Abstract
Background
Recent animal studies have shown that the level of stress-responsive arginine vasopressin (AVP) gene expression in the amygdala is increased during early withdrawal from long-term heroin or cocaine administration. The selective AVP V1b receptor antagonist SSR149415 (capable of exerting antidepressant-like and anxiolytic effects in animal models) also blocked stress-induced reinstatement of drug-seeking behavior. The present study was undertaken to investigate the effects of alcohol and to determine whether: (1) there are genetically determined differences in basal AVP mRNA levels in the medial/central amygdala (Me/CeA) and medial hypothalamus (MH) between selectively bred Sardinian alcohol-preferring (sP) and -nonpreferring (sNP) rats; (2) the AVP mRNA levels are altered by long-term alcohol drinking in sP rats; and (3) the V1b receptor antagonist SSR149415 alters alcohol drinking in sP rats.
Methods
In Experiment 1, AVP mRNA levels were measured in the Me/CeA and MH of alcohol-naive sP and sNP rats, and sP rats exposed to the the standard, homecage 2-bottle “alcohol vs water” choice regimen 24 hours/day for 17 days. In Experiment 2, SSR149415 (0, 3, 10, or 30 mg/kg; i.p.) was acutely administered 30 min before lights off to alcohol-experienced sP rats. Alcohol, water, and food intake were monitored 6 and 24 hours later.
Results
We found higher basal AVP mRNA levels in both Me/CeA and MH of alcohol-naive sP than sNP rats; alcohol consumption decreased AVP mRNA levels in both brain regions of sP rats, suggesting genetically determined differences between the two rat lines and in the effects of alcohol drinking in sP rats. Acute treatment with SSR149415 significantly reduced alcohol intake of sP rats.
Conclusion
The stress-responsive AVP/V1b receptor system is one component of the neural circuitry underlying high alcohol drinking in sP rats.
Keywords: arginine vasopressin, V1b receptor, amygdala, alcohol drinking, Sardinian alcohol-preferring (sP) and -nonpreferring (sNP) rats, gene expression
Introduction
Since the 1980’s, evidence has emerged implicating arginine vasopressin (AVP) in the control of alcohol drinking. In fact, systemic administration of desglycinamide-(Arg8)-vasopressin (DGAVP, a centrally effective AVP fragment [de Wied et al., 1972]) decreased alcohol intake in rhesus monkeys (Kornet et al., 1991). In studies exploring the role of AVP in Brattleboro homozygote rats lacking vasopressin, DGAVP reduced alcohol intake (Rigter and Crabbe, 1985). AVP mRNA levels in the bed nucleus of the stria terminalis (BNST), paraventricular nucleus (PVN), and supraoptic nucleus (SON) of the hypothalamus were decreased in C57BL/6NCR mice after prolonged exposure to an alcohol-containing diet, suggesting that chronic alcohol interfered with AVP gene expression (Ishizawa et al., 1990; Gulya et al., 1991). Reduction of the number of AVP-immunoreactivity (ir) neurons and the AVP mRNA levels in the hypothalamus after chronic alcohol consumption has also been demonstrated in humans (Harding et al., 1996) and rats (Silva et al., 2002). Two recent studies using mice with targeted disruptions of two central receptor subtypes (V1a and V1b) have found different results: Caldwell et al (2006) reported no effects of either V1a or V1b deletion on alcohol intake; Sanbe et al. (2008) showed increased alcohol intake after V1a deletion.
There is evidence suggesting that increased AVP neuronal activity may represent an important step in the neurobiology of stress-related behaviors in several rodent models. Specifically: (a) acute stress increases extracellular AVP-immunoreactivity levels and AVP gene expression in the rat amygdala and hypothalamus (Ebner et al., 2002; Wigger et al., 2004; Zhou et al., 2005); and (b) activation of AVP V1b receptors (especially in the amygdala) is involved in “anxiety”-related and “depression”-like behaviors in rats and mice (Griebel et al., 2002; Salome et al., 2006).
Several laboratories have explored the role of AVP and its central receptors (V1a and V1b subtypes) in heroin and cocaine addiction. The AVP-like immunoreactivity level in the amygdala was increased after acute cocaine injection (Sarnyai et al., 1992). We reported that amygdalar AVP gene expression levels were increased in acute cocaine withdrawal (Zhou et al., 2005). These findings parallel the results of our recent studies that showed an increased AVP mRNA level in the amygdala during early opiate withdrawal (Zhou et al., 2008). Notably, the systemically active, highly selective, non-peptide V1b receptor antagonist SSR149415 dose-dependently blocked stress (foot shock) -induced reinstatement of heroin-seeking behavior (Zhou et al., 2008). Together, these data suggest that the AVP/V1b system in the amygdala may be one of the critical components of the neural circuitry contributing to stress, anxiety, drug withdrawal and drug-seeking behaviors.
We therefore designed a set of novel experiments addressing three research questions. Since selectively bred Sardinian alcohol-preferring (sP) rats display more inherent “anxiety”-related behaviors than Sardinian alcohol-nonpreferring (sNP) rats (Colombo et al., 1995; Richter et al., 2000; Cagiano et al., 2002; Leggio et al., 2003; Roman and Colombo, 2009), our first research question was whether there is a genetically determined difference in basal AVP mRNA levels in the medial/central amygdala (Me/CeA) or medial hypothalamus (MH) between sP and sNP rats. The second question was whether long-term exposure to alcohol drinking alters the AVP gene expression in sP rats. To answer this question, we assessed the effect of 17-day alcohol drinking on AVP mRNA levels in brain regions of sP rats exposed to the standard, homecage 2-bottle “alcohol (10%, v/v) vs. water” choice regimen with unlimited access for 24 hours/day (see Colombo et al., 2006). The third question was whether AVP modulates alcohol drinking behavior through a V1b receptor-mediated mechanism. To this end, we tested the effect of the acute administration of V1b receptor antagonist SSR149415 on alcohol drinking, using the same 2-bottle choice model. SSR149415 is highly selective for the V1b receptor (60-to 800-fold more than for the V1a receptor), and displays both anxiolytic and antidepressant properties, as well as blocking neurochemical (noradrenaline release) and autonomic (hyperthermia) responses to various stressors and heroin-seeking behavior in rodents (Griebel et al., 2002; Serradeil-Le Gal et al., 2003; Overstreet and Griebel, 2005; Salome et al., 2006; Zhou et al., 2008).
Materials and Methods
All experimental procedures employed in the present study were in accordance with the Principles of Laboratory Animal Care (NIH Publication No 86-23, 1996), the European Communities Council Directive (86/609/EEC), and the subsequent Italian Law on the “Protection of animals used for experimental and other scientific reasons”. During all experimental procedures, the number of animals and their potential suffering were minimized.
Experiment 1. Genetically determined differences and effects of alcohol drinking on AVP gene expression levels in sP and sNP rats
This study was designed to examine the AVP gene expression levels in the Me/CeA, basolateral amygdala, and MH of both sP and sNP rats with (alcohol-experienced) or without (alcohol-naive) exposure to the 2-bottle “alcohol vs water” choice regimen for 17 consecutive days.
The Me/CeA, basolateral amygdala and MH were selected because recent studies have demonstrated that activation of the AVP/V1b receptor system in the amygdala and hypothalamus is involved in “anxiety”-related and “depression”-like behaviors in rodents (Ebner et al., 2002; Wigger et al., 2004; Salome et al., 2006).
1.1. Animals
Male sP and sNP rats from the 67th generation, approximately 75 days old at the start of each study, were used. The animal facility was under an inverted 12:12 hour light-dark cycle (lights on at 09:00 pm), at a constant temperature of 22 ± 2°C and relative humidity of approximately 60%. Starting from the age of 60 days, all rats were individually housed in standard plastic cages with wood chip bedding. Standard rat chow (Mucedola, Settimo Milanese, Italy) was always available.
1.2. Alcohol drinking procedure
Both sP and sNP rats were exposed to 10% (v/v) alcohol and water under the standard, homecage 2-bottle “alcohol vs. water” choice regimen, with unlimited access for 24 hours/day, 17 consecutive days. Bottles were refilled every day with fresh solution or water and their left-right positions interchanged at random to avoid development of position preference. Alcohol, water, and food intake were monitored by weighing both bottles and food pellets (0.1-g accuracy) once daily immediately before the start of the dark phase. Body weight was recorded once every other day.
To summarize, rats of both lines were divided into two subgroups of eight individuals, yielding a total of four treatment groups: alcohol-naive sP and sNP rats, exposed only to water; alcohol-experienced sP and sNP rats, exposed to the above-mentioned 2-bottle choice regimen.
1.3. Preparation of RNA extracts
On day 18, animals were sacrificed 30 min after removal of alcohol and/or water bottles and 3 hours after lights on. Each rat brain was removed from the skull and placed in a chilled rat brain matrix (ASI Instruments, Houston, Texas). Coronal slices containing the brain regions of interest were removed from the matrix and placed on a chilled petri dish. Dissection was carried out under a dissecting microscope using razor blades and forceps. The brain regions of interest were identified according to the Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 1986), as described in detail (Zhou et al., 2006). The Me/CeA (including the medial nucleus and central nucleus of the amygdala) and basolateral amygdala on the section (Bregma −2.56 to −3.80 mm), and MH (including the PVN, but not supraoptic nucleus) on the section (Bregma −1.80 to −2.56 mm) were dissected on ice, homogenized in guanidinium thiocyanate buffer and extracted with acidic phenol and chloroform. After the final ethanol precipitation step, each extract was resuspended in DEPC-treated H2O and stored at −80 °C.
1.4. Solution hybridization ribonuclease (RNase) protection-trichloroacetic acid (TCA) precipitation assay
The solution hybridization RNase protection-TCA precipitation protocol has been described in detail in an earlier report (Zhou et al., 2006). A 502 bp fragment from rat AVP cDNA was cloned into the polylinker region of pCR II (Invitrogen, Carlsbad, CA). The plasmid pS/E (a pSP65 derivative) was used to synthesize riboprobe for the 18S rRNA to determine total RNA. 33P-labeled cRNA antisense probes and unlabeled RNA sense standards were synthesized using an SP6 transcription system. A denaturing agarose gel containing 1.0 M formaldehyde showed that a single full-length transcript had been synthesized from each plasmid.
RNA extracts were dried in 1.5 ml Eppendorf tubes and resuspended in 30 μl that contained 150,000 to 300,000 cpm of a probe in 2 × TESS (10 mM N-Tris[hydroxy-methyl]methyl-2-aminoethane sulfonic acid, pH 7.4; 10 mM EDTA; 0.3 M NaCl; 0.5% SDS). Samples were covered with mineral oil and hybridized overnight at 75°C. For RNase treatment, 250 μl of a buffer that contained 0.3 M NaCl; 5 mM EDTA; 10 mM Tris-HCl (pH 7.5), 40 μg/ml RNase A (Worthington, Biochemicals, Freehold, NJ) and 2 μg/ml RNase T1 (Calbiochem, San Diego, CA) were added and each sample was incubated at 30°C for 1 hour. TCA precipitation was effected by the addition of 1 ml of a solution that contained 5% TCA and 0.75% sodium pyrophosphate. Precipitates were collected onto a filter in sets of 24 by using a cell harvester (Brandel, Gaithersburg, MD) and were measured in a scintillation counter with liquid scintillant (Beckman Instruments, Palo Alto, CA).
The procedure to measure mRNA levels involved a comparison of values obtained from experimental samples (brain extracts) to those obtained for a set of calibration standards. The calibration standards had known amounts of an in vitro sense transcript whose concentration was determined by optical absorbance at 260 nm. The set of calibration standards included those with no added sense transcript and those that contained between 1.25 and 80 pg of the sense transcript (Zhou et al., 2006). A new standard curve was generated each time experimental samples were analyzed and all extracts of a particular tissue were assayed for each mRNA in a single assay. Total cellular RNA concentrations were measured by hybridization of diluted extracts to a 33P-labeled probe complementary to 18S rRNA at 75°C. The calibration standards for this curve contained 10 μg of E. coli tRNA plus either 0.0, or from 2.5 to 40 ng of total RNA from rat brain whose concentration was determined by optical absorbance at 260 nm.
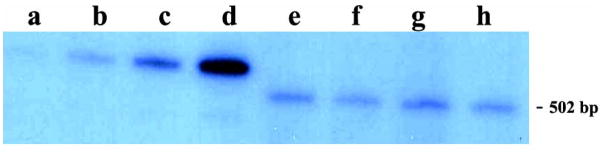
Selected samples of the Me/CeA or MH were subjected to solution hybridization and RNase treatment followed by gel electrophoresis. Fig. 1 shows the size distribution of rat AVP antisense probe surviving solution hybridization and RNase treatment. The protected RNA:RNA hybrids were phenol-chloroform extracted, precipitated with 100% ethanol, and electrophoresed through nondenaturing 4% polyacrylamide gels. The gels were dried and exposed to X-film. The protected species was approximately 502 bp, corresponding to an RNA:RNA hybrid formed by hybridization of the full-length cRNA probe with total cytoplasmic RNA samples extracted from the Me/CeA and MH of sP or sNP rats.
Figure 1.
Representative autoradiograms of the AVP sense transcript standards with total cytoplasmic RNA samples after hybridization with the rat AVP cRNA probe and RNase digestion. The size of the main protected RNA:RNA hybrid is about 502 bp. Lanes a to d: 5, 10, 20 or 40 pg of the AVP sense transcript standards. Lanes e and f: 3.8 μg of total cytoplasmic RNA samples extracted from the Me/CeA of alcohol-naïve sP and sNP rats, respectively. Lanes g and h: 0.75 μg of total cytoplasmic RNA samples extracted from the MH of alcohol-naïve sP and sNP rats, respectively.
1.5. Statistical Analyses
Data on daily alcohol intake in each rat line (sP or sNP) were analyzed using separate one-way analyses of variance (ANOVAs) for repeated measures. Group differences in AVP mRNA levels were analyzed using two-way (alcohol exposure; rat line) ANOVA for all four rat groups, followed by the Newman-Keuls post hoc test (p<0.05).
Experiment 2. Effects of the selective V1b receptor antagonist SSR149415 on alcohol drinking in sP rats
2.1. Animals
Male sP rats from the 70th generation, approximately 75 days old at the start of the study, were used. Environmental features of the animal facility were identical to those described above (see section 1.1.). Rats were individually housed as described above and given ad libitum access to rat chow.
2.2. Alcohol drinking and SSR149415 administration
In order to minimize stress induced by handling and drug injection, rats underwent daily sessions of habituation to handling and i.p. administration starting from the age of 60 days. Rats were exposed to the 2-bottle “alcohol (10%, v/v) vs. water” choice regimen for 4 consecutive weeks before administration of SSR149415 (this procedure produced “alcohol-experienced” rats at the time of drug testing). On the test day, rats were divided into four groups (n=8), which were matched for body weight and daily alcohol, water, and food intake over the 3 preceding days. SSR149415 (a gift from Sanofi Aventis, Montpellier, France) was suspended in saline with a few drops of Tween 80. SSR149415 was administered intraperitoneally 30 min before lights off. SSR149415 was administered at doses of 3, 10, or 30 mg/kg; this SSR149415 dose-range was selected on the basis of previous studies in which it was behaviorally active [producing anxiolytic effect (Griebel et al., 2002; Hodgson et al., 2007; Ishizuka et al., 2010) and also attenuating heroin-seeking behavior (Zhou et al., 2008) in rats and mice]. Control rats were treated with an identical volume of saline with a few drops of Tween 80. Alcohol, water, total fluid (the sum of alcohol solution and water), and food intake, as well as preference ratio (the ratio between intake of alcohol solution and total fluid intake) were measured 6 and 24 hours after lights off, by weighing bottles and food pellets (0.1-g accuracy). Data on alcohol, water, total fluid, and food intake were expressed in g/kg pure alcohol, ml/kg water, ml/kg alcohol solution plus water, and g/kg food, respectively. Data on the preference ratio were expressed as percent of alcohol solution consumed over total fluid intake.
2.4. Statistical Analyses
Data on the effect of SSR149415 on alcohol, water, total fluid, food intake and preference ratio were analyzed by separate one-way ANOVAs at 6- and 24-hour recording times, followed by the Newman-Keuls post hoc test.
Results
Experiment 1. Genetically determined differences and effects of alcohol drinking on AVP gene expression levels in the Me/CeA, basolateral amygdala or MH of sP and sNP rats
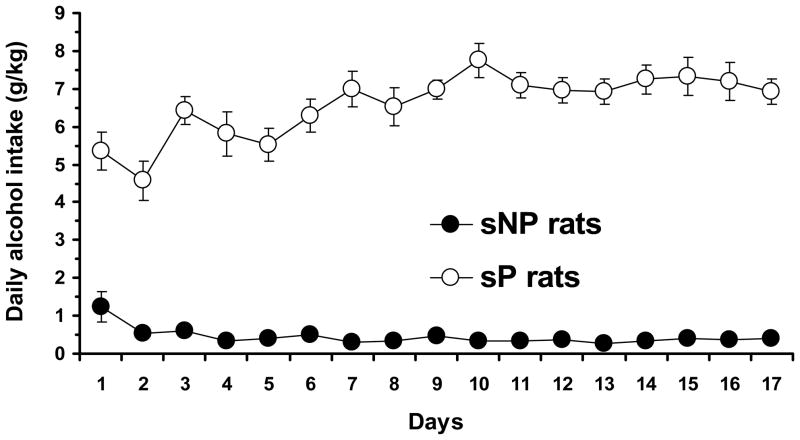
All sP rats exposed to the 2-bottle “alcohol vs. water” choice regimen rapidly acquired alcohol drinking behavior, as indicated by daily alcohol intakes higher than 4 g/kg [i.e., the selection criterion adopted in the breeding program of sP rats (see Colombo et al., 2006)] by day 3 in each rat; subsequently, daily alcohol intake rose progressively, averaging approximately 6.5 g/kg/day throughout the 17-day period of exposure [F(16,112)=6.19, p<0.0001] (Fig. 2). These data were very similar to those repeatedly recorded in sP rats exposed to alcohol and water under the 2-bottle choice regimen (see Colombo et al., 2006). Conversely, daily alcohol intake in alcohol-experienced sNP averaged – with the sole exception of the first three days – less than 0.5 g/kg [F(16,112)=5.64, p<0.0001] (Fig. 2). These data are consistent with those repeatedly recorded in sNP rats (see Colombo et al., 2006).
Figure 2.
Daily alcohol intake in previously alcohol-naive, Sardinian alcohol-preferring (sP) and Sardinian alcohol-nonpreferring (sNP) rats exposed to the homecage, 2-bottle “alcohol (10% v/v) vs water” choice regimen with unlimited access for 24 hours/day for 17 consecutive days. Each point is the mean ± SEM for eight rats.
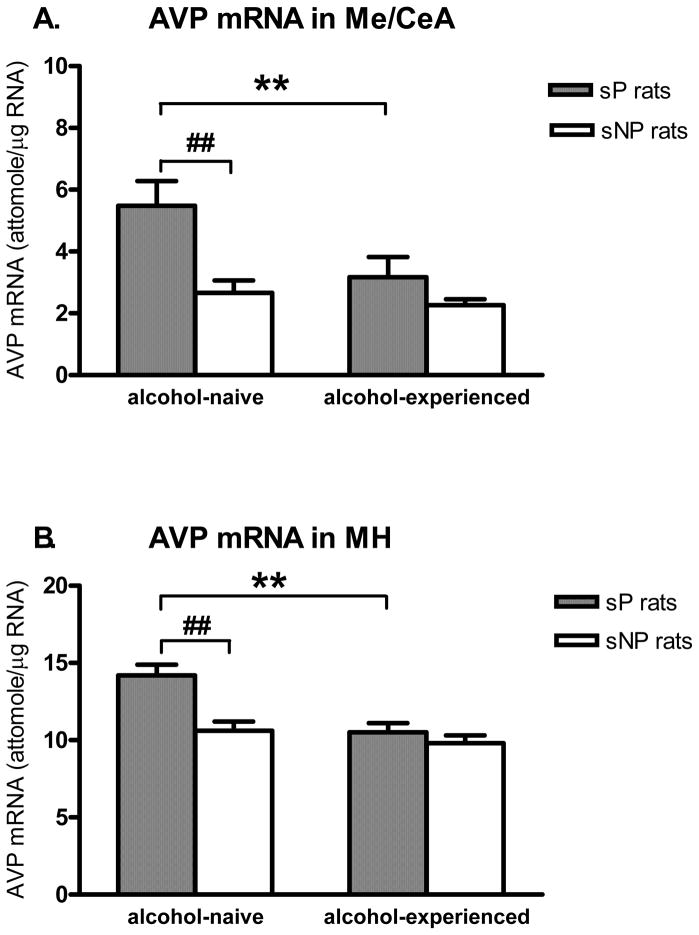
In the Me/CeA (Fig. 3A), two-way ANOVA showed significant effects for alcohol exposure (F(1,28)=5.81, p<0.05) and rat line (F(1,28)=11.0, p<0.005). Post-hoc analysis revealed significantly higher basal levels of AVP mRNA in the Me/CeA of alcohol-naive sP than sNP rats (p<0.005). AVP mRNA levels were significantly lower in alcohol-experienced than -naive sP rats (p<0.01); no difference was found between alcohol-experienced and -naive sNP rats.
Figure 3.
Genetically determined differences between selectively bred, Sardinian alcohol-preferring (sP) and -nonpreferring (sNP) rats and effects of prolonged alcohol drinking on AVP mRNA levels (attomole/μg total RNA) in the medial/central amygdala (Me/CeA) (A) and medial hypothalamus (MH) (B). Both sP and sNP rats were offered either water as the sole fluid available (alcohol-naive rats) or a free choice between 10% (v/v) alcohol and water for 17 consecutive days (alcohol-experienced rats). Rat line differences: ## p<0.01; Alcohol exposure differences: ** p<0.01, n=7–8. Note, different axes showing relative abundance of AVP mRNA levels in those two regions.
In the MH (Fig. 3B), two-way ANOVA showed significant effects for alcohol exposure (F(1,27)=13.6, p<0.005), rat line (F(1,27)=11.9, p<0.005) and the alcohol exposure x rat line interaction (F(1,27)=5.35, p<0.05). Basal levels of AVP mRNA were significantly higher in alcohol-naive sP than sNP rats (p<0.0005). AVP mRNA levels were significantly lower in alcohol-experienced sP than -naive sP rats (p<0.001), while no difference was observed between alcohol-experienced and -naive sNP rats.
There was a very low expression level of the AVP gene in the basolateral amygdala in both sP and sNP rats. In two alcohol-naive rats of each line, a pilot study did not show significant difference in AVP mRNA levels in the basolateral amygdala (data not shown). Therefore, we did not further determine the effects of alcohol drinking in this brain region.
Experiment 2. Effects of the selective V1b receptor antagonist SSR149415 on alcohol drinking in sP rats
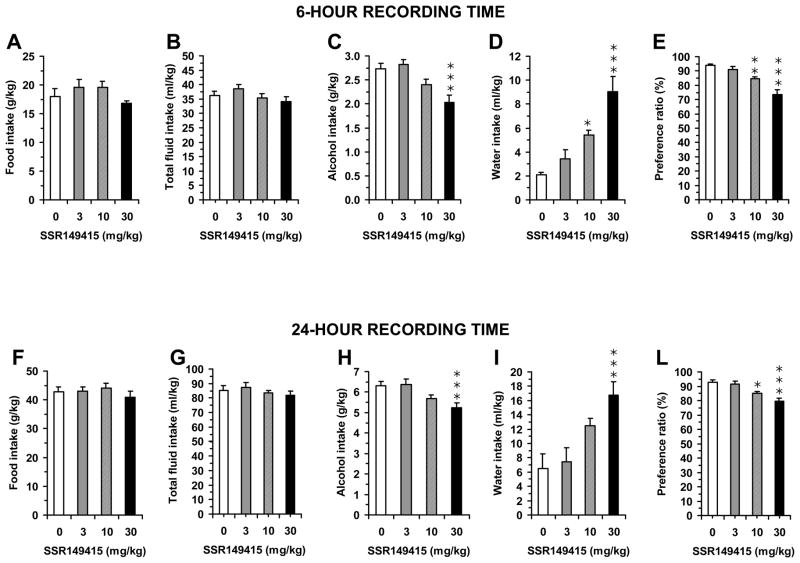
One-way ANOVA revealed a significant effect of acute treatment with SSR149415 on alcohol intake in sP rats at either 6-h [F(3,28)=8.99, p<0.0005] or 24-h [F(3,28)=5.83, p<0.005] recording times. Post hoc analysis revealed that 30 mg/kg SSR149415 significantly reduced alcohol intake at either recording time (Fig. 4C and 4H). In comparison to vehicle-treated rats, mean alcohol intake in 30 mg/kg SSR149415-treated rats was reduced by approximately 25% and 17% at 6- and 24-h recording times, respectively.
Figure 4.
Effect of the acute, intraperitoneal administration of the selective AVP V1b receptor antagonist SSR149415 (0, 3, 10, or 30 mg/kg) on alcohol, water, total fluid, and food intake, and preference ratio (defined as the ratio between intake of alcohol solution and total fluid intake) in alcohol-experienced, Sardinian alcohol-preferring (sP) rats exposed to the homecage, 2-bottle “alcohol (10% v/v) vs water” choice regimen with unlimited access for 24 hours/day. Data were collected 6 (panels A–E) and 24 (panels F–L) hours after lights off. SSR149415 was administered 30 min before lights off. Each bar is the mean ± SEM for eight rats. *: p<0.05, **: p<0.01, and ***: p<0.005 with respect to 0 mg/kg SSR149415-treated rat group.
At both recording times, SSR149415-induced reduction in alcohol intake was associated with a fully compensatory increase in water intake [6-h data: F(3,28)=15.29, p<0.0001; 24-h data: F(3,28)=7.41, p<0.001] (Fig. 4D and 4I), leaving total fluid intake virtually unchanged [6-h data: F(3,28)=1.69, p>0.05; 24-h data: F(3,28)=0.59, p>0.05] (Fig. 4B and 4G). One-way ANOVA revealed also a significant effect of SSR149415 treatment on preference ratio at either 6-h [F(3,28)=19.30, p<0.0001] or 24-h [F(3,28)=9.92, p<0.0001] recording times. Post hoc analysis revealed that both 10 and 30 mg/kg SSR149415 significantly reduced preference ratio at either recording time (Fig. 4E and 4L).
The specificity of the action of SSR149415 on alcohol intake was further suggested by the lack of any effect of SSR149415 administration on food intake at 6-h [F(3,28)=1.36, p>0.05] or at 24-h [F(3,28)=0.50, p>0.05] (Fig. 4A and 4F).
Discussion
Previous lines of experimental evidence have suggested sP rats as an animal model of genetically determined “anxiety”. Indeed, alcohol-naive sP rats displayed more “anxiety”-related behaviors than alcohol-naive sNP rats when tested on the elevated plus maze (Colombo et al., 1995; Richter et al., 2000; Leggio et al., 2003), the elevated zero maze (Cagiano et al., 2002), the open field (Agabio et al, 2001), and the multivariate concentric square field (Roman and Colombo, 2009). Recent evidence suggests that increased AVP neuronal activity may be involved in “anxiety”-related and “depression”-like behaviors in rodents (Griebel et al., 2002; Wigger et al., 2004; Salome et al., 2006). Therefore, in the present study, our first objective was to examine whether there are genetically determined differences in AVP gene expression levels between sP and sNP rats in two key brain areas, Me/CeA and MH. We found sP rats displayed markedly higher basal AVP mRNA levels in both the Me/CeA and MH than sNP rats. Our results may be interpreted to suggest a role for the high basal AVP gene expression (probably a higher AVP biosynthesis rate with resultant higher peptide levels) in the genetically determined tendency of sP rats towards “anxiety”-related and “depression”-like states (Colombo et al., 1995; Ciccocioppo et al., 1998). These emotional states, and the search for the anxiolytic and antidepressant-like effects of alcohol, might in turn contribute to promoting the high levels of alcohol preference and consumption that characterize sP rats. Indeed, voluntary alcohol intake reduces “anxiety”-related (Colombo et al., 1995) and “depression”-like (Ciccocioppo et al., 1998) behaviors in sP rats; these data suggest that sP rats may consume alcohol, at least in part, to ameliorate their high negative emotional states.
The results of the present study are consistent with higher basal AVP mRNA levels found in the PVN of two other lines of rats selectively bred (using breeding procedures and criteria similar to those of sP/sNP rats) for high alcohol preference and consumption: Indiana alcohol-preferring P and high alcohol-drinking (HAD) rats, when compared with their counterparts, alcohol-nonpreferring NP and low alcohol-drinking (LAD) rats, respectively (Hwang et al., 1998).
To investigate whether alcohol drinking would alter AVP gene expression, we measured AVP mRNA levels in the Me/CeA and MH of sP rats exposed to 17-day alcohol drinking. We found that this long-term consumption of high amounts of alcohol by sP rats was associated with decreases in AVP mRNA levels in both brain regions. This effect seemed to be gene-specific, since in the MH, for instance, we observed no effect of 17-day alcohol drinking on corticotropin-releasing factor (CRF) mRNA levels in sP rats (data not shown). Furthermore, our results are in good agreement with many earlier studies showing that after prolonged alcohol exposure there is a decrease in AVP mRNA levels in several brain areas (including the BNST, PVN, and SON) in rodents (e.g., Gulya et al., 1991; Silva et al., 2002). In parallel with mRNA changes, prolonged alcohol consumption was also associated with decreases in the levels of AVP-like immunoreactivity in the mouse PVN, SON and BNST projection to the lateral septum (Gulya et al., 1991). Together, our data indicate that high basal AVP gene expression in the Me/CeA and MH of sP rats was decreased by high alcohol consumption, likely as a consequence of reduced AVP biosynthesis and peptide levels. Conversely, no change in AVP mRNA levels was observed in alcohol-experienced sNP rats; however, their extremely low levels of alcohol drinking (<0.5 g/kg/day on most days) made any alcohol-induced change highly unlikely.
Central AVP binds to two different G protein-coupled receptor subtypes: V1a and V1b, and both are highly expressed in the rat extended amygdala, with high concentrations in the nucleus accumbens, BNST, and CeA (Veinante and Freund-Mercier, 1997). V1a receptors have been found to play a prominent role in modulation of social behavior in animal models (Donaldson and Young, 2008; Insel, 2010). A recent study found that AVP gene expression and protein levels in the nucleus accumbens were increased after cocaine conditioning, and the blockade of V1a receptors attenuated the expression of cocaine conditioned response, suggesting an involvement of V1a receptor in cocaine rewarding processes (Rodriguez-Borrero et al., 2010). In contrast, neuroanatomical distribution of V1b receptor is prominent in the amygdala, hypothalamus, anterior pituitary, and hippocampus (Lolait et al., 1995; Hernando et al., 2001).
Our third objective in the present study was to investigate the potential of the V1b receptor blockade in reducing alcohol drinking in alcohol-experienced sP rats. Acute administration of 30 mg/kg SSR149415 (a systemically active, selective, non-peptide V1b receptor antagonist), significantly reduced alcohol intake in sP rats. It is unlikely that the effect of SSR149415 in reducing alcohol intake was secondary to a general suppression of appetitive or consummatory behaviors, and/or nonspecific sedative effects, since no tested dose of SSR149415 affected – even minimally – food intake; further, SSR149415-induced reduction of alcohol intake was associated with a fully compensatory increase in water intake (accordingly, treatment with SSR149415 produced a dose-dependent reduction in preference for alcohol over water). These results suggest that stress-responsive AVP activation on V1b receptors may play a role in modulating alcohol drinking in sP rats. This finding is in line with the results of our recent study showing that SSR149415 dose-dependently blocked foot shock stress-induced reinstatement of heroin-seeking behavior (Zhou et al., 2008).
It was found that removal of endogenous AVP by injecting AVP antiserum directly into the cerebrospinal fluid led to facilitation of heroin self-administration behavior, suggesting that AVP may be physiologically involved in central reinforcement processes (Van Ree and De Wied, 1977). Of interest, the AVP fragment DGAVP, which lacks the classical endocrine actions of vasopressin and is only centrally active (de Wied et al., 1972), was found to inhibit alcohol self-administration in monkeys (Kornet et al., 1991) and reduce alcohol intake in Brattleboro homozygote rats (Righter and Crabbe, 1985). This AVP analog was also reported to reduce the acquisition of heroinand of cocaine intravenous self-administration in rats (Van Ree et al., 1999). Recent studies using V1a or V1b receptor knockout mice have found no effect of either V1a or V1b receptor deletion on alcohol consumption (Caldwell et al., 2006). Furthermore, another group recently reported that V1a receptor knockout mice displayed an increased alcohol consumption and preference (Sanbe et al., 2008). Together, these findings suggest that the relation of AVP systems and alcohol drinking is complex, and that the effect of AVP receptor agonists or antagonists, or deletion on alcohol drinking may be dependent on the activation of selective AVP receptor V1a or V1b subtypes and the genotype of animals.
Although interesting and novel, the results of the current study should be interpreted with caution for at least two reasons. First, analysis of AVP mRNA levels in the Me/CeA or MH extracts did not provide anatomical resolution between the Me and CeA. However, using semi-quantitative in situ hybridization technique, AVP mRNA expression is only found in the Me (but not CeA) (Szot and Dorsa, 1994). Therefore, our data suggest that a genetically determined difference and effect of alcohol drinking occurred in the Me, but not CeA. In the MH region, measurement of AVP mRNA levels could be confounded by potentially differential responses of AVP mRNA levels in parvocellular and magnocellular cells. Second, we did not measure AVP peptide levels, and thus our findings are limited to gene expression for biosynthesis. In order to confirm that the effects observed on the AVP mRNA level could be translated to the protein level, we have conducted several experiments using immunohistochemistry for AVP in rats and mice. It is worth mentioning that we had difficulties in visualizing the AVP-ir neurons in the amygdala of either rats or mice, using commercial anti-AVP antibodies with fluorescent microscopy (unpublished data).
In summary, our results demonstrate the existence of genetically determined high basal levels of AVP gene expression in the Me/CeA and MH of selectively bred alcohol-preferring sP rats, in comparison with their alcohol-nonpreferring counterpart (sNP rats). We also found a significant decrease in AVP gene expression levels in both the Me/CeA and MH of sP rats after 17-day alcohol consumption. Because increases of AVP neuronal activity are likely involved in “anxiety”-related and depressive-like behaviors, we suggest that the observed alterations in the AVP system may contribute to the emotional state of sP rats and, therefore, to their high alcohol drinking. Finally, the selective V1b receptor antagonist SSR149415 attenuated alcohol drinking in sP rats, indicating that AVP contributes in the modulation of alcohol drinking behavior through a V1b receptor-mediated mechanism.
Acknowledgments
The authors would like to thank Dr. G. Aguilera for providing the rat AVP cDNA, Drs. T. Nilsen and P. Maroney for the 18S DNA, and Mrs. Carla Acciaro for rat breeding. The work was supported by NIH-NIDA Center Grant DA-P60-05130 (M.J.K.) and CNR grant to Commessa “Neurobiologia dell’alcolismo” (G.C.).
References
- Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Cagiano R, Cassano T, Coluccia A, Gaetani S, Giustino A, Steardo L, Tattoli M, Trabace L, Cuomo V. Genetic factors involved in the effects of developmental low-level alcohol induced behavioral alterations in rats. Neuropsychopharmacology. 2002;26:191–203. doi: 10.1016/S0893-133X(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS, 3rd, Wersinger SR. The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides. 2006;40:325–337. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa G, Massi M. Antidepressant-like effect of ethanol revealed in forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology. 1998;144:151–7. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: A genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MAM, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- de Wied D, Greven HM, Lande S, Witter A. Dissociation of the behavioral and endocrine effects of lysine vasopressin by tryptic digestion. Br J Pharmacol. 1972;45:118–122. doi: 10.1111/j.1476-5381.1972.tb09582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak C, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci. 2002;15:384–388. doi: 10.1046/j.0953-816x.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K, Dave JR, Hoffman PL. Chronic ethanol ingestion decreases vasopressin mRNA in hypothalamic and extrahypothalamic nuclei of mouse brain. Brain Res. 1991;557:129–35. [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JL, Harper CG, Kril JJ. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Froehlich JC, Hwang WS, Lumeng L, Li T-K. More vasopressin mRNA in the paraventricular hypothalamic nucleus of alcohol-preferring rats and high alcohol-drinking rats selectively bred for high alcohol preference. Alcohol Clin Exp Res. 1998;22:664–669. doi: 10.1111/j.1530-0277.1998.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa H, Dave JR, Liu LI, Tabakoff B, Hoffman PL. Hypothalamic vasopressin mRNA levels in mice are decreased after chronic ethanol ingestion. Eur J Pharmacol. 1990;189:119–27. doi: 10.1016/0922-4106(90)90015-p. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Abe H, Tanoue A, Kannan H, Ishida Y. Involvement of vasopressin V1b receptor in anti-anxiety action of SSRI and SNRI in mice. Neurosci Res. 2010;66:233–237. doi: 10.1016/j.neures.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Ribbens LG, Van Ree JM. The effect of desglycinamide-(Arg8)-vasopressin (DGAVP) on the acquisition of free-choice alcohol drinking in rhesus monkeys. Alcohol Clin Exp Res. 1991;15:72–79. doi: 10.1111/j.1530-0277.1991.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, Colombo G, de Montis MG. Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res. 2003;973:275–284. doi: 10.1016/s0006-8993(03)02533-2. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacol Biochem Behav. 2005;82:223–227. doi: 10.1016/j.pbb.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; New York, NY: 1986. [DOI] [PubMed] [Google Scholar]
- Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–1772. [PubMed] [Google Scholar]
- Rigter H, Crabbe JC. Vasopressin and ethanol preference. I. Effects of vasopressin and the fragment DGAVP on altered ethanol preference in Brattleboro diabetes insipidus rats. Peptides. 1985;6:669–676. doi: 10.1016/0196-9781(85)90170-6. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Borrero E, Rivera-Escalera F, Candelas F, Montalvo J, Muñoz-Miranda WJ, Walker JR, Maldonado-Vlaar CS. Arginine vasopressin gene expression changes within the nucleus accumbens during environment elicited cocaine-conditioned response in rats. Neuropharmacology. 2010;58:88–101. doi: 10.1016/j.neuropharm.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Colombo G. Lower risk taking and exploratory behavior in alcohol-preferring sP rats than in alcohol non-preferring sNP rats in the multivariate concentric square field™ (MCSF) test. Behav Brain Res. 2009;205:249–258. doi: 10.1016/j.bbr.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology. 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Takeo S, Tsujimoto G, Tanoue A. Alcohol preference in mice lacking the Avpr1a vasopressin receptor. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1482–R1490. doi: 10.1152/ajpregu.00708.2007. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Vecsernyés M, Laczi F, Bíró E, Szabó G, Kovács GL. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23:27–31. doi: 10.1016/0143-4179(92)90006-i. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Derick S, Brossard G, Manning M, Simiand J, Gaillard R, Griebel G, Guillon G. Functional and pharmacological characterization of the first specific agonist and antagonist for the V1b receptor in mammals. Stress. 2003;6:199–206. doi: 10.1080/1025389032000114524. [DOI] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM. Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res. 2002;925:76–88. doi: 10.1016/s0006-8993(01)03261-9. [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM. Expression of cytoplasmic and nuclear vasopressin RNA following castration and testosterone replacement: evidence for transcriptional regulation. Mol Cell Neurosci. 1994;5:1–10. doi: 10.1006/mcne.1994.1001. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, de Wied D. Heroin self-administration is under control of vasopressin. Life Sci. 1977;21:315–319. doi: 10.1016/0024-3205(77)90511-2. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Gerrits MAFM, Vanderschuren LJMJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Reviews. 1999;51:341–396. [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Wigger A, Sánchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor JT, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation. Neuroscience. 2005;134:1391–1397. doi: 10.1016/j.neuroscience.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cumming E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and its V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]