Abstract
Increasing the pool of cells at early T-cell developmental stages enhances thymopoiesis and is especially beneficial when T-cell production is compromised by radiation or aging. Within the immature double-negative (DN; CD4−CD8−) thymocyte subpopulation, the DN1 subset contains the most primitive cells including the rare early T-cell progenitors (ETPs). In the present study, a human MCL1 transgene, under the control of its endogenous promoter, resulted in enlargement of an undistorted thymus in C57/BL6 mice. Enlargement occurred in females but not males, being seen at 1 month of age and maintained during progression into adulthood as the thymus underwent involution. The small DN1 subset was expanded disproportionally (ETPs increasing from ∼0.016 to 0.03% of thymocytes), while more mature thymocytes were increased proportionally (1.5-fold) along with the stroma. DN1 cells from transgenic females exhibited increased viability with maintained proliferation, and their survival in primary culture was extended. Exposure of transgenic females to γ-irradiation also revealed an expanded pool of radioresistant DN1 cells exhibiting increased viability. While the viability of DN1 cells from transgenic males was equivalent to that of their non-transgenic counterparts directly after harvest, it was enhanced in culture—suggesting that the effect of the transgene was suppressed in the in vivo environment of the male. Viability was increased in ETPs from transgenic females, but unchanged in more mature thymocytes, indicating that primitive cells were affected selectively. The MCL1 transgene thus increases the viability and pool size of primitive ETP/DN1 cells, promoting thymopoiesis and radioresistance in peripubescent females and into adulthood.
Keywords: double negative, early T-cell progenitor, MCL1, radioresistance, thymus, viability
Introduction
The regulation of cell viability is an integral part of development along the T-lymphoid lineage (1). The thymus generates large numbers of thymocytes, but only those that bear a functional non-self-reactive TCR remain viable through multiple stages of selection. Control of viability is also important at early stages, prior to expression of the TCR. This is because survival underlies the ability of primitive cells to expand and further develop and thus to maintain an ongoing stream of cells available for selection and naive T-cell production.
The CD4-CD8- double-negative (DN) thymocyte subpopulation gives rise to CD4+CD8+ double-positive (DP) cells and then single-positive (SP; CD4+CD8- or CD4-CD8+) cells. The most primitive of the DN1–DN4 subsets, DN1, represents ∼0.5 to 1% of thymocytes (2–4). Within DN1, the minute population of early T-cell progenitors (ETPs) derives from precursors emigrating from the bone marrow and gives rise to essentially all maturing T-lymphocytes (5–9).
Thymopoiesis can be compromised by a variety of types of damage or stress—such as infections, malnutrition or treatment with radiation/chemotherapy—as well as during normal aging-related thymic involution (3, 10–13). This can result in a diminished ability to respond to a variety of foreign antigens and therefore increased susceptibility to infections and cancer (11–14). The restoration of thymopoiesis occurs very slowly, as seen after bone marrow transplantation.
Various strategies are being pursued to enhance thymopoiesis (14–16). IL-7 promotes the production of T cells and their peripheral expansion (17, 18). Keratinocyte growth factor (KGF) stimulates expansion of the thymic epithelium, allowing for an increase in ETPs and thymic enlargement (15, 19). The ablation of gonadal hormones increases chemokine ligand 25 (CCL25) and has similar overall effects (20–24).
The ability to affect the viability of early stage thymocytes might also provide an avenue for promoting thymopoiesis and the cellularity of the thymus. Prolongation of the survival of these cells might increase their pool size. Further amplification and development from this expanded pool might, in turn, allow for an increase in more mature thymocytes. Enhanced survival of DN stage cells might also be reflected in an increase in the radioresistant pool (25–27).
MCL1 is an antiapoptotic member of the BCL2 family that was identified in ML-1 immature myeloblastic leukemia cells, where its expression is markedly stimulated early in monocytic differentiation induced with 12-O-tetradecanoyl phorbol acetic acid (TPA) (28). MCL1 promotes viability at critical stages in the differentiation of various lineages (29,30). Accordingly, a model has been proposed suggesting that, by affecting viability in particular—often early—stages along the differentiation continuum, MCL1 modulates the pools of cells available for further amplification and development (31). This serves to regulate the production of cell types and lineages as needed by the organism. Conditional knockout of MCL1 with Lck-Cre prevents progression beyond the DN2/3 stage, resulting in a profound decrease in thymocyte number (30, 32). MCL1 therefore represents a candidate that might be capable of enhancing the viability of primitive cells and promoting the expansion of developing thymocytes.
Transgenic mice containing human MCL1 under the control of its endogenous promoter express the transgene-encoded protein in hematolymphoid tissues (at levels approaching those seen upon stimulation of ML-1 cells with TPA) and exhibit splenic enlargement (∼1.5-fold) (33). Some of the mice exhibited thymic enlargement, although this was not initially followed up on because it was not seen in other animals.
The present work showed that enlargement of an undistorted thymus occurs in MCL1 transgenic females, where it is seen at 1 month and maintained until at least 7 months of age. Unexpectedly, this was not associated with increased viability in the major DP and SP cell subpopulations. Instead, the small DN1 cell subset exhibited enhanced survival, and this was reflected in disproportional expansion and an increase in the radioresistant pool of these cells. The minute ETP population within DN1 also exhibited increased viability and disproportional expansion. In sum, the MCL1 transgene increases the viability and pool size of primitive ETP/DN1 cells, enhancing thymopoiesis in females.
Methods
Animals and whole-body irradiation
Hybrid C57/BL6 X SJL MCL1 transgenic mice [B6; SJL-Tg (MCL1)8caig] were previously generated using a mini-gene construct containing human genomic DNA; the purpose was to utilize the transgene under the control of its endogenous promoter, with the anticipation that the additional copies of MCL1 would be expressed in tissues that normally express this gene product, including hematolymphoid tissues such as the spleen and thymus (33). These mice were mated to inbred C57/BL6 mice (Taconic) for >10 generations. Purity was confirmed by genome scanning (Jackson Laboratories, Bar Harbor, ME, USA), and the MCL1 C57/BL6 line was designated B6.SJL-Tg (MCL1)8caig/caig. Genotyping was carried out using primers that detect the human MCL1 transgene but not the endogenous mouse homologue (34), with the RedExtract-N-AMP tissue PCR kit (Sigma–Aldrich).
For irradiation, mice were irradiated with two doses of 4.75 Gy each, 3–4 h apart, in a cesium-137 γ irradiator (J.L. Shepherd, San Fernando, CA, USA).
The animal work in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth Medical School (IACUC protocol No: 11-01-02; the institutional Animal Welfare Assurance No: A3259-01).
Thymocyte and bone marrow cell collection
After grinding the thymus with a 1 ml syringe plug, cells were either passed through a 75 μm cell strainer (BD Biosciences, Bedford, MA, USA) followed by a 100 μm sterile filter membrane (Labcor Products, Frederick, MD, USA) or removed by agitation in RPMI medium at 4°C followed by digestion with Collagenase/Dispase/DNase I (Roche) twice for 15 min each with intermittent shaking. Bone marrow cells were flushed out of the mouse femur using a 3 ml syringe and 25G5/8 needle. The RBCs were lysed with ammonium chloride before staining with surface markers. Cell counts were determined by Coulter counter.
Western blotting for MCL1 transgene expression
Protein was extracted in ice-cold radioimmunoprecipitation assay buffer (Pierce, Rockford, IL, USA) containing fresh protease (Roche) and phosphatase (Sigma–Aldrich) inhibitor cocktails, using an ultrasonic homogenizer (Sonics and Materials, Inc., Newtown, CT, USA). Equal amounts of protein [BCA protein assay (Pierce)] were loaded on 12.5% polyacrylamide gels and subjected to western blotting. Blots were probed using a rabbit polyclonal anti-MCL1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-819, 1:1000) under conditions that detect human but not endogenous mouse MCL1 (35); they were then re-probed with anti-GAPDH (sc-25778, Santa Cruz Biotechnology; 1:1000) or anti-β actin (AC-15, Abcam; 1:1000). Densitometric scanning of non-saturated blots was carried out using an Umax PowerLook III scanner, and the bands were quantified using Labworks 4 software (UVP Imaging and Analysis, Inc., Upland, CA, USA).
Immunofluorescence staining for expression of keratins 5 and 8
After embedding the freshly isolated thymus in optimal cutting temperature compound (Sakura, Torrance, CA, USA), serial 8 μm sections were cut and fixed in cold acetone, blocked with normal goat serum plus 2.4G2 antibody (PharMingen, San Diego, CA, USA) and incubated with anti-keratin 8 (C-04, Abcam) and anti-keratin-5 antibody (Covance, Berkeley, CA, USA). The secondary antibodies used were Dylight 488-conjugated goat anti-mouse IgG and Dylight 594-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Images were acquired with a Nikon 90i fluorescence microscope (×10 objective lens) equipped with a Coolsnap HQ2 camera and Nikon Elements software.
Flow cytometry
The fluorochrome-conjugated antibodies PE-B220, -CD19, -IgM, -Gr-1, -TER-119, -CD3ϵ, -NK1.1, -CD11b, -CD8α, -CD127 and PerCP-Cy5.5-EpCAM were purchased from BioLegend (San Diego, CA, USA). PE-TCRβ, -TCRγσ, -Flt3, APC-CD25 and APC-CD117 (c-kit) antibodies were from BD Pharmingen. FITC-Sca-1 and PE/Cy5-CD44 antibodies were from eBioscience (San Diego, CA, USA), and the FITC-CD117 antibody was from Southern Biotechnology (Birmingham, AL, USA).
DP, SP4 and SP8 cells were assayed by staining with APC-CD4 plus PE-CD8α, after using the dot plot of forward scatter versus side scatter to gate out debris. DN (Lin−) cells were assayed by eliminating cells staining with the PE-lineage markers B220, CD19, IgM, Gr-1, TER-119, CD3ϵ, NK1.1, CD11b, CD8α, anti-mouse TCRβ and anti-mouse TCRγσ. The percentages of cells in these subsets, along with the total thymocyte number, were used to calculate the number of cells in each subpopulation.
Cells in the DN1–DN4 quadrants were assessed by staining DN (Lin−) cells with PE/Cy5-CD44 and APC-CD25. It is noted that cells in the DN4 quadrant should be considered as CD44−CD25− cells and may not all be true DN4 stage thymocytes. For example, cell death occurs throughout thymocyte development and dying cells that have lost markers but not yet been cleared could fall within the DN4 quadrant. The purpose of the present work was to examine cells in these quadrants in non-transgenic versus transgenic animals. Therefore, the results obtained for the DN4 quadrant are presented, not as a precise assessment of DN4 stage cells, but for the purpose of completeness. Another point is that, when results from multiple experiments were combined, the percentages of cells in the DN1–DN4 quadrants were calculated relative to the total thymocyte population rather than the DN (Lin−) subpopulation. This was because of experiment-to-experiment variability in the percentage of cells in the DN4 quadrant calculated relative to the DN (Lin−) subpopulation. This artifactually affected the percentages in the other subsets calculated relative to the DN (Lin−) subpopulation. Using total thymocytes as a basis for calculating the percentages of cells in the DN subsets, in cases where multiple replicate experiments were carried out, provided a denominator that circumvented fluctuations in the DN4 subset in experiments carried out on different days.
ETPs were identified by gating on PE-Lin−/PE-CD127−/APC-CD25− cells and then identifying PE/Cy5-CD44hi/FITC-CD117hi cells. The lineage-negative, sca-1-poistive c-kit positive cells (LSK) population in the bone marrow was assessed by gating out PE-lineage markers and then staining with FITC-Sca-1 and APC-CD117. Large Lin− EpCAM+ cells representing thymic epithelial cells were assessed as described (36). Data were collected using the BD FACScan installed with a 25 mW diode red laser and Rainbow software (Cytek development, Fremont, CA, USA) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Assessment of TCR-alpha recombination
Assay for signal-joint T-cell receptor excision circle (sjTREC) formation was performed as described (37) with modifications. DNA was extracted from 5 × 106 thymocytes using the DNeasy blood and tissue kit (Qiagen), dissolved in 100 μl DNase-free water, measured using a Nano-drop (Thermo Scientific), and 5 μl was used as a template in real-time PCR. The primers (forward, 5′-CATTGCCTTTGAACCAAGCTG-3′; reverse, 5′-TTATGCACAGGGTGCAGGTG-3′) and probe (5′-FAM-TGCTGTGTGCCCTGCCCTGCC-TAMRA-3′) were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Using TaqMan fast universal PCR master mix (Applied Biosystems), PCR was run at 95°C for 10 min followed by 45 cycles of 95°C for 30 s and 62°C for 1 min (ABI 7500 fast system). The absolute quantification method was used with standard curves generated from serial dilutions of pCR2-sjTREC plasmid (from Dr Gregory Sempowski) along with no-template controls. Each sample was tested two to three times in triplicate. The results were normalized as sjTREC molecules/microgram genomic DNA, and total sjTREC formation per thymus was calculated.
Annexin V and 5-bromo-2-deoxyuridine staining
To assay viability, cells were stained with cell surface markers to differentiate thymocyte subpopulations, washed with annexin V binding buffer and stained with FITC-annexin V for 15 min on ice. In some experiments, 7-amino-actinomycin D (7-AAD) was added to distinguish early versus late apoptosis. To assay viability upon explantation into primary culture, freshly isolated thymocytes were plated in DMEM medium containing 10% fetal bovine serum (5 × 106 cells per well of a 48 well plate) and cells were monitored for up to 3 days.
The above approach for co-staining with cell surface markers allowed for the detection of annexin V+ DN1–DN4 and more mature cells but was not sufficiently sensitive for assay of the minute ETP population even when large numbers of thymocytes were collected during flow cytometry. However, annexin V staining could be successfully applied after enrichment of ETPs by the removal of CD8 expressing thymocytes. To this end, thymocytes were incubated with anti-CD8 mAb (clone 53–6.7; BD Pharmingen) and rabbit complement (Cedarlane, Ontario, Canada) for 30 min at 37°C. Cells were then purified on Cedarlane lympholyte-M density cell separation medium, stained with FITC-annexin V, PE-lineage markers, PE-CD127, PE-CD25, PE/Cy5-CD44 and APC-CD117 and subjected to flow cytometry with the collection of 106 events. This approach was found to be preferable to other enrichment methods such as flow cytometric sorting, as the latter did not reliably preserve ETP viability. However, neither of these ETP enrichment approaches was feasible for use along with permeabilization to assay 5-bromo-2-deoxyuridine (BrdU) incorporation because ETPs are present at levels below the sensitivity of this assay even when optimal cell permeabilization procedures are used (38).
To assess cell proliferation, mice were injected twice with 1 mg of BrdU (i.p.), with a 1.5 h interval between injections, and thymocytes were harvested 2 h after the second injection. They were then stained with cell surface markers followed by fixation, permeabilization, DNase treatment and incubation with FITC-anti-BrdU for detection as described in the FITC-BrdU flow cytometry kit from BD Pharmingen.
Real-time PCR for CCL25 and p-selectin expression
The RNeasy mini kit (Qiagen) was used to extract RNA from the stromal tissue remaining after thymocyte removal by agitation and brief digestion with Collagenase/Dispase/DNase (Roche), using previously used procedures (39). The RNA was transcribed into cDNA with ProtoScript first-strand cDNA synthesis kit (New England Biolabs). cDNA was used as the template using primers for CCL25 (forward, 5′-AGGCACCAGCTCTCAGGACC-3′; reverse, 5′-GTCTTCAAAGGCACCTTGGGCATGG-3′), p-selectin (forward, 5′-ATGCCTGGCTACTGGACACT-3′; reverse, 5′-CTTCATCGCACATGAACTGG-3′) and GAPDH (forward, 5′-AACTTTGGCATTGTGGAAGG-3′; reverse, 5′-GGATGCAGGGATGATGTTCT-3′). Real-time PCR was carried out with SYBR Green PCR master mix (Applied Biosystems) and run in a 7500 fast real-time PCR system (Applied Biosystems) using the following program: 95°C for 3 min, followed by 45 cycles at 95°C for 30 s, 60°C for 40 s and 68°C for 40 s. A dissociation reaction was performed to check the specificity of the amplification in each experiment. Expression of CCL25 and p-selectin was normalized to that of GAPDH utilized the 2ΔΔCt method.
Bone marrow transplantation and generation of chimeric mice
C57BL/6 CD45.1 congenic mice (B6.SJL-Ptprca/BoyAiTac) were purchased from Taconic and maintained in the animal facility in Dartmouth Medical School. For use as bone marrow recipients, non-transgenic females (CD45.1; 1–2 months old) were irradiated with 9.5 Gy (1Gy/min) on a cesium-137 irradiator, where the dose was delivered in two fractions of 4.75 Gy separated by 3 h. Recipients then received 1 × 107 freshly isolated bone marrow cells, which were extracted from either non-transgenic or transgenic females (CD45.2; 1–2 months old) and delivered via the retro-orbital route. The thymi of reconstituted animals were examined after 7 weeks. Similar procedures were used to transplant non-transgenic versus transgenic recipient females with bone marrow donor cells from CD45.1 congenic females.
Experimental design and statistical analysis
Since a limited number of animals could be processed in a given experiment, transgenic and non-transgenic animals (typically two of each) matched for age and sex were assayed in parallel, and a series of such experiments was carried out. Two-way analysis of variance (ANOVA) was carried out with post-hoc testing using the Holm–Sidak method (SigmaStat software using log- or square root-transformed data). Because some experiment-to-experiment variability was noted in monitoring the small fraction of cells in the DN1 (Lin−) subset, data from matched non-transgenic and transgenic animals assayed in the same experiment were considered as paired (repeated) measures and analyzed by RMANOVA. The transgenic/non-transgenic ratio of cell numbers was calculated by averaging the number of cells present in transgenic and non-transgenic animals assayed in the same experiment. The ratios obtained in the series of experiments were averaged, and appropriate methods were used to test for changes in this ratio (two-tailed test) (40). The half-life of disappearance of viable cells in culture was estimated by fitting exponential decay curves using non-linear regression (SigmaStat).
Results
Thymocyte numbers are increased in MCL1 transgenic females and remain elevated during involution
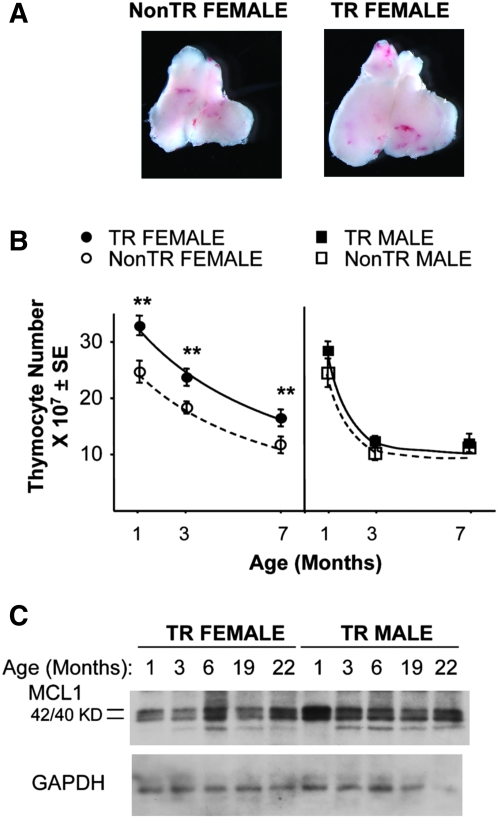
A large cohort of 1- to 7-month-old males and females was examined because of the possibility that thymic enlargement might be prominent at a certain age or in animals of a particular sex (13, 24). The findings obtained showed that enlargement occurred in transgenic females (Fig. 1A), where it was seen in peripubescent (1 month old) as well as adult animals (Fig. 1B). These initial findings allowed us to focus on females in the study below, which was aimed at gaining a better understanding of the effects of the MCL1 transgene in the thymus. This organ, like other components of the immune system, exhibits sex-related differences and is affected in complex ways by reproductive and other hormones (24, 42). Although the underlying mechanisms for this are poorly understood (24, 43–45), these may play a role in the observed lack of a significant effect in vivo in MCL1 transgenic males as this was not due to an absence of transgene expression (Fig. 1C). Overall, the MCL1 transgene resulted in thymic expansion in young females and this was maintained into adulthood as the thymus underwent involution.
Fig. 1.
Thymic enlargement in MCL1 transgenic females. (A) The thymus from a 7-month-old MCL1 transgenic female (right panel) and an age-matched female non-transgenic control (left panel) were visualized using a Leica MZFIII stereomicroscope equipped with a Plan 1X 0.14(0.025–0.125) objective lens (Leica, Heerbrugg, Switzerland). (B) Total thymocyte number was assayed in females and males of various ages. The data shown are from a total of 119 animals assayed in a series of 31 experiments in which age- and sex-matched transgenic and non-transgenic animals were compared in parallel. The points represent the mean of 7–17 females or 4–8 males. Asterisks in this and subsequent figures indicate differences between MCL1 transgenic and non-transgenic animals (**P < 0.05). (C) Expression of the MCL1 transgene was monitored in females and males of increasing age. Equal amounts of protein were loaded for western blotting with large format gels, which separate the two bands of the closely spaced 42/40 KD MCL1 doublet that derives from full-length mRNA (41). Transgene expression in the C57/BL6 mice used in the present work did not differ significantly from that seen on the original hybrid background, where the 22-month-old mice in the western blot shown were from the original hybrid line. No significant difference between the sexes was seen upon western blotting for MCL1 transgene expression in an additional three males and three females at 3 months of age (not shown).
MCL1 transgenic females exhibit proportional expansion of the major thymocyte subpopulations along with the stroma but no change in the viability of DP or SP cells
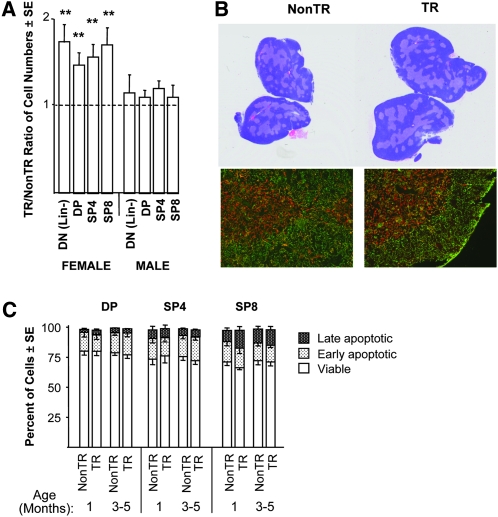
The numbers of DN (Lin−), DP, SP4 and SP8 thymocytes were increased to a similar extent in transgenic females (1.5- to 1.7-fold on average, Fig. 2A), such that the relative proportions of cells in these major thymocyte subpopulations remained constant (Supplementary Figure 1 is available at International Immunology Online). Total thymic sjTREC formation was likewise increased ∼1.5-fold (Fig. 2A). Hematoxylin and eosin staining showed that thymic architecture was normal (Fig. 2B, upper photographs). Immunofluorescent keratin staining suggested that areas containing medullary versus cortical epithelial cells were expanded comparably, with the density of these cells being unaffected (Fig. 2B, lower photographs). Accordingly, EpCAM+ thymic epithelial cells were increased as were thymocytes (Fig. 2B). In brief, transgenic females exhibited overall expansion of a grossly unaltered thymus.
Fig. 2.
Proportional expansion of the major thymocyte subpopulations, without a change in their viability, in MCL1 transgenic females. (A) The numbers of cells in the DN (Lin−), DP and SP subpopulations were assessed in experiments in Fig. 1(B), and the ratio of these numbers in transgenic versus non-transgenic animals was calculated. The ratios shown represent 15 experiments for DN (Lin−) cells and 20 experiments for DP and SP cells. These ratios did not vary with age and, for females, the ratios for DN (Lin−), DP and SP cells differed from 1 (**P < 0.05) but did not differ significantly from each other. The cell numbers used to calculate these ratios are shown in Supplementary Figure 1A (available at International Immunology Online). sjTREC formation/thymus was increased 1.5-fold [±0.17; standard error (SE)] in transgenic as compared with non-transgenic females (10 each, assayed in five separate experiments) examined at 1–3 months of age. (B) Sections of thymi of 1-month-old females were stained with hematoxylin and eosin (upper photographs), or frozen sections (lower photographs) were stained with anti-keratin 5 (red) and anti-keratin 8 (green). Large EpCAM+ cells (36) were increased 1.3-fold and to the same extent as thymocytes in 1-month-old transgenic as compared with non-transgenic females (two each). (C) The DP, SP4 and SP8 subpopulations were assayed for the percentages of viable (annexin V−/7-AAD−), early apoptotic (annexin V+/7-AAD−) and late apoptotic (annexin V+/7-AAD+) cells. The values shown represent the mean of 3–8 female mice. For clarity, the SE is shown in the downward direction in some cases. Non-transgenic and transgenic males did not exhibit a significant difference in the viability of DP, SP4 or SP8 cells (not shown).
DP and SP cells, which account for the majority of thymocytes, did not exhibit increased viability in the presence of the transgene (Fig. 2C), which was surprising in view of the well-documented role of MCL1 in the promotion of viability (31). Nonetheless, this was true upon examination directly after harvest of thymocytes, as well as upon their incubation in primary culture (Supplementary Figure 2 is available at International Immunology Online).
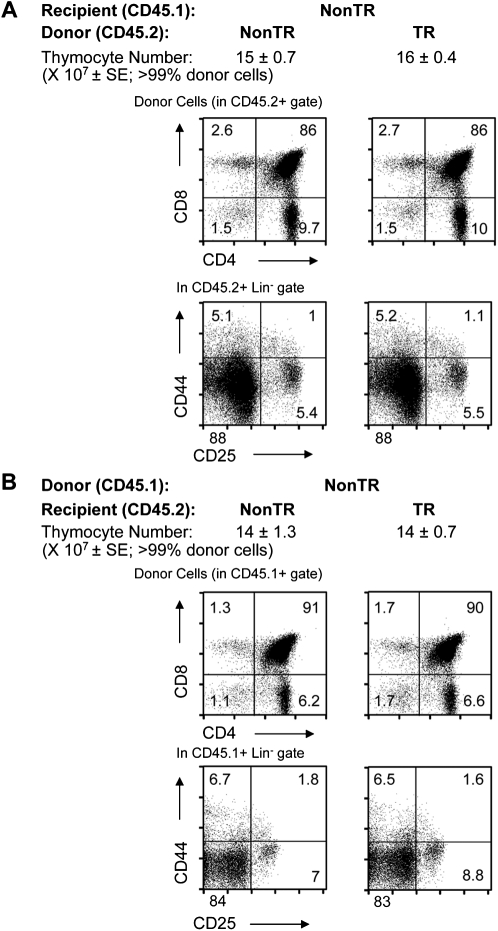
In view of the above observation (Fig. 2A), females were also assessed for thymic enlargement in the setting of bone marrow transplantation using either transgenic donor cells or transgenic recipients. To this end, bone marrow donor cells from MCL1 transgenic versus non-transgenic females (CD45.2) were transferred into lethally irradiated non-transgenic female recipients (CD45.1). Comparable total thymocyte numbers and subset proportions were seen 7 weeks later when >99% of thymocytes were donor-derived (Fig. 3A). Similar observations were made in converse experiments involving the transfer of bone marrow cells from non-transgenic female donors into transgenic versus non-transgenic recipients (Fig. 3B). Since no change was seen in the setting of bone marrow transplantation in the presence of either transgenic thymocytes or stroma, the studies below focused on intact transgenic animals. Another option for future studies will be to use fetal tissue, where initial experiments show that transgenic as well as non-transgenic enriched Lin− bone marrow cells from females can develop in fetal organ tissue culture (Supplementary Figure 3 is available at International Immunology Online). Since thymic enlargement is seen at a young age (Fig. 1) and could involve events that occur during development, it may be recapitulated in fetal lobe transplantation.
Fig. 3.
Lack of thymic enlargement after irradiation/bone marrow transplantation using MCL1 transgenic females as either a source of donor cells or as recipients. (A) Non-transgenic female mice (CD45.1) were subjected to lethal irradiation and transplanted with bone marrow cells from either non-transgenic or MCL1 transgenic congenic (CD45.2) females. After 7 weeks of reconstitution, >99% of thymocytes were of donor origin as assessed by expression of CD45.2. The thymocyte numbers shown represent the mean ± SE of four animals assayed in two independent experiments. Donor-derived cells in the DP and SP (upper panels) as well as the DN1–DN4 (lower panels) subpopulations are shown, where the percentages of cells in each quadrant are shown on the dot plots. (B) Irradiated non-transgenic or MCL1 transgenic females (CD45.2) were transplanted with bone marrow cells from non-transgenic congenic (CD45.1) females. After 7 weeks of reconstitution, >99% of thymocytes were of donor origin as assessed by expression of CD45.1. The thymocyte numbers shown represent the mean ± SE of four animals assayed in three independent experiments. Donor-derived cells in the DP and SP (upper panels) as well as the DN1–DN4 (lower panels) subpopulations are shown, where the percentages of cells in each quadrant are shown on the dot plots.
DN1 cells in MCL1 transgenic females exhibit disproportional expansion and increased viability but not proliferation
While it was puzzling that transgenic females exhibited proportional thymic enlargement without a change in the viability of DP and SP cells, this suggested the possibility that thymocytes at an early stage of development might be affected. For example, an overall increase in thymocyte numbers could relate to an increase in a small population of primitive cells capable of amplification and further development. Indeed, examination of the DN (Lin−) subsets showed that the small percentage of thymocytes in DN1 was consistently increased in transgenic females, while the percentages of cells in the other DN (Lin−) subsets were not significantly altered (Fig. 4A). In contrast, disproportional expansion of DN1 cells was not seen in males (Fig. 4A).
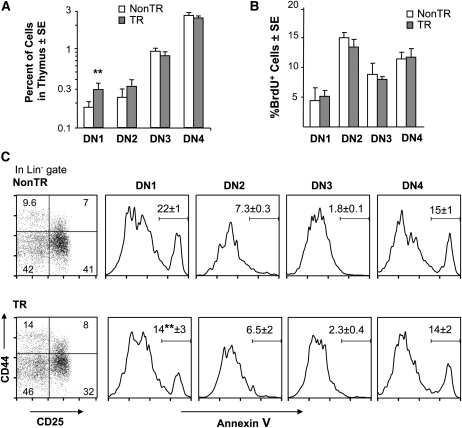
Fig. 4.
Disproportional expansion of DN1 cells, which exhibit increased viability and the maintenance of proliferation, in MCL1 transgenic females. (A) The percentages of cells in the DN1–DN4 subsets were assayed in females in six independent experiments. The values shown represent the mean percentage of cells in the indicated subset out of total thymocytes (**P < 0.05), where these percentages are shown on a log scale to accommodate the small size of the DN1 and DN2 populations. The values for DN1 cells in non-transgenic and transgenic males averaged 0.21% ± 0.04 (SE) and 0.22% ± 0.03, respectively, where the presence of the transgene similarly did not significantly affect the values for the other DN subsets. (B) The percentages of BrdU+ cells in the DN1–DN4 quadrants were assessed in 1- to 2-month-old females, where the values shown represent the mean of three to four animals. (C) The percentages of viable versus apoptotic (annexin V+) cells in the DN1–DN4 quadrants were assayed in 1 month females. In the leftmost panels, the values on the dot plots represent the percentage of cells in each quadrant [% of the DN (Lin−) population; mean of three animals]. Representative histograms are shown for the annexin V assay, where the values listed on the histograms represent the mean percentage annexin V+ cells (±SE) for the three animals. These results were analyzed along with those from 7-month-old females (Supplementary Figure 4A is available at International Immunology Online), a significant difference being seen in DN1 cells from transgenic versus non-transgenic animals (**P < 0.05).
The increase in DN1 cells could relate to an enhancement of their viability, given the known antiapoptotic effects of MCL1 (31). This proved to be the case as the viability of DN1 cells was elevated in transgenic as compared with non-transgenic females, a decrease in the percentage of apoptotic DN1 cells being seen at 1 month (Fig. 4C) as well as 7 months of age (Supplementary Figure 4A is available at International Immunology Online). In contrast, viability was unchanged in cells in the other DN quadrants, the viability of DN2/3 cells being high (>80%) in the absence as well as the presence of the transgene. No change in viability in any of these subsets was seen in thymocytes harvested from transgenic versus non-transgenic males (Supplementary Figure 4B is available at International Immunology Online).
An increase in DN1 cells and in overall thymocyte numbers could also relate to a stimulation of proliferation/cell cycling, although this seemed an unlikely possibility in the case of MCL1 because proliferation is not affected in stably transfected cell lines (33) and can be inhibited in transient transfectants (46). Initial examination of BrdU incorporation in total thymocytes showed that the percentage of BrdU+ cells was comparable in transgenic and non-transgenic females (Supplementary Figure 5A is available at International Immunology Online). In other words, the numbers of BrdU+ thymocytes as well as the numbers of total thymocytes were increased, such that the percentage of BrdU+ cells was unchanged. Examination of the thymocyte subpopulations likewise showed that transgenic and non-transgenic females did not differ in terms of the percentages of BrdU+ cells present in the DN1–DN4 (Fig. 4B) or the DP or SP subsets (Supplementary Figure 5A is available at International Immunology Online). Proliferation thus did not appear to be stimulated in the presence of the transgene. Because DN1 cells were disproportionally expanded in transgenic females (Fig. 4A), with the same percentage being BrdU+ (Fig. 4B), the number of BrdU+ DN1 cells was increased (Supplementary Figure 5B is available at International Immunology Online). The ratio of BrdU+ DN1 cell numbers in transgenic versus non-transgenic females averaged 2.7. This ratio for BrdU+ DN2–DN4, DP and SP cell numbers in transgenic versus non-transgenic females averaged 1.3–1.7, mirroring the 1.4-fold increase in thymocyte numbers (Supplementary Figure 5B is available at International Immunology Online). The fact that among the DN2–DN4, DP and SP subsets, a particular subset did not stand out as differing from the others in terms of a stimulation of BrdU incorporation agreed with the above finding that the relative proportions of cells in these subpopulations were unchanged (Supplementary Figures 1 and 4A are available at International Immunology Online). While it is possible that a stimulation of growth occurred in one of these subsets but was not detected by BrdU incorporation, this did not result in an ostensible skewing of sjTREC formation relative to thymocyte numbers (Fig. 2A). It remains possible that small changes in BrdU incorporation occurred in various thymocyte subsets and thus were not detected and did not result in discrete changes in the proportions of a particular subpopulation. If changes occurred equivalently before and after sjTREC formation (47), this parameter could also be unaltered. In addition, such changes were not detected in female mice reconstituted with bone marrow from transgenic or non-transgenic female donors (Fig. 3A). Overall, the above experiments detected a difference in transgenic as compared with non-transgenic females in that the small DN1 subset was disproportionally expanded, with an increased proportion of these cells being viable and an unchanged proportion undergoing proliferation.
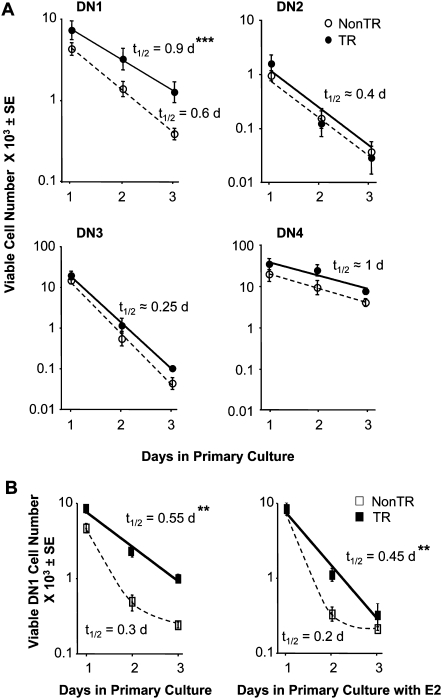
DN1 cells from MCL1 transgenic females as well as males exhibit enhanced viability upon explantation into primary culture
Explantation of splenocytes from MCL1 transgenic animals into primary culture had shown that their viability was lost more slowly than that of non-transgenic control cells (35). The same approach was therefore used to follow viable DN (Lin−) thymocytes in the various subsets upon incubation in vitro. Viable DN1 cells from non-transgenic females were lost from primary cultures with a half-life of ∼0.6 days, whereas this value for their transgenic counterparts was significantly extended to ∼0.9 days (Fig. 5A). Cells in the other DN (Lin−) subsets did not exhibit a significant change in survival, where DN2 and DN3 cells underwent rapid loss in the presence or absence of the transgene. Thus, a selective effect of the MCL1 transgene on the viability of DN1 stage thymocytes was seen, not only upon examination directly after harvest but also upon incubation in primary culture in the absence of stroma.
Fig. 5.
Extension of DN1 cell survival in primary cultures of thymocytes from MCL1 transgenic females as well as males. (A) Thymocytes from females were placed into primary culture and monitored on days 1–3 for the number of viable cells in the DN1–DN4 quadrants. Two independent experiments were carried out, the first with a non-transgenic versus a transgenic female and the second with two females of each type, where the points represent the average number of viable (annexin V-) cells from the three animals. Duplicate wells were followed for each animal, and the half-life of loss of viable cells (t1/2) was calculated by non-linear regression (***P = 0.01). (B) An identical experiment was carried out using thymocytes from males [three non-transgenic animals and four transgenic animals (**P < 0.05)]. Viable DN2/3 cells from these animals were lost rapidly and could not be followed in culture. Cells from each animal were cultured in either the absence (left panel) or the presence (right panel) of 10 nM beta-estradiol (E2).
Unexpectedly, examination of thymocytes from males revealed that the loss of viable DN1 cells from transgenic animals likewise occurred more slowly in primary culture than did that of their non-transgenic counterparts (Fig. 5B, left panel, and see legend regarding the rapid loss of other DN cells). In other words, while no change was seen upon examination of DN1 cells directly after harvest from transgenic males (Supplementary Figure 4B is available at International Immunology Online), an extension of viability was seen upon incubation in vitro (Fig. 5B). This was also seen in parallel cultures exposed to beta-estradiol in this experiment (Fig. 5B, right panel). These findings suggest that factors present in the in vivo host environment of the male counteract the effect of MCL1 and that the effect of the transgene is unmasked upon removal from this environment and incubation in primary culture.
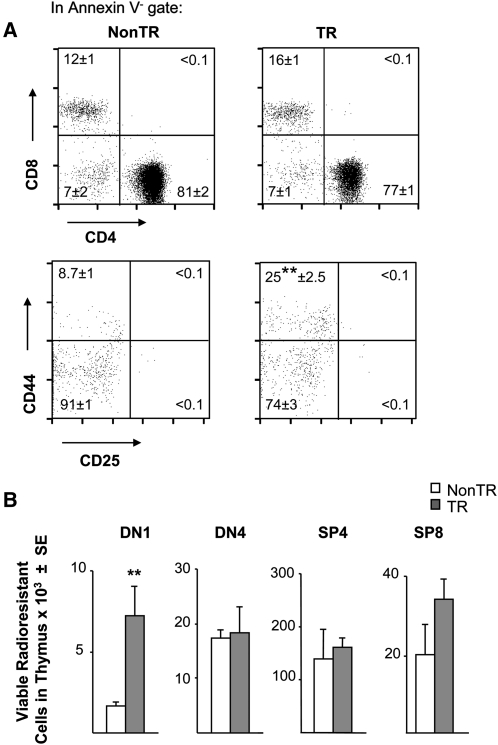
MCL1 increases radioresistant DN1 cells and enhances their viability during recovery from radiation
Thymocytes are highly sensitive to damage induced by whole-body irradiation (25, 26). However, DN cells are more radioresistant than DP cells, the former demonstrating a loss of ∼1 log under conditions that cause 2- to 3-log kill of the latter (25, 26). Based on the increased viability of DN1 cells seen in transgenic females in vivo and in vitro (Figs 4 and 5), it seemed likely that an increased pool of radioresistant DN1 cells might remain present upon exposure of these animals to irradiation. Indeed, upon irradiation with doses that killed essentially all DP cells (Fig. 6A, upper pair of dot plots), more viable DN1 cells remained present in transgenic as compared with non-transgenic females (Fig. 6A, lower pair of dot plots and Fig. 6B). In contrast, essentially, no viable DN2 or DN3 cells remained present, their sensitivity to radiation recalling the rapid loss of these cells seen upon in vitro culture (Fig. 5).
Fig. 6.
Elevated radioresistant DN1 cell pool in MCL1 transgenic females. (A) Female mice were exposed to whole-body irradiation and assayed after 2 days for remaining viable (annexin V-) cells in the DP and SP subpopulations (upper panels) and the DN1–DN4 quadrants (lower panels). A representative dot plot is shown where the mean percentage of cells in each quadrant is indicated (±SE of three animals; **P < 0.05). (B) The total number of viable cells in the various subsets was calculated for the above animals.
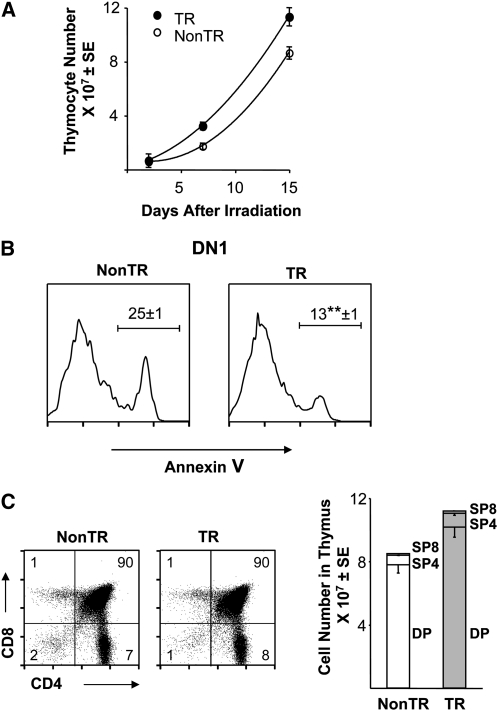
While radioresistant immature thymocytes cannot provide permanent reconstitution, they can reinitiate growth and development and thus provide a ‘first wave’ in the recovery of the thymus after transplantation (25, 26). Examination at 1–2 weeks after irradiation demonstrated increased thymocyte numbers in transgenic as compared with non-transgenic females (Fig. 7A), with enhanced DN1 cell viability being maintained in the transgenic animals (Fig. 7B). The fact that DP cells were essentially completely killed (Fig. 6) but that the recovering population consisted primarily of DP and SP cells (Fig. 7C) indicated that the immature cells remaining after irradiation were capable of proliferation and further development. In sum, irradiated transgenic females exhibited enhanced DN1 cell viability and robust regrowth of developing thymocytes.
Fig. 7.
Augmentation of DN1 cell viability and thymocyte numbers in MCL1 transgenic females recovering from radiation. (A) Female mice (1–2 months of age) were exposed to whole-body irradiation (9.5 Gy) and assayed for total thymocyte numbers after 2–15 days. Each point represents the mean ± SE of three to six animals assayed in two independent experiments. Animals to be assayed at 15 days underwent bone marrow transplantation directly after irradiation to allow for their survival, where essentially all thymocytes (>99%) present at 15 days were of host rather than donor origin. (B) The DN1 quadrant was assayed for viable versus apoptotic cells at 15 days post-irradiation, where the values on the histograms represent the mean percent annexin V+ cells (±SE of three animals; **P < 0.05). (C) DP and SP cells were assayed at 15 days post-irradiation. A representative dot plot is shown where percentage of cells in each quadrant is indicated. The bar graph indicates the total cell number in these subpopulations (mean ± SE of three animals), where the SE is shown in the downward direction in some cases for clarity.
MCL1 increases viable ETPs but not bone marrow LSK precursors
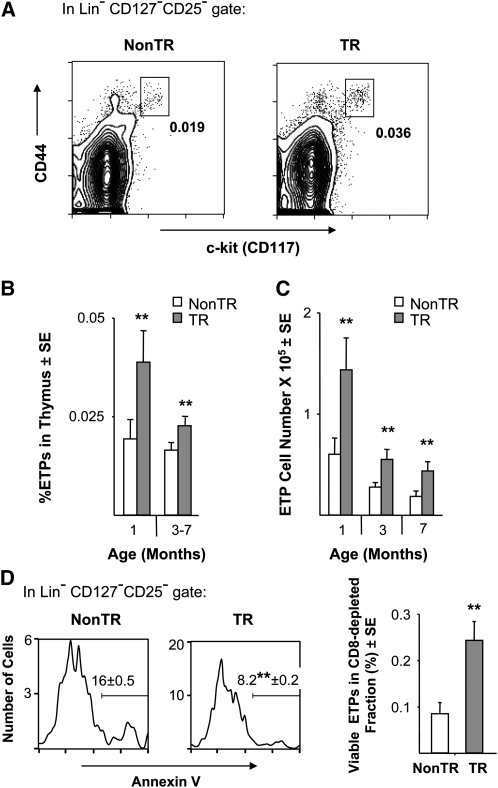
ETPs constitute only a fraction of DN1 cells, estimates of their percentage in total thymocytes being in the range of 0.007–0.04% and depending on age and possibly on the markers considered (2–6). This minute population is nonetheless critically important as it gives rise to DN2–DN4 and more mature cells. Like DN1 cells, ETPs were increased in transgenic females, both in terms of their numbers and in terms of proportion to the augmented thymocyte population (Fig. 8A and B). The effects on ETPs were similar to the effects on total thymocytes in that expansion was not seen in males, and ETP pools declined with age as expected (3, 4) but remained elevated in the presence of the transgene (Fig. 8C).
Fig. 8.
Increase in viable ETPs in MCL1 transgenic females. (A) ETPs in representative 3-month-old females are shown in the boxes, where the percentage of ETPs within the total thymocyte population is indicated. (B) The percentage ETPs was assessed in females in 19 of the experiments in Fig. 1(B). The points shown represent the mean of 6–10 animals at 1 month and 10–17 animals at 3–7 months of age (**P < 0.05). In non-transgenic and transgenic males, respectively, the percentage of ETPs was 0.022% ± 0.003 (SE) and 0.026% ± 0.003 at 1 month of age and 0.009% ± 0.004 and 0.015 ± 0.004 at 3–7 months. (C) The total number of ETPs present in the thymus was calculated from the experiments in panel B, where the transgenic/non-transgenic ratio for ETP cell number averaged 2.4 [±0.26 (SE); **P < 0.05]. (D) ETP viability was monitored after enrichment of immature thymocytes from females by anti-CD8 complement mediated lysis. Three independent experiments were carried out using pairs of 1-month-old transgenic versus non-transgenic females. In the panels on the left, the mean percentage of ETPs that were annexin V+ (±SE) is listed on the histograms (**P < 0.05; Student’s t-test). In the graph on the right, viable (annexin V-) ETPs as a percent of the enriched cell population is shown.
Because of the rarity of ETPs, enrichment by depletion of CD8+ cells was carried out in order to assay their viability. Higher viability was observed in ETPs from transgenic as compared with non-transgenic females (Fig. 8D, left side), where the values obtained from non-transgenic animals agreed very closely with the findings of others (3, 4). In addition, the recovery of viable ETPs after enrichment was increased ∼3-fold (Fig. 8D, right side), in keeping with the increase in the total number of these cells (Fig. 8C) and in their viability (Fig. 8D left side). Unfortunately, the sensitivity of assay for apoptosis could not be further enhanced to accurately monitor ETPs after irradiation or upon explantation into primary culture, where cell numbers and viability are further reduced.
The bone marrow precursors to ETPs lie within the Lin−Sca1+c-kit+ (LSK) stem/progenitor cell population, although the hierarchical relationships leading to ETP formation are an area of active investigation (6, 8, 9, 48). In contrast to ETPs in the thymus, the LSK reservoir in the bone marrow was not expanded in transgenic females (Supplementary Figure 6 is available at International Immunology Online). The homing of precursors to the thymus is mediated by the expression of adhesion molecules and cytokine ligands on thymic stromal cells (e.g. p-selectin and CCL25) (49). Thymic expansion induced by androgen ablation involves the up-regulation of CCL25 on thymic epithelial cells (20). However, in contrast to androgen ablation, stromal tissue from transgenic females did not exhibit an increase in CCL25 mRNA, as expression was 1.5-fold ± 0.64 that seen in non-transgenic controls (SE of 2–3 experiments). This ratio for p-selectin was 1.2 (±0.1). These findings thus did not provide evidence for an increase in bone marrow precursors or for elevated expression of thymic stromal components involved in their recruitment in MCL1 transgenic females but demonstrated that apoptosis is reduced in thymocytes at the earliest stages of development.
Discussion
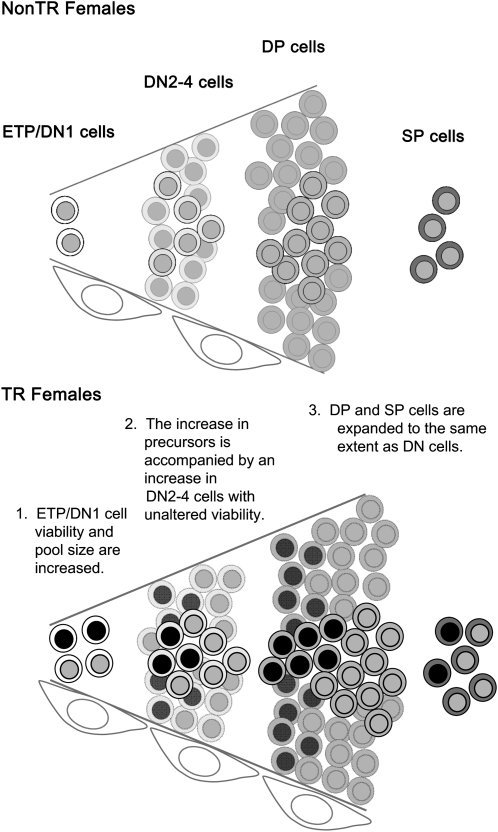
The present findings show that MCL1 transgenic females exhibit an enlarged but undistorted thymus (Figs 1 and 2), DN1 cells being increased disproportionally and other thymocyte subsets proportionally (Figs 2A and 4A). DN1 cells, but not more mature thymocytes, exhibited increased viability, which was at least partially cell autonomous as it was seen both upon examination directly after harvest and upon incubation in primary culture (Figs 2C,4C and 5). Extended survival with the maintenance of proliferation thus appeared to underlie the steady state increase in the DN1 cell population. The ETP population within DN1 similarly exhibited disproportional expansion and increased viability (Fig. 8), although its paucity precluded other analyses such as BrdU incorporation. However, nearly all viable ETPs are in a cycling (non-G0) state in young animals (3, 4), suggesting that reduced ETP apoptosis may likewise allow for the expansion of cells capable of proliferation. In sum, the MCL1 transgene results in increased viability and the expansion of early stage cells in females, and this is associated with overall enlargement of an apparently normal thymus. Figure 9 suggests a model to account for these findings, which are in line with other observations indicating that MCL1 can promote lineage and tissue expansion by enhancing the survival of cells at pivotal stages along the developmental continuum (31).
Fig. 9.
MCL1-induced promotion of thymocyte viability beginning at primitive stages. The diagram depicts thymocyte development in non-transgenic (upper panel) and transgenic (lower panel) females, where the cells with the darker nuclei represent the additional thymocytes present in transgenic females. The pattern seen in non-transgenic as well as transgenic males is comparable to that shown in the upper panel. Increasing maturity is indicated by darkening cytoplasm. Thymocytes are normally subject to extensive proliferation during early stages and then positive and negative selection. Since the amplification and subsequent selection are more extensive than can be represented literally, cells in the background are muted to indicate that they symbolize these processes. Elongated cells at the lower edge represent thymic stroma.
The thymic expansion seen in the presence of the MCL1 transgene is consistent with the profound thymocyte loss seen upon MCL1 knockout with Lck-Cre (30, 32). This knockout becomes fully effective in DN2/3 (48, 50) and arrests development beyond these stages (30, 32). However, DN2/3 cell viability was high in non-transgenic as well as transgenic females. One possible explanation for these observations is that MCL1 is required in DN2/3 (its knockout aborting development) but that endogenous MCL1 expression is more than sufficient in DN2/3 cells such that their viability is not further elevated in the presence of the transgene. Findings with promoter mutations that selectively reduce MCL1 expression in the thymus support this possibility (51). These mutations caused a decrease in DN1 cell numbers but did not decrease cells at later DN stages, suggesting that reduced endogenous MCL1 expression has a more pronounced effect on cells in DN1 than in later stages.
The present findings are also consistent with recent studies utilizing a mouse MCL1 transgene under the control of the vav promoter, where the major thymocyte subpopulations were not altered in young males (52). A difference was that peripheral lymphocytes accumulated and enhanced survival was seen upon in vitro incubation of the total thymocyte population, possibly because of the high efficacy of vav promoter (53). Both types of MCL1 transgenic mice have a high probability of developing tumors with long latency, those used here developing B- (but not T) cell lymphoma (34). The fact that specific elements in the MCL1 promoter selectively control expression in thymus (51) suggests the possibility of promoting expression in this tissue, for a defined period of time, to aid thymopoiesis while minimizing tumorigenesis.
Androgen ablation and/or growth factors that promote stromal expansion increase ETP numbers and result in enhanced thymopoiesis (15, 22). In contrast, ETP numbers decrease during aging-related involution (4, 24, 54, 55). Interestingly, when ETP numbers are reduced by the knockout of homing molecules (56), thymocyte numbers are not reduced proportionally. This suggests the existence of compensatory mechanisms, which operate at early stages because the proportions of cells in DN1–DN4 and the following stages are not affected. It is possible, although speculative, that these mechanisms involve increased viability and proliferation of DN1 cells. In any case, the present findings are in accord with results from other studies indicating that early stages of development represent an important point of control in thymopoiesis.
Thymocytes as well the stroma were increased in MCL1 transgenic females, and the role of the stroma remains to be further investigated. This is particularly true in view of the fact that thymic enlargement did not occur after bone marrow transplantation to produce chimeric mice in which either the thymocytes or the stroma derived from transgenic animals (Fig. 3). Interestingly, knockout of proapoptotic BID was unexpectedly found to reduce the ability of donor bone marrow to reconstitute the thymus (57). The actions of thymocytes and thymic epithelial/stromal cells are closely intertwined (54, 58–61), and the BCL2 family plays a role in both tissues. In thymocytes, BCL2 promotes viability in DN2 stage cells (53, 62, 63), A1 aids survival during β-selection (64–68) and BCLX and BCL2 inhibit apoptosis in DP and SP cells (64, 65, 69). In the thymic stroma, knockout of proapoptotic BAD results in increased thymocyte numbers after irradiation (70). BAX/BAK knockout causes ETPs to expand but impairs their development (71) and, like knockout of BIM, allows the survival of autoreactive cells (72–74). The present studies suggest that, in an appropriate host environment, MCL1 can expand the pool of primitive thymocytes without interfering with their further differentiation.
The finding that the transgene has prominent in vivo effects in females but not males also remains to be further investigated. The observation that DN1 cells from transgenic males exhibit an extension of viability in primary culture suggests that the effect of MCL1 is suppressed in the environment of the male. Testosterone is known to act through effects on the stroma to suppress thymopoiesis, and castration or chemical sex steroid ablation of adult wild-type males results in an increase in thymus size (4, 20, 45). It is possible that endogenous MCL1 contributes to this effect, in other words that, upon androgen ablation, the effect of MCL1 can become manifest. However, factors present in the environment of the female may also be important, particularly as female reproductive hormones are known to have complex effects, suppressing thymus size in adult animals but promoting fetal development of the organ (24). Further experiments with male and female reproductive hormones, such as in organ culture, may be useful in this regard as it is difficult to predict in advance what effect sex steroid ablation will have in transgenic animals since thymus size is expected to increase in non-transgenic adult females as well as males (24).
The ability to target MCL1 to expand the thymus in a normal fashion has implications for conditions in which enhancement of thymopoiesis would be useful (e.g. treatment with radiation/chemotherapy or in chronic infections such as AIDS), as well as for normal aging (13, 15). It has potential utility after bone marrow transplantation because slow thymic reconstitution increases susceptibility to infections particularly in the elderly (14). Finally, the present finding in the thymus raises the possibility that viability-regulating genes might be exploitable for engineering other tissues (76, 77).
Supplementary data
Supplementary Figures 1–6 are available at International Immunology Online.
Funding
National Institutes of Health (R01 CA057359 to R.W.C.); Veteran’s Administration Merit Review Grant [(2009-2013) to J.A.K.].
Supplementary Material
Acknowledgments
We thank Drs Roy A. Fava [Dartmouth Medical School (DMS)] and Christopher H Lowrey (DMS) for helpful discussions and advice. We thank Ann Lavanway for help with the fluorescence microscopy and Drs Shanna Nifoussi and Mary Jo Turk for many useful suggestions regarding the manuscript. Conflict of Interests: The authors declare no conflict of interests.
References
- 1.Hernandez JB, Newton RH, Walsh CM. Life and death in the thymus—cell death signaling during T cell development. Curr. Opin. Cell Biol. 2010;22:865. doi: 10.1016/j.ceb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balciunaite G, Ceredig R, Rolink AG. The earliest subpopulation of mouse thymocytes contains potent T, significant macrophage, and natural killer cell but no B-lymphocyte potential. Blood. 2005;105:1930. doi: 10.1182/blood-2004-08-3087. [DOI] [PubMed] [Google Scholar]
- 3.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 4.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 2005;175:2982. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 5.Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 2003;4:168. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 6.Shortman K, Wu L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 1996;14:29. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J. Exp. Med. 2008;205:1187. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akashi K. Lymphoid lineage fate decision of hematopoietic stem cells. Ann. N. Y. Acad. Sci. 2009;1176:18. doi: 10.1111/j.1749-6632.2009.04570.x. [DOI] [PubMed] [Google Scholar]
- 9.Saran N, Lyszkiewicz M, Pommerencke J, et al. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood. 2010;115:1137. doi: 10.1182/blood-2009-07-230821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez-Gerpe L, Rey-Mendez M. Evolution of the thymus size in response to physiological and random events throughout life. Microsc. Res. Tech. 2003;62:464. doi: 10.1002/jemt.10408. [DOI] [PubMed] [Google Scholar]
- 11.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl Acad. Sci. USA. 2006;103:8447. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorshkind K, Swain S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr. Opin. Immunol. 2009;21:404. doi: 10.1016/j.coi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollander GA, Krenger W, Blazar BR. Emerging. strategies to boost thymic function. Curr. Opin. Pharmacol. 2010;10:443. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DK, Hakim FT, Gress RE. The thymus and the immune system: layered levels of control. J. Thorac. Oncol. 2010;5:S273. doi: 10.1097/JTO.0b013e3181f20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chidgey AP, Seach N, Dudakov J, Hammett MV, Boyd RL. Strategies for reconstituting and boosting T cell-based immunity following haematopoietic stem cell transplantation: pre-clinical and clinical approaches. Semin. Immunopathol. 2008;30:457. doi: 10.1007/s00281-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall R, Pido-Lopez J, Imami N, et al. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 18.Alpdogan O, Muriglan SJ, Eng JM, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J. Clin. Invest. 2003;112:1095. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimond M, Leonard WJ, Spolsk R, et al. Thymic stromal lymphopoietin is not necessary or sufficient to mediate the thymopoietic effects of keratinocyte growth factor. Blood. 2008;111:969. doi: 10.1182/blood-2007-09-113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KM, Lucas PJ, Bare CV, et al. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112:3255. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg GL, Dudakov JA, Reiseger JJ, et al. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J. Immunol. 2010;184:6014. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen NJ, Kovacs WJ. Evidence that androgens modulate human thymic T cell output. J. Investig. Med. 2011;59:32. doi: 10.2310/jim.0b013e318200dc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell. Immunol. 2008;252:122. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Penit C, Ezine S. Cell proliferation and thymocyte subset reconstitution in sublethally irradiated mice: compared kinetics of endogenous and intrathymically transferred progenitors. Proc. Natl Acad. Sci. USA. 1989;86:5547. doi: 10.1073/pnas.86.14.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penit C, Vasseur F. Sequential events in thymocyte differentiation and thymus regeneration revealed by a combination of bromodeoxyuridine DNA labeling and antimitotic drug treatment. J. Immunol. 1988;140:3315. [PubMed] [Google Scholar]
- 27.Bosco N, Swee LK, Benard A, Ceredig R, Rolink A. Auto-reconstitution of the T-cell compartment by radioresistant hematopoietic cells following lethal irradiation and bone marrow transplantation. Exp. Hematol. 2010;38:222. doi: 10.1016/j.exphem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL-2. Proc. Natl Acad. Sci. USA. 1993;90:3516. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 30.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 31.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation, and tumorigenesis. Leukemia. 2002;16:444. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 32.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J. Immunol. 2008;181:521. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, Qian L, Bieszczad CK, et al. Mcl1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226. [PubMed] [Google Scholar]
- 34.Zhou P, Xie H, Qian L, et al. MCL1 transgenic mice exhibit a high incidence of B cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97:3902. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 35.Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89:630. [PubMed] [Google Scholar]
- 36.Irla M, Hugues S, Gill J, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol. Immunol. 2002;38:841. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 38.Rothaeusler K, Baumgarth N. Evaluation of intranuclear BrdU detection procedures for use in multicolor flow cytometry. Cytometry A. 2006;69:249. doi: 10.1002/cyto.a.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 2007;19:1201. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein A. Biostatistics, an Introductory Text. New York: MacMillan Publishing Co., Inc.; 1964. [Google Scholar]
- 41.De Biasio A, Vrana JA, Zhou P, et al. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J. Biol. Chem. 2007 doi: 10.1074/jbc.M700938200. 282:23919. [DOI] [PubMed] [Google Scholar]
- 42.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 2010;9:494. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lustig A, Carter A, Bertak D, et al. Transcriptome analysis of murine thymocytes reveals age-associated changes in thymic gene expression. Int. J. Med. Sci. 2009;6:51. doi: 10.7150/ijms.6.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lustig A, Weeraratna AT, Wood WW, et al. Transcriptome analysis of age-, gender- and diet-associated changes in murine thymus. Cell Immunol. 2007;245:42. doi: 10.1016/j.cellimm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 46.Fujise K, Zhang D, Liu J, Yeh ET. Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen. J. Biol. Chem. 2000;275:39458. doi: 10.1074/jbc.M006626200. [DOI] [PubMed] [Google Scholar]
- 47.Dulude G, Cheynier R, Gauchat D, et al. The magnitude of thymic output is genetically determined through controlled intrathymic precursor T cell proliferation. J. Immunol. 2008;181:7818. doi: 10.4049/jimmunol.181.11.7818. [DOI] [PubMed] [Google Scholar]
- 48.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 2008;8:9. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gossens K, Naus S, Corbel SY, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J. Exp. Med. 2009;206:761. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee PP, Fitzpatrick DR, Beard C, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 51.Yang CY, Lin NH, Lee JM, et al. Promoter knock-in mutations reveal a role of Mcl-1 in thymocyte-positive selection and tissue or cell lineage-specific regulation of Mcl-1 expression. J. Immunol. 2009;182:2959. doi: 10.4049/jimmunol.0803550. [DOI] [PubMed] [Google Scholar]
- 52.Campbell KJ, Bath ML, Turner ML, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116:3197. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc. Natl Acad. Sci. USA. 1999;96:14943. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on early B- and T-cell development. Immunol. Rev. 2005;205:7. doi: 10.1111/j.0105-2896.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 55.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 56.Rossi FM, Corbel SY, Merzaban JS, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat. Immunol. 2011;6:626. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 57.Shen H, Yu H, Liang PH, et al. Bid is a positive regulator for donor-derived lymphoid cell regeneration in gamma-irradiated recipients. Exp. Hematol. 39:947. doi: 10.1016/j.exphem.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 59.Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G. Checkpoints in the development of thymic cortical epithelial cells. J. Immunol. 2009;182:130. doi: 10.4049/jimmunol.182.1.130. [DOI] [PubMed] [Google Scholar]
- 60.Roberts NA, Desanti GE, Withers DR, et al. Absence of thymus crosstalk in the fetus does not preclude hematopoietic induction of a functional thymus in the adult. Eur. J. Immunol. 2009;39:2395. doi: 10.1002/eji.200939501. [DOI] [PubMed] [Google Scholar]
- 61.Jenkinson WE, Bacon A, White AJ, Anderson G, Jenkinson EJ. An epithelial progenitor pool regulates thymus growth. J. Immunol. 2008;181:6101. doi: 10.4049/jimmunol.181.9.6101. [DOI] [PubMed] [Google Scholar]
- 62.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 63.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhang N, Hartig H, Dzhagalov I, Draper D, He YW. The role of apoptosis in the development and function of T lymphocytes. Cell Res. 2005;15:749. doi: 10.1038/sj.cr.7290345. [DOI] [PubMed] [Google Scholar]
- 65.Opferman JT. Apoptosis in the development of the immune system. Cell Death Differ. 2008;15:234. doi: 10.1038/sj.cdd.4402182. [DOI] [PubMed] [Google Scholar]
- 66.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 67.Kondo M, Akashi K, Dome J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 68.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1-/- mice. Cell. 1997;89:1011. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 69.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 70.Kelly PN, White MJ, Goschnick MW, et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ. 2010;17:1655. doi: 10.1038/cdd.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biswas S, Shi Q, Matise L, Cleveland S, Dave U, Zinkel S. A role for proapoptotic Bax and Bak in T-cell differentiation and transformation. Blood. 2010;116:5237. doi: 10.1182/blood-2010-04-279687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 2002;3:932. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 73.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 2004;200:1189. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bouillet P, Purton JF, Godfrey DI, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 76.Arbour N, Vanderluit JL, Le Grand JN, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J. Neurosci. 2008;28:6068. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sitailo LA, Jerome-Morais A, Denning MF. Mcl-1 functions as major epidermal survival protein required for proper keratinocyte differentiation. J. Invest. Dermatol. 2009;129:1351. doi: 10.1038/jid.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.