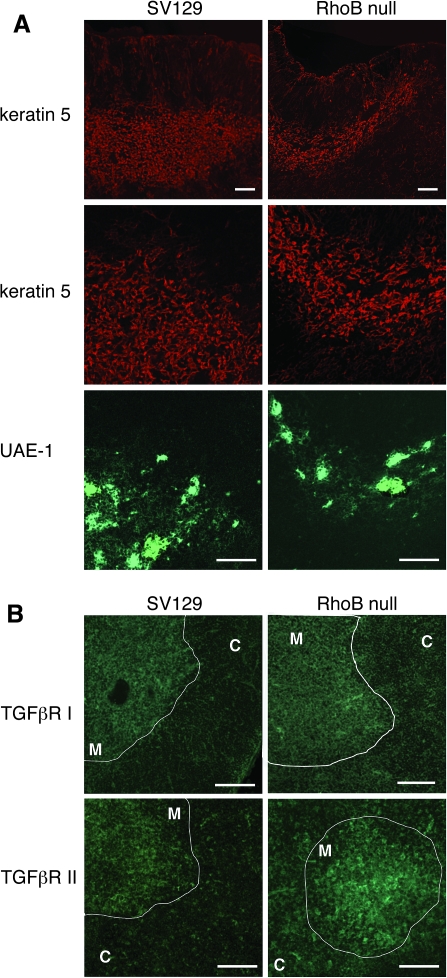
To investigate whether the lack of RhoB also affected thymic architecture and phenotype of thymic epithelia, thymus sections from wild-type and RhoB-deficient mice at the age of 6 weeks were stained with two reagents recognizing thymus medullary epithelial cells, anti-keratin 5 antibody and UEA-1. Although typically RhoB-null thymi displayed smaller size than wild-type thymi, RhoB-deficient thymic lobes showed a regular separation into distinct cortical and medullary compartments. However, no detectable difference in the distribution of keratin 5 and the binding to UEA-1 between RhoB-null animals and SV129 was observed (Fig. 4A). Specifically, at 6–8 weeks of age, no significant difference in the thymic vasculature between RhoB-null mice and SV129 mice (data not shown).
Fig. 4.
Lack of RhoB induces increase the expression of TGFβRII in thymic medullary epithelium. Organization of thymic medullary epithelium in 6-week-old SV129 and RhoB-null mice. Immunofluorescence staining of thymus sections of both strains was performed to detect keratin 5 (red) and the binding to UEA-1 (green). (B) Increased expression of TGFβRII in thymic medullary epithelium of RhoB-null mice. Immunofluorescence staining of thymus sections of both mice strains was performed to detect TGFβRI (green) or TGFβRII expressions (green). High TGFβRII expression is detected throughout medullary epithelium of RhoB-null thymus. The level of TGFβRI in medullary epithelium of RhoB-null thymus is similar to wild-type thymus. C, Cortex; M, medulla. Data are representative of three independent experiments with six mice per group. Scale bars = 100 μm.
TGF-β induces a heteromeric complex of type I and type II serine/threonine kinase receptors after ligand binding. Subsequent signal transduction results as the TGF-β type II receptor (TGFβRII) phosphorylates the type I receptor (TGFβRI) chain that leads to the activation of downstream signaling compo-nents (44). We have previously demonstrated the expression of TGFβRI and TGFβRII in thymus medullary region by immunochemical analysis (41). RhoB is reported to inhibit TGFβRII expression in epithelial cells (45). We analyzed the expression of TGFβRI and TGFβRII in SV129 thymi by confocal microscopy analysis (Fig. 4B). TGFβRI was expressed in the medulla, particularly in the epithelium, and TGFβRII was found in the medullary epithelium. In addition, extensive expression of TGFβRII was observed in thymic vascular smooth muscle cells (data not shown). The distribution of TGFβRI in RhoB-deficient thymus was similar to that in wild-type thymus (Fig. 4B). By contrast, high expression of TGFβRII was detectable throughout medullary epithelium in Rho-null thymus (Fig. 4B). It seems that RhoB deficiency mediates an increased TGFβRII expression in thymic medullary epithelium.
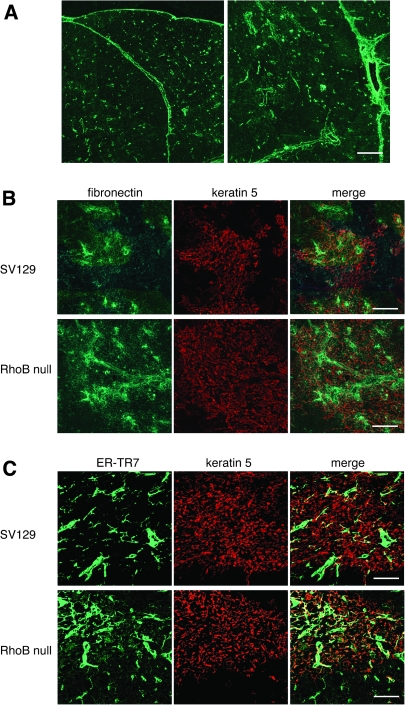
The inhibition of RhoB was reported to lead to an increase in TGFβRII expression, resulting in enhanced TGF-β binding and signaling (45, 46). TGF-β preferentially stimulates the synthesis of the extracellular matrix proteins such as fibronectin (47, 48). To identify whether RhoB deficiency might induce aberrant TGF-β signaling in thymic medullary epithelium, we compared immunolocalization of fibronectin in 6-week-old SV129 and RhoB-null thymi. As expected from a previous study (49), fibronectin was distributed especially in the capsule, septa and pervasuclar cells around blood vessels in the wild-type thymus (Fig. 5A). We observed a marked enhancement of fibronectin in RhoB-null thymus. The double staining for fibronectin and keratin 5 revealed that fibronectin was occasionally associated with epithelium in medullary region (Fig. 5B). Recent studies have also demonstrated that thymic atrophy is associated with an increase in thymic mesenchymal fibroblast content (50). The distribution of mesenchyme in RhoB-null thymus was addressed using pan-mesenchymal marker ER-TR7. As previously described (40), ER-TR7 marked the cells in the capsule, septa and prevascular cells in control SV129 thymus. The frequent accumulation of mesenchyme in the medullary region of RhoB-null thymus was observed (Fig. 5C). Images by fluorescence microscopy can give a quantitative analysis for cell number by counting positive cells in the region of thymic medulla (e. g. Aire or FoxN1). However, the quantitative analysis by fluorescence intensity has never been widely used in thymus sections. The determination of background values in thymus tissue sections for comparison analyses is frequently difficult. We could not include the quantitative data.
Fig. 5.
Lack of RhoB induces the increased expression of fibronectin in thymic medullary epithelium. (A) Expression of fibronectin in the thymus of adult mouse. Immunofluorescence staining of thymus sections of 6-week-old SV129 mice was performed to detect fibronectin. Fibronectin is detectable in the capsule, septa and perivascular cells. (B) Increased expression of fibronectin in thymic medullary region of RhoB-null mice. Immunofluorescence staining of thymus sections of 6-week-old male SV129 and RhoB-null thymus was performed to detect fibronectin (green) and keratin 5 (red). RhoB deficiency increases expression of fibronectin in thymic medullary region. Fibronectin associated with medullary epithelium is frequently seen. (C) Increased distribution of mesenchymal cells in thymic medullary region of RhoB-null mice. Thymus sections of 6-week-old SV129 and RhoB-null thymus were stained with ER-TR7 and anti-keratin 5 Ab. In RhoB-null thymus, accumulation of mesenchymal cells in thymic medullary region is frequently detectable. Data are representative of three independent experiments with six mice per group. Scale bars = 100 μm.
Discussion
Here, we identified that RhoB was expressed in thymic medullary epithelium. RhoB is reported to be present in the epithelial cells of various tissues, such as neural crest (51), lens (52), saliva gland (53) and lung (54). Our data support a physiological role of RhoB in the development and maintenance of thymus using RhoB-deficient mice. RhoB deficiency exhibited a marked decrease in thymus weight and cellularity as early as 5 weeks of age. RhoB-deficient mice exhibited an accumulation of DN thymocytes at the earliest CD44+CD25−development stage and a reduction of mature thymocytes, suggesting that T-cell development is arrested during the most immature developmental stage in the mutant mice.
RhoB-deficient mice displayed a similar distribution of keratin 5- and UEA-1-positive thymic medullary epithelial cells to normal mice. The expression of MHC class II in RhoB-null thymic medullary epithelium was comparable with that in wild-type thymus (data not shown). The histological examinations revealed that RhoB deficiency increased the expression of TGFßRII, but not TGFßRI in thymic medullary epithelium, consistent with previous studies demonstrating the inhibition of TGFβRII expression by RhoB (45). It has recently been suggested that thymic epithelial cells undergo dynamic changes throughout the life span and age-associated thymic involution occurs as a result of inability to maintain steady state levels of thymic epithelial cell populations (38). Mice deficient for the expression of TGFβRII in thymic epithelial cells display diminished thymic atrophy, indicating that TGF-β signaling in thymic epithelial cells is directly associated with the phenomenon of thymic involution (55). Interestingly, the absence of TGF-β signaling in the epithelial cells influences the pool size of single-positive mature thymocytes (55). Our findings also support a role of TGF-β signaling in thymic epithelium in thymus development and maintenance. Fibronectin is shown to be up-regulated in the thymus in an age-dependent manner (56). We detected the increased expression of fibronectin in the RhoB-null thymus. It is likely that the elevated TGF-β signaling is involved in the increment of fibronectin. Together, the deficiency of RhoB may result in an increase in TGFβRII expression, leading to the enhancement of TGF-β signaling in thymic medullary epithelial cells. Our results also indicated that thymic atrophy results from a failure of the thymic microenvironment to support thymopoiesis.
Thymic size in mouse is shown to reach a peak at 4 weeks of age (38, 42). RhoB-knockout mice do not exhibit any apparent defects in thymus development by 4 weeks of age. They displayed thymic atrophy after 5 weeks of age. We therefore concluded that RhoB deficiency leads to accelerate thymic involution. The level of RhoB is reported to be gradually reduced in lung and skeletal muscle with age, although there is no age-related change of RhoB expression in thymus (57). We analyzed the expression of RhoB mRNA in SV129 thymus by real-time quantitative RT–PCR, and the age-related decrease of RhoB in the thymus was undetectable by 1 year of age (data not shown). It is, therefore, unlikely that RhoB is directly associated with age-related thymic involution. We suggest that RhoB regulates the expression of TGFβRII in thymic medullary epithelial cells, resulting in the inhibition of TGF-β signaling which plays a role in thymic atrophy.
The early thymic atrophy due to the lack of RhoB was more prominent in male mice than in female mice. The striking thymic atrophy was detectable in male mice at 5 weeks of age. Androgen receptor is present on thymic epithelial cells, and the action of androgen on thymic epithelium is required for the induction of thymic atrophy (37). Altered expression of androgen receptor in RhoB-null thymus was undetectable (data not shown). The susceptibility to deficiency of RhoB in male mice remains unclear. Several explanations between thymic atrophy and increasing thymic TGF-β levels have been proposed (28–30). Thymic TGF-β1 mRNA expression was up-regulated in RhoB-knockout male. Although TGF-β2 expressed both in the thymic microenvironment and in the hematopoietic system is shown to accelerate thymic involution in mice (30), the level of TGF-ß2 mRNA detected in RhoB-null thymus was not higher than that in control thymus. Down-regulation of IL-7 levels in the thymus plays a role in age-associated thymic involution (31). However, the overexpression of IL-7 within the thymus cannot fully restore thymic defects in aged mice (58). In our study, thymic atrophy in RhoB deficiency was linked to increased TGF-β1 and decreased IL-7 expression in the thymus. While there is still controversy as to whether thymic involution is attributed to age-related changes in the production of TGF-β1 and IL-7, the changes of TGF-β1 and IL-7 might follow the induction of thymic atrophy in RhoB-null mice.
In conclusion, we show that RhoB deficiency leaded to marked thymic atrophy during the post-natal period. We demonstrate that RhoB regulates the expression of TGFβRII in thymic medullary epithelial cells. Our data supported a novel link between TGF-β signaling and thymic atrophy. The mechanism by which RhoB modulates TGF-β signaling transduction in thymic medullary epithelial cells leading to thymic atrophy remains to be elucidated.
Funding
This work was supported by the American Diabetes Association Grant (705RA10); and the National Institutes of Health grant (HL071049 to L.E.B.)
Acknowledgments
We are grateful to Dr Carl-Henrik Heldin (Uppsala University, Sweden) for providing anti-TGFβRII antibody.
References
- 1.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 2004;301:43. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho. J. Cell Sci. 2004;117:1301. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 5.Du W, Prendergast GC. Geranylgeranylated RhoB mediates suppression of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res. 1999;59:5492. [PubMed] [Google Scholar]
- 6.Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti SM. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation, induce apoptosis and suppress human tumor growth in nude mice. J. Biol. Chem. 2000;275:17974. doi: 10.1074/jbc.C000145200. [DOI] [PubMed] [Google Scholar]
- 7.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 2001;21:6906. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazieres J, Tillement V, Allal C, et al. Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells. Exp. Cell Res. 2005;304:354. doi: 10.1016/j.yexcr.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Forget MA, Desrosiers RR, Moumdjian M, et al. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin. Exp. Metastasis. 2002;19:9. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]
- 10.Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin. Cancer Res. 2002;8:2225. [PubMed] [Google Scholar]
- 11.Mazieres J, Antonia T, Daste G, et al. Loss of RhoB expression in human lung cancer progression. Clin. Cancer Res. 2004;10:2742. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 12.Jiang K, Delarue FL, Sebti SM. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene. 2004;23:1136. doi: 10.1038/sj.onc.1207236. [DOI] [PubMed] [Google Scholar]
- 13.Canguilhem B, Pradines A, Baudouin C, et al. RhoB protects human keratinocytes from UVB-induced apoptosis through epidermal growth factor receptor signaling. J. Biol. Chem. 2005;280:43257. doi: 10.1074/jbc.M508650200. [DOI] [PubMed] [Google Scholar]
- 14.Milia J, Teyssier F, Dalenc F, et al. Farnesylated RhoB inhibits radiation-induced mitotic cell death and controls radiation-induced centrosome overduplication. Cell Death Differ. 2005;12:492. doi: 10.1038/sj.cdd.4401586. [DOI] [PubMed] [Google Scholar]
- 15.Adamson P, Paterson HF, Hall A. Intracellular localization of the p.21rho proteins. J. Cell Biol. 1992;119:617. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 2001;152:111. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J. Cell Sci. 2004;117:3221. doi: 10.1242/jcs.01193. [DOI] [PubMed] [Google Scholar]
- 18.Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase RhoB. Curr. Biol. 1999;9:955. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- 19.Adini I, Rabinowitz I, Sun JF, Prendergast GC, Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev. 2003;17:2721. doi: 10.1101/gad.1134603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 2001;21:6906. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp. Cell. Res. 2007;313:3505. doi: 10.1016/j.yexcr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Aspinall R, Andrew D, Pido-Lopez J. Age-associated changes in thymopoiesis. Springer Semin. Immunopathol. 2002;24:87. doi: 10.1007/s00281-001-0098-z. [DOI] [PubMed] [Google Scholar]
- 23.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol. Rev. 2005;205:72. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 24.Tosi P, Kraft R, Luzi P, et al. Involution pattern of the human thymus. I. Size of the cortical area as a function of age. Clin. Exp. Immunol. 1982;47:497. [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke AG, Kendall MD. Histological changes in the thymus during mouse pregnancy. Thymus. 1989;14:65. [PubMed] [Google Scholar]
- 26.Barr IG, Khalid BA, Pearce P, et al. Dihydrotestosterone and estradiol deplete corticosensitive thymocytes lacking in receptors for these hormones. J. Immunol. 1982;128:2825. [PubMed] [Google Scholar]
- 27.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell. Immunol. 2008;252:122. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Olsen NJ, Zhou P, Ong H, Kovacs WJ. Testosterone induces expression of transforming growth factor-beta 1 in the murine thymus. J. Steroid Biochem. Mol. Biol. 1993;45:327. doi: 10.1016/0960-0760(93)90001-d. [DOI] [PubMed] [Google Scholar]
- 29.Sempowski GD, Hale LP, Sundy JS, et al. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J. Immunol. 2000;164:2180. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Langer JC, Snoeck HW. Transforming growth factor-beta2 is involved in quantitative genetic variation in thymic involution. Blood. 2006;107:1974. doi: 10.1182/blood-2005-04-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspinall R, Andrew D. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 2002;37:455. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 32.Tyan ML. Age-related decrease in mouse T progenitors. J. Immunol. 1977;118:846. [PubMed] [Google Scholar]
- 33.Eren R, Globerson A, Abel L, Zharhary D. Quantitative analysis of bone marrow thymic progenitors in young and aged mice. Cell. Immunol. 1990;127:238. doi: 10.1016/0008-8749(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 34.Min H, Montecino-Rodriquez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 35.Aspinall R. Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J. Immunol. 1997;158:3037. [PubMed] [Google Scholar]
- 36.Farr AG, Dooley JL, Erickson M. Organization of thymic medullary epithelial heterogeneity: implications for mechanisms of epithelial differentiation. Immunol. Rev. 2002;189:20. doi: 10.1034/j.1600-065x.2002.18903.x. [DOI] [PubMed] [Google Scholar]
- 37.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 38.Gray DH, Seach N, Ueno T, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 39.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 2007;19:1201. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 40.Odaka C. Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J. Histochem. Cytochem. 2009;57:373. doi: 10.1369/jhc.2008.952895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosendahl A, Speletas M, Leandersson K, Ivars F, Sideras P. Transforming growth factor-beta- and Activin-Smad signaling pathways are activated at distinct maturation stages of the thymopoeisis. Int. Immunol. 2003;15:1401. doi: 10.1093/intimm/dxg139. [DOI] [PubMed] [Google Scholar]
- 42.Pazirandeh A, Jondal M, Okret S. Glucocorticoids delay age-associated thymic involution through directly affecting the thymocytes. Endocrinology. 2004;145:2392. doi: 10.1210/en.2003-1660. [DOI] [PubMed] [Google Scholar]
- 43.Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol. Ther. 2003;98:257. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 44.Massagué J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell. Biol. 2000;1:169. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 45.Adnane J, Seijo E, Chen Z, et al. RhoB, not RhoA, represses the transcription of the transforming growth factor beta type II receptor by a mechanism involving activator protein 1. J. Biol. Chem. 2002;277:8500. doi: 10.1074/jbc.M104367200. [DOI] [PubMed] [Google Scholar]
- 46.Engel ME, Datta PK, Moses HL. RhoB is stabilized by transforming growth factor beta and antagonizes transcriptional activation. J. Biol. Chem. 1998;273:9921. doi: 10.1074/jbc.273.16.9921. [DOI] [PubMed] [Google Scholar]
- 47.Ignotz RA, Endo T, Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J. Biol. Chem. 1987;262:6443. [PubMed] [Google Scholar]
- 48.Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J. Clin. Invest. 1987;79:1285. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lannes-Vieira J, Dardenne M, Savino W. Extracellular matrix components of the mouse thymus microenvironment: ontogenetic studies and modulation by glucocorticoid hormones. J. Histochem. Cytochem. 1991;9:1539. doi: 10.1177/39.11.1918928. [DOI] [PubMed] [Google Scholar]
- 50.Aw D, Silva AB, Maddick M, von Zglinicki T, Palmer DB. Architectural changes in the thymus of aging mice. Aging Cell. 2008;7:158. doi: 10.1111/j.1474-9726.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 51.Henderson DJ, Ybot-Gonzalez P, Copp AJ. RhoB is expressed in migrating neural crest and endocardial cushions of the developing mouse embryo. Mech. Dev. 2000;95:211. doi: 10.1016/s0925-4773(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 52.Maddala R, Peng YW, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev. Dyn. 2001;222:534. doi: 10.1002/dvdy.1202. [DOI] [PubMed] [Google Scholar]
- 53.Crema VO, Fossati AC, Hamassaki DE, Santos MF. Distribution of small Rho GTPases in the developing rat submandibular gland. J. Mol. Histol. 2008;39:519. doi: 10.1007/s10735-008-9192-z. [DOI] [PubMed] [Google Scholar]
- 54.Mazieres J, Antonia T, Daste G, et al. Loss of RhoB expression in human lung cancer progression. Clin. Cancer Res. 2004;10:2742. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 55.Hauri-Hohl MM, Zuklys S, Keller MP, et al. TGF-beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood. 2008;112:626. doi: 10.1182/blood-2007-10-115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elcüman EA, Akay MT. Age-dependent immunolocalization of fibronectin and histological changes in the thymus of rats. Vet. Res. Commun. 1998;22:525. doi: 10.1023/a:1006141719909. [DOI] [PubMed] [Google Scholar]
- 57.Yoon YS, Choo JH, Yoo T, Kang K, Chung JH. RhoB is epigenetically regulated in an age- and tissue-specific manner. Biochem. Biophys. Res. Commun. 2007;362:164. doi: 10.1016/j.bbrc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J. Immunol. 2004;173:4867. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]