Abstract
The prophylactic efficacy of a schistosome antigen (Sm-p80) was tested in a nonhuman primate model, the baboon. Using a total of 28 baboons, different vaccination strategies were used including recombinant Sm-p80 protein formulated in Toll-like receptor 7 and Toll-like receptor 9 agonists, and DNA priming followed by boosting with protein plus adjuvants. Recombinant protein approaches provided levels of prophylactic efficacy of 52%–58%, whereas prime-boost approaches conferred 38%–47% protection in baboons. An appropriately balanced pro-inflammatory (T-helper 17 [Th17] and Th1) and anti-inflammatory (Th2) type of response was generated; the Th1 and Th17 types of immune responses appear to be indicative of increased prophylactic efficacy. Production and expression of several cytokines (interleukin 2 [IL-2], interferon γ, IL-12α, IL-1β, IL-6, and IL-22) were up-regulated in vaccinated animals. Human correlate studies revealed Sm-p80 reactivity with immunoglobulin G in human serum samples from schistosome-infected individuals. In addition, a complete lack of prevailing Sm-p80–specific immunoglobulin E in a high-risk or infected population was observed, thus minimizing the risk of hypersensitivity reaction following vaccination with Sm-p80 in humans. This study provided the proof of concept to move Sm-p80 forward into further preclinical development leading to human clinical trials.
Schistosomiasis (Bilharzia) is a major neglected tropical disease of public health concern to a billion people (200 million people are currently infected; 779 million are at risk to acquire the infection) in 74 countries; 85% of these countries are in Africa [1, 2]. The disease carries high morbidity, and some estimates of disability-adjusted life-years (up to 70 million years annually) actually rank schistosomiasis ahead of malaria [3, 4]. Current schistosomiasis control strategies have been geared toward repeated treatment with praziquantel, a drug discovered in the 1970s [2, 5, 6]. The present standard practices of monitoring, evaluation, and delivery of mass drug administration for neglected tropical diseases in general, and schistosomiasis in particular, are inconsistent and inadequate [7]. Reliance on the drug therapy approach alone is barely adequate in the short term because this approach has had little bearing on the reduction of disease transmission; in addition, there is always the inherent threat of development of drug resistance by the parasite [8–10]. Reduction in the disease sequelae and transmission can only be attained through long-term protection via prophylactic vaccination coupled with drug treatment [9, 11]. A prophylactic vaccine that provides at least 50% protection would play an important role in the reduction of schistosomiasis morbidity. Vaccine-generated immune responses could lead to reduced worm burdens and lower egg production that would ultimately result in lessened transmission [12–14].
One major consideration when developing a vaccine for helminths is the potential risk of augmenting harmful immunopathogenic responses, as has been the unfortunate case with the ASP-2–based hookworm vaccine [12]. In clinical trials, higher levels of ASP-2–specific immunoglobulin E (IgE) antibodies, already present in human volunteers from their previous hookworm infections prior to vaccination, resulted in severe hypersensitivity reactions upon immunization that ultimately resulted in the cessation of further development of the vaccine [12]. To avoid a similar situation in a future clinical trial with any schistosome vaccine candidate, we have also performed human correlate studies in which the presence of IgG and lack of preexisting IgE responses to our vaccine candidate were determined in human serum samples from both pediatric and adult populations of areas in Kenya where schistosomiasis is endemic.
To develop a defined molecular vaccine for intestinal schistosomiasis, we have targeted a key schistosome protein (Sm-p80) that is easily accessible to the host’s immune system [15, 16]. This antigen plays a crucial role in the escape of the parasite from immune killing by the host and thus is an ideal vaccine candidate [14, 16]. Sm-p80 has been tested for its prophylactic efficacy in different vaccine formulations and approaches in 2 experimental animal models (mouse and baboon) of infection and disease [14, 16–30]. In the present study, we have tested the prophylactic efficacy of Sm-p80–based vaccine formulations in both recombinant protein and DNA-prime/protein-boost approaches using the baboon model.
MATERIALS AND METHODS
Animals and Parasites
Baboons (Papio anubis) of both sexes, 2.3–6.29 years old, were obtained from the University of Oklahoma Health Sciences Center (OUHSC; Oklahoma City, OK) baboon breeding colony and housed in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities at OUHSC. Baboons were prescreened for intestinal and blood parasites and for antibodies that were cross-reactive to Sm-p80 and were found negative for both. Schistosoma mansoni–infected snails (Biomphalaria glabrata) were acquired from the Schistosomiasis Resource Center, Biomedical Research Institute (Rockville, MD). This study was approved by the institutional animal care and use committee.
Preparation of Vaccine Formulations
DNA immunogen was prepared as described elsewhere [18, 19, 28, 29]. Briefly, a full-length coding sequence of Sm-p80 was subcloned into BamHI/BglII sites of VR-1020 (Vical, Inc). For DNA vaccination, the Sm-p80-VR-1020 plasmid was isolated via the conventional alkaline lysis method and purified on Sepharose CL4B columns [18, 19, 28, 29]. The protein immunogen (rSm-p80) was generated as described elsewhere [17, 20]. Briefly, the full-length coding sequence of Sm-p80 was cloned into the pCold II vector (GenScript Corp). The expressed proteins were purified via Ni-nitrilotriacetic acid-agarose followed by a Sephadex G-150 column. Endotoxin levels in both DNA and protein samples were analyzed with a Limulus amebocyte lysate assay (Charles River Laboratories International) [17, 20].
Baboon Vaccinations, Parasite Challenge, and Worm Burden Determination
The complete vaccine formulations and their administration frequencies as well as schedules for challenge and necropsy of baboons are outlined in detail in Table 1. Briefly, the adjuvants used were resiquimod (R848; Toll-like receptor 7 [TLR7] agonist in baboons) and CpG ODN (TLR9 agonist). Challenge infections, necropsies, and determination of the percentage of protection were performed as described elsewhere [19, 28].
Table 1.
Immunization, Challenge, and Necropsy Schedule of Baboons
| Study week, compound (dose) or action |
|||||||
| Vaccine group | 0 | 4 | 8 | 12 | 20 or 21 | Mean worm burden (SE) | Reduction in worm burden, %a |
| Recombinant vaccine | |||||||
| Control ODN (n = 4) | Control ODN(250 μg) | Control ODN(250 μg) | Control ODN(250 μg) | Challenge | Sacrifice | 184.25 (54.97) | |
| rSm-p80 + ODN-10104 (n = 4) | rSm-p80 (250 μg) + ODN-10104(250 μg) | rSm-p80 (250 μg) + ODN-10104(250 μg) | rSm-p80 (250 μg) + ODN-10104(250 μg) | Challenge | Sacrifice | 77.75 (17.78) | 57.80 |
| Control R848 (n = 4) | BA (250 μg) +R848 (50 μg) | BA (250 μg) +R848 (50 μg) | BA (250 μg) +R848 (50 μg) | Challenge | Sacrifice | 178 (29.97) | |
| rSm-p80 + R848 (n = 4) | rSm-p80 (250 μg) + R848 (50 μg) | rSm-p80 (250 μg) +R848 (50 μg) | rSm-p80 (250 μg) +R848 (50 μg) | Challenge | Sacrifice | 85.25 (9.67) | 52.10 |
| DNA-prime/protein-boost vaccine | |||||||
| Control prime-boost VR-1020 + ODN (n = 3) | VR-1020(500 μg) | Control ODN(250 μg) | Control ODN(250 μg) | Challenge | Sacrifice | 684.33 (25.00) | |
| Prime-boost Sm-p80-VR-1020 + rSm-p80-ODN-10104 (n = 3) | Sm-p80-VR-1020 (500 μg) | rSm-p80 (250 μg) + ODN-10104(250 μg) | rSm-p80 (250 μg) + ODN-10104(250 μg) | Challenge | Sacrifice | 360.33 (76.09) | 47.34 |
| Control prime-boost VR-1020 + R848 (n = 3) | Sm-p80-VR-1020 (500 μg) | BA (250 μg) +R848 (50 μg) | BA (250 μg) +R848 (50 μg) | Challenge | Sacrifice | 809.66 (31.67) | |
| Prime-boost Sm-p80-VR-1020 + rSm-p80 + R848 (n = 3) | Sm-p80- VR-1020 (500 μg) | rSm-p80 (250 μg) + R848 (50 μg) | rSm-p80 (250 μg) + R848 (50 μg) | Challenge | Sacrifice | 505.00 (20.78) | 37.62 |
Mean worm burden and resulting prophylactic efficacy of Sm-p80–based vaccine formulations using recombinant protein and DNA-prime/protein-boost regimens. Baboons were challenged with 1000 cercariae. Abbreviations: BA, baboon albumin; R848, Resiquimod.
P ≤ .001.
Collection of Blood Samples and Peripheral Blood Mononuclear Cell Isolation
Blood samples were collected just before the first immunization, at every booster (ie, 4 and 8 weeks), and before challenge infection (12 weeks). The collected serum samples were used in enzyme-linked immunosorbent assays (ELISAs) [19, 28]. Peripheral blood mononuclear cells (PBMCs) from the blood were isolated using Histopaque-1077 (Sigma-Aldrich) [19, 28].
Baboon Serum Antibody Assays
Serum samples from each individual animal were used to determine antibody levels and titers for IgG, IgG subtypes (IgG1, IgG2, IgG3, and IgG4), IgM, IgA, and IgE antibodies as described elsewhere [19, 26, 28].
PBMC and Splenocyte Proliferation Assays and Estimation of Cytokine Production
PBMCs from the 2 groups of baboons were isolated as described above. Splenocytes were isolated from the macerated spleens of individual baboons obtained after the animals were euthanized. The in vitro proliferation assays were performed as follows: in a 96-well flat-bottom plate, 5 × 105 PBMCs or splenocytes, 200 μL/well, were stimulated with either 0.5 μg of ConA, 1.2 μg of recombinant Sm-p80, or 1.2 μg of ovalbumin and incubated at 37°C with 5% CO2. After 48 hours of incubation, an aliquot of the supernatant was removed for the estimation of cytokine production and the remainder was used for the (MTT 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, a yellow tetrazole) assay, as described elsewhere [19, 28]. Using a baboon T-helper 1 (Th1) and Th2 ELISA panel kit (U-cyTech, the Netherlands), the production of cytokines (interleukin 2 [IL-2], IL-4, IL-10, and interferon γ [IFN-γ]) by the proliferating PBMCs and splenocytes were estimated as described elsewhere [19, 28].
RNA Extraction, Complementary DNA Synthesis, and Reverse-Transcription Polymerase Chain Reaction
PBMCs, spleens, and lymph node cells were cultured in the presence or absence of Sm-p80 antigen. The cultures were maintained at 37°C for 24 hours. Total RNA was extracted from cells via the TRIzol method (Invitrogen). Reverse-transcription reactions for first-strand complementary DNA synthesis were performed as described elsewhere [17, 30]. Expression levels of different cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12α, IL-12β, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IL-19, IL-20, IL-21, IL-22, IL-23, tumor necrosis factor α [TNF-α], IFN-γ, transforming growth factor β1 [TGF-β1], and TGF-β2) and glyceraldehyde 3-phosphate dehydrogenase were determined via reverse-transcription polymerase chain reaction (PCR) using published protocols [31–33]. PCR products were analyzed on 2% agarose gels, and relative differences in expression levels of cytokines were determined using the Quantity One program (version 4.6.2; Bio-Rad).
Flow Cytometry Analysis
To obtain Th1 and Th2 profiles, PBMCs were seeded into 24-well plates (2 × 106cells/well). The cells were incubated with or without rSm-p80 (12 μg/mL) overnight; GolgiPlug (1:1,000 dilution) was added in the last 10 hours of the incubation period. The cells maintained in phorbol 12-myristate 13-acetate (100 ng/mL) and ionomycin (1 μg/mL) were processed as positive controls. Direct staining was performed to identify CD3+ and CD8+ cells using fluorescein isothiocyanate (FITC) conjugated mouse anti-human CD3 and PE-Cy7 mouse anti-human CD8 antibodies, respectively. Similarly, staining was performed to identify CD3+ and CD4+ cells using FITC conjugated mouse anti-human CD3 and PerCP-Cy5.5 mouse anti-human CD4 antibodies, respectively. Intracellular staining was performed to detect IFN-γ– or IL-4–secreting cells using PE-mouse anti-human IL-4, PE-mouse anti-human IFN-γ, or APC-mouse anti-human IL-4. All of the immunological reagents and antibodies were obtained from BD Biosciences. The data were collected using CellQuset Pro software (BD Biosciences). The data were analyzed using Flowjo software (Tree Star).
Human Correlate Studies
This component of the study was approved by the institutional review board of Boston University (Boston, Massachusetts), the Scientific Steering Committee of the Kenya Medical Research Institute (KEMRI; Kisumu, Kenya), and the National Ethics Review Committee of Kenya. The study site was along the shores of Lake Victoria, ∼8 km from Kisumu City in western Kenya, among adult males exposed by their work as car washers and fisherman (n = 43). Occupationally exposed laborers have relatively longer contacts with the lake water, which raises their average rates of infection. Children aged 8–10 years were recruited from a primary school located within 3 km of Lake Victoria in the Asembo Bay area of the Nyanza Province in western Kenya (n = 9). Stool samples were examined for S. mansoni eggs and for other helminth ova by the modified Kato-Katz method (2 slides each, 3 stool specimens obtained over several days). Subjects positive for S. mansoni were treated with 40 mg/kg praziquantel; those positive for other helminth ova were treated with 400 mg of albendazole as described elsewhere [34, 35]. Uninfected Kenyan adult subjects were recruited from KEMRI (n = 9). Informed consent was obtained for adult subject participation. Written informed consent was obtained from the parent or guardian of each child before enrollment, and the children gave verbal assent for participation in the study. Blood samples were obtained by venipuncture and collected into heparinized tubes. Plasma samples were collected and stored at −80°C until use.
Anti-Sm-p80 IgG and IgE levels were measured with standard ELISA. For measurement of IgG levels, plates were coated with purified Sm-p80 (1 μg/mL). Human plasma was used at 1:500 dilution and incubated for 2 hours at room temperature. Rabbit anti-human IgG (Southern Biotech) was used at 1:10 000 dilution. For detection of Sm-p80–specific IgE, plasma was used at 1:10 dilution and incubated overnight at 4°C. Goat anti-human IgE (Sigma) was used at 1:1000 dilution. For comparison, ELISA plates were also coated with 5 μg/mL schistosomal egg antigen (SEA; gift from W. Evan Secor, Centers for Disease Control and Prevention, Atlanta, GA) and serum levels of SEA-specific IgE were measured as above.
Statistical Analyses
Significance between the 2 groups was calculated via an independent sample t test, using the SPSS computer program (version 13.0; SPSS). Bonferroni adjustments were included for multiple comparisons, to reduce the risk of reaching false conclusions based on chance. P values obtained by these methods were considered to be significant if they were < .05.
RESULTS
Reduction in Worm Burden Following Vaccination With Sm-p80–Based Vaccine Formulations
Using 2 different vaccination strategies, (1) immunization with rSm-p80 protein formulated in adjuvant and (2) priming with Sm-p80 plasmid DNA and boosting with rSm-p80 protein formulated in adjuvant, the protective potential of Sm-p80 was determined (Table 1). Baboons immunized with recombinant protein formulations of vaccine showed 52%–58% reduction in worm burden (Table 1). In baboons immunized by use of DNA-prime/protein-boost approaches, the reduction in numbers of adult parasites ranged from 38% to 47% (Table 1).
Antibody Responses to Sm-p80 in Vaccinated Baboons
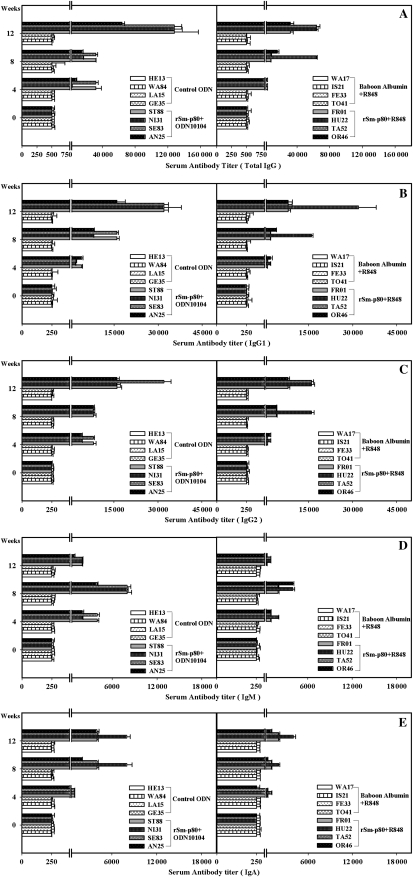
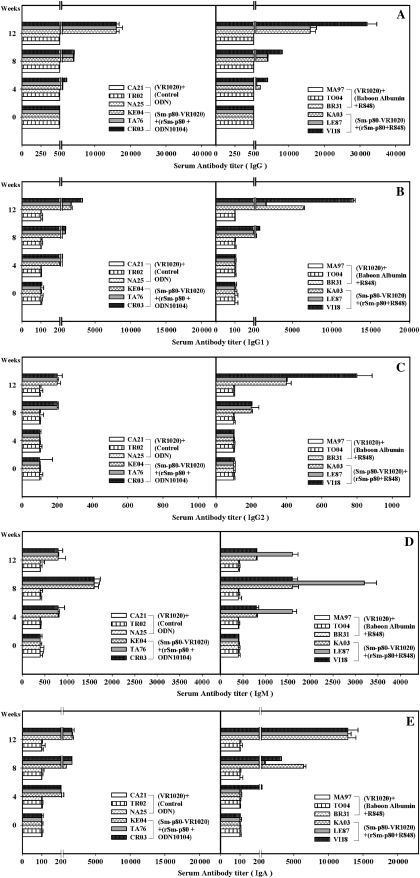
Class-specific antibody titers were measured in the serum samples of animals immunized with the Sm-p80–based vaccine delivered in the recombinant protein approach (Figure 1) and by the DNA-prime/protein-boost regimen (Figure 2). In rSm-p80 vaccine groups, total IgG, IgG1, IgG2, and IgA titers peaked at week 12 and total IgM titers peaked at week 8 in all individual baboons receiving Sm-p80 vaccine (Figure 1). Similarly, in the prime-boost groups, total IgG, IgG1, IgG2, and IgA titers peaked at week 12 and total IgM titers peaked at week 8 in all individual baboons receiving Sm-p80 vaccine (Figure 2). Reactivity to IgG3 and IgG4 was not detected in any of the vaccinated animals. In addition, no detectable levels of Sm-p80–specific antibodies (total IgG or IgG subtypes) were detected in the group of animals immunized with control formulations.
Figure 1.
Titers of anti–Sm-p80 antibodies in immunized baboons. Enzyme-linked immunosorbent assay was performed with serum samples from each baboon in their respective control and experimental groups of recombinant protein vaccine formulations (rSm-p80 + CpG ODN or Resiquimod [R848]). Shown are total immunoglobulin G (IgG) (A), IgG1 (B), IgG2 (C), IgM (D), and IgA (E) levels in individual control (HE13, WA84, LA15, and GE35) and vaccinated (ST88, NI31, SE83, and AN25) baboon serum samples collected every 4 weeks. The values represent the mean (± SE) of 3 experiments.
Figure 2.
Titers of anti–Sm-p80 antibodies in immunized baboons. Enzyme-linked immunosorbent assay was performed with serum samples from each baboon in their respective control and experimental groups of DNA-prime and protein-boost vaccine formulations (Sm-p80-VR-1020 + rSm-p80 + ODN-10104; Sm-p80 + VR-1020 + rSm-p80 + R848). Shown are total immunoglobulin G (IgG) (A), IgG1 (B), IgG2 (C), IgM (D), and IgA (E) levels in individual control (CA21, TR02, and NA25) and vaccinated (KE04, TA76, and CR03) baboon serum samples collected every 4 weeks. The values represent the mean (± SE) of 3 experiments.
T-Cell Proliferative Responses to Sm-p80 and Cytokine Production in Vaccinated Baboons
PBMCs and splenocytes of all of the animal groups immunized with Sm-p80 vaccine showed higher stimulation indices compared with their respective controls, but the stimulation indices were markedly lower in comparison with the stimulation induced by ConA (data not shown). As shown in Table 2, the high degree of proliferation by PBMCs was also correlated with the IFN-γ and IL-2 production. PBMCs from both recombinant protein groups produced 9-fold more IL-2 and 6–8-fold higher levels of IFN-γ. Similarly, PBMCs from both prime-boost groups produced 20–26-fold more IL-2 and 16–22-fold higher levels of IFN-γ (Table 2). IL-4 and IL-10 production by PBMCs in all of the 4 groups was present but was not as remarkable as IFN-γ and IL-2 production (Table 2).
Table 2.
Cytokine Production by Peripheral Blood Mononuclear Cells Induced by Recombinant Sm-p80 After 48 Hours of Culture In Vitro
| Mean cytokine level (SD), pg/mL |
|||||
| Vaccine, baboon ID | Vaccine group | IL-2 | IL-4 | IL-10 | IFN-γ |
| Recombinant vaccine | |||||
| RE13 | Control ODN | 59.13 (0.00) | 123.84 (34.72) | 77.76 (11.85) | 121.92 (6.71) |
| WA84 | Control ODN | 60.48 (15.23) | 121.92 (15.11) | 73.92 (12.46) | 135.36 (21.93) |
| LA15 | Control ODN | 61.44 (10.00) | 117.12 (5.24) | 66.24 (4.63) | 95.68 (9.63) |
| GE35 | Control ODN | 58.56 (5.24) | 139.20 (11.03) | 71.04 (4.32) | 38.40 (3.33) |
| ST88 | rSm-p80 + ODN-10104 | 600.96 (6.13) | 252.80 (12.15) | 250.56 (35.55) | 723.84 (34.37) |
| NI31 | rSm-p80 + ODN-10104 | 635.52 (34.80) | 122.88 (5.00) | 258.24 (19.03) | 734.40 (44.30) |
| SE83 | rSm-p80 + ODN-10104 | 536.64 (18.89) | 160.64 (17.17) | 191 (12.86) | 981.12 (21.60) |
| AN25 | rSm-p80 + ODN-10104 | 486.72 (53.01) | 142.40 (18.69) | 204.48 (30.04) | 680.64 (9.47) |
| WA17 | Baboon albumin + R848 | 54.72 (11.22) | 44.80 (4.44) | 63.36 (9.69) | 119.04 (12.90) |
| IS21 | Baboon albumin + R848 | 56.64 (5.42) | 41.28 (12.40) | 66.24 (9.27) | 96.00 (9.65) |
| FE33 | Baboon albumin + R848 | 53.76 (6.57) | 38.08 (2.68) | 64.32 (4.29) | 167.04 (17.57) |
| TO41 | Baboon albumin + R848 | 59.52 (5.23) | 43.20 (7.92) | 49.92 (4.10) | 120.00 (18.57) |
| FR01 | rSm-p80 + R848 | 515.52 (19.66) | 144.00 (13.51) | 229.12 (11.62) | 750.72 (16.77) |
| HU22 | rSm-p80 + R848 | 571.20 (20.97) | 134.72 (6.08) | 122.88 (7.35) | 791.04 (27.96) |
| TA52 | rSm-p80 + R848 | 426.24 (15.13) | 140.48 (8.74) | 233 (19.74) | 821.76 (6.72) |
| OR46 | rSm-p80 + R848 | 534.72 (10.34) | 154.24 (15.89) | 186.24 (17.86) | 674.88 (14.56) |
| DNA-prime/protein-boost vaccine | |||||
| CA21 | VR-1020 + control ODN | 12.1 (0.23) | 10.44 (2.11) | 7.66 (1.50) | 11.10 (0.11) |
| TR02 | VR-1020 + control ODN | 8.88 (0.29) | 12.12 (1.13) | 9.22 (1.42) | 15.60 (1.13) |
| NA25 | VR-1020 + control ODN | 14.14 (1.00) | 17.13 (3.23) | 6.24 (4.63) | 9.88 (1.36) |
| KE04 | Sm-p80-VR-1020 + rSm-p80 + ODN-10104 | 358.51 (15.24) | 145.30 (11.00) | 69.08 (8.23) | 538.10 (13.93) |
| TA76 | Sm-p80-VR-1020 + rSm-p80 + ODN-10104 | 210.16 (16.31) | 42.83 (2.11) | 20.60 (5.50) | 123.40 (4.70) |
| CR03 | Sm-p80-VR-1020 + rSm-p80 + ODN-10104 | 335.20 (23.81) | 39.68 (3.01) | 28.41 (10.30) | 139.30 (15.20) |
| MA97 | VR-1020 + baboon albumin + R848 | 11.42 (1.20) | 14.30 (2.41) | 13.60 (2.90) | 20.04 (2.10) |
| TO04 | VR-1020 + baboon albumin + R848 | 16.40 (5.42) | 11.81 (2.10) | 17.40 (3.70) | 10.00 (1.51) |
| BR31 | VR-1020 + baboon albumin + R848 | 13.60 (1.17) | 8.80 (0.89) | 14.20 (1.29) | 17.14 (2.71) |
| KA03 | Sm-p80-VR-1020 + rSm-p80 + R848 | 389.21 (18.39) | 53.29 (3.29) | 49.49 (5.20) | 139.00 (8.70) |
| LE87 | Sm-p80-VR-1020 + rSm-p80 + R848 | 225.29 (17.60) | 44.10 (5.19) | 89.12 (10.29) | 254.82 (20.70) |
| VI18 | Sm-p80-VR-1020 + rSm-p80 + R848 | 191.30 (10.79) | 39.22 (5.98) | 32.18 (2.20) | 190.03 (21.60) |
The values represent the mean (SD) of 3 independent experiments.
Assessment of Cytokine Messenger RNA Profiles
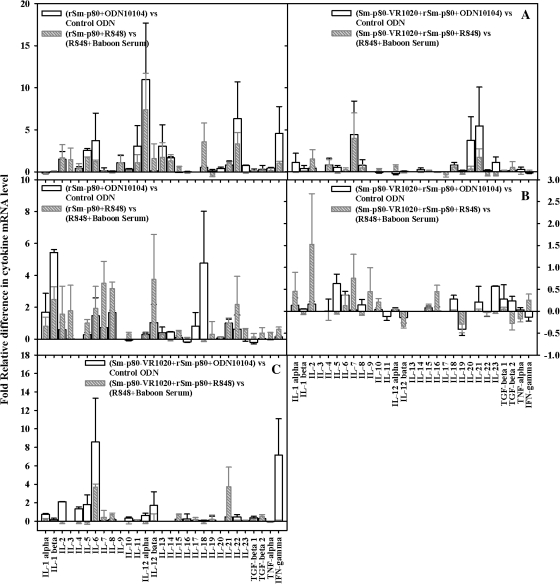
A large number of cytokines was studied and relative differences in their expression levels were recorded in PBMCs, spleens, and lymph node cells following stimulation in vitro with recombinant Sm-p80, for all 4 of the vaccination approaches (Figure 3). Many of cytokines were found to be up-regulated in this study, but some key ones include IL-12α, IL-22, and IFN-γ (PBMCs) and IL-1β (spleen) in the recombinant protein vaccine approach. In the prime-boost approach, up-regulated cytokines included IL-21 (PBMCs) and IFN-γ and IL-6 (lymph nodes) (Figure 3).
Figure 3.
Relative fold differences in cytokine messenger RNA expression levels by peripheral blood mononuclear cells (A), splenocytes (B), and lymph node cells (C) after 24 hours of stimulation with recombinant Sm-p80 in vitro. The relative cytokine messenger RNA expression level was calculated by comparing the differences in the levels of the control group with those of the respective experimental group after standardization using respective glyceraldehyde 3-phosphate dehydrogenase.
Identification of IFN-γ– and IL-4–Producing Cell Populations by Flow Cytometry
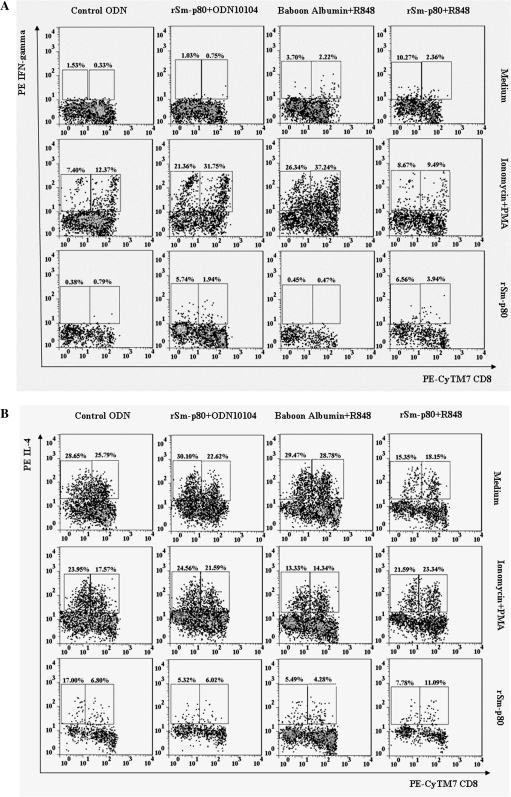
The secretion of IFN-γ and IL-4 by CD3+CD8+, CD3+CD8−, CD3+CD4+, and CD3+CD4− cells was analyzed in PBMCs of vaccinated and control baboons (Figure 4). Analyses revealed a 16.54%–20.27% increase in population of CD3+CD8− cells in the Sm-p80 + ODN-10104 vaccinated group of baboons compared with cells from control animals (Figure 4). The baboons that received rSm-p80 + R848 did not show a significant increase in CD3+CD8− cells, compared with the respective control group (Figure 4). However, in both of these experiments, a 6.5%–9.5% increases in IFN-γ–producing cells was detected (Figure 4). In both prime-boost experiments, differences in subtypes of CD3+CD4+ and CD3+CD4− populations of cells were not distinct (Figure 4). Similar to the recombinant protein vaccine group, in the prime-boost groups, the IFN-γ–secreting cell populations increased by up to 5.0% compared with control groups (Figure 4). The population of IL-4–secreting cells mostly came from CD8 cells (Tc2) in all of the vaccinated groups (Figure 4).
Figure 4.
Quantification of intracellular interferon γ (IFN-γ)– and interlekin 4 (IL-4)–producing CD3+CD4+ and CD3+CD8+ cells from control and vaccinated baboon peripheral blood mononuclear cells. Initial gating was performed using the CD3 marker. A, IFN-γ–producing cells in recombinant Sm-p80 + CpG ODN and Sm-p80 + R848 groups. B, IL-4–producing cells in recombinant Sm-p80 + CpG ODN and Sm-p80 + R848 groups. C, IFN-γ–producing cells in prime-boost groups (Sm-p80− VR-1020 + rSm-p80 + ODN-10104; Sm-p80-VR-1020 + rSm-p80 + R848). D, IL-4–producing cells in prime-boost groups (Sm-p80-VR-1020 + rSm-p80 + ODN-10104; Sm-p80-VR-1020 + rSm-p80 + R848).
IgG and IgE Reactivity to Sm-p80 in Human Serum Samples From Schistosome-Infected Patients
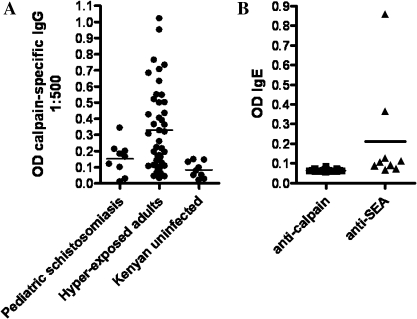
It is evident from the data that the serum samples from both at-risk children and hyperexposed adults contain varying levels (< 0.3 optical density (OD) in children and up to 1.0 OD in adults at 1:500 dilution) of Sm-p80–specific IgG (Figure 5). Even though there is pronounced IgE reactivity to schistosome soluble egg antigens in infected individuals, there is no to negligible IgE immunoreactivity with Sm-p80 (Figure 5).
Figure 5.
Antibody responses to Sm-p80 in human serum samples from an area in Kenya where schistosomiasis is endemic. A, Immunoglobulin G (IgG) reactivity to Sm-p80 in serum samples obtained from schistosome-carrying pediatric patients (n = 9) and hyperexposed adults (n = 43) as well as uninfected adults (n = 9) in Kenya. P > .05 for pediatric patients with schistosomiasis versus hyperexposed adults; P > .05 for pediatric patients with schistosomiasis versus uninfected adults; P < .01 for hyperexposed adults versus uninfected results (Dunn multiple comparison test). B, IgE reactivity to Sm-p80 and schistosome soluble egg antigen (SEA) in the serum samples of schistosome-infected individuals. P < .001 for anticalpain antibodies versus anti-SEA antibodies (Mann–Whitney U test).
DISCUSSION
Data generated in this study indicate that from the 2 different vaccination strategies used with Sm-p80–based vaccine, recombinant Sm-p80 formulated in TLR7 and TLR9 agonists provides the best levels of prophylactic efficacy in baboons (52%–58%). These levels of protection have not been previously obtained with any schistosome antigen in large animal models. In addition, there is now consensus that a vaccine that confers an initial 50% protection in humans should be effective in reducing overall morbidity and mortality [9, 11, 36], and in all likelihood would be an appropriate first-generation schistosomiasis vaccine [37]. Because baboons present a disease of schistosomiasis comparable to that in humans [38] and provide an excellent model for vaccine efficacy determination studies [14, 39], detection of high levels of Sm-p80–mediated protection in this model further strengthens the validity of Sm-p80 as a vaccine candidate.
A mixed and balanced response of the pro-inflammatory (Th17 and Th1) and anti-inflammatory (Th2) type was observed in this study, but the generation of Th1 response appears to be important in Sm-p80–mediated protection in baboons. The predominant antibody in protected animals is IgG and its subtypes IgG1 and IgG2. In humans, IgG1 is implicated in Th2-type responses in many cases [40–42], but it has also been shown to be driven by IL-12 and IFN-γ in a few systems [43, 44]. IgG2, on the other hand, in most cases is dependent on IL-12 and IFN-γ [42, 45]. Similarly, enhanced production of IL-2 and IFN-γ in cultures and higher levels of IL-12α messenger RNA in PMBCs stimulated with Sm-p80 further reinforce the importance of Th1 type of responses in animals vaccinated with recombinant protein formulation. The Th17 type of responses may also play some role in providing Sm-p80–mediated protection, as evidenced by the increase in IL-1β, IL-6, and IL-22 messenger RNA levels in PBMCs, splenocytes, and lymph node cells from vaccinated baboons. Essential and synergistic roles for some of these cytokines have been reported in human Th17 differentiation directed by TLR ligand-activated dendritic cells [46]. A clear increase in the population of CD3+CD4+ cells in the rSm-p80 + ODN-10104 group (the best performing strategy) indicates that the vaccine is effective in expanding T cell counts. CD4+ T cells have been reported to be involved in conferring some resistance to humans from reinfection with schistosomes, perhaps by orchestrating many of the effector mechanisms required for human protective immunity. [47–49].
It is also evident from human correlate studies that some schistosome-hyperexposed individuals develop anti–Sm-p80 IgG, but how this correlates with resistance to infection is still unclear. Furthermore, the pediatric population (8–10 years old), which is highly susceptible to schistosome infection, has very low levels, if any, of anti–Sm-p80 IgG and thus a Sm-p80–based vaccine should greatly augment protective immunity. Finally, the lack of prevailing Sm-p80–specific IgE in the human schistosome-infected population provides a good level of confidence that the risk of hypersensitivity reactions following vaccination with Sm-p80 is minimal.
The data presented here provide a clear proof of concept in a nonhuman primate model of schistosomiasis using a recombinant Sm-p80 protein formulation as the basis for further preclinical vaccine development leading to human clinical trials.
Notes
Acknowledgments.
We thank Maria Chavez-Suarez and James Patin for their excellent technical assistance with the baboon studies. We also thank Patrick Whitworth for assistance with human correlate studies. We are grateful to Alicia Gauna for excellent assistance in making the tables and figures.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01AI071223 to A. A. S.); and the Thrasher Research Fund (award 02824-5 to A. A. S.). The baboon facility is supported by the National Center for Research Resources (grant P40RR012317 to G. L. W.); and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R27AI074843 to L. G.-L.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–6. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 4.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utzinger J, N'Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.08.020. 2010; doi:10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Parker M, Allen T. Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res Policy Syst. 2011;9:3. doi: 10.1186/1478-4505-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doenhoff MJ, Hagan P, Cioli D, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–35. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 9.Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–42. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–7. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Bethony JM, Cole RN, Guo X, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev. 2011;239:237–70. doi: 10.1111/j.1600-065X.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–814. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008;102:825–33. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 15.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–56. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui AA, Zhou Y, Podesta RB, et al. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:44–37. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad G, Zhang W, Torben W, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–77. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009;31:156–61. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad G, Zhang W, Torben W, et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine. 2009;27:2830–7. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad G, Zhang W, Torben W, Noor Z, Siddiqui AA. Protective effects of Sm-p80 in the presence of resiquimod as an adjuvant against challenge infection with Schistosoma mansoni in mice. Int J Infect Dis. 2010;14:e781–7. doi: 10.1016/j.ijid.2010.02.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–40. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 22.Hota-Mitchell S, Clarke MW, Podesta RB, Dekaban GA. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine. 1999;17:1338–54. doi: 10.1016/s0264-410x(98)00391-0. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui AA, Phillips T, Charest H, et al. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003;21:2882–9. doi: 10.1016/s0264-410x(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui AA, Phillips T, Charest H, et al. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003;71:3844–18. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005;12:3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005;23:1451–6. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Torben W, Ahmad G, Zhang W, Siddiqui AA. Role of antibodies in Sm-p80-mediated protection against Schistosoma mansoni challenge infection in murine and nonhuman primate models. Vaccine. 2011;29:2262–71. doi: 10.1016/j.vaccine.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Ahmad G, Torben W, et al. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis. 2010;201:1105–2. doi: 10.1086/651147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Ahmad G, Torben W, Siddiqui AA. Sm-p80-based DNA vaccine made in a human use approved vector VR1020 protects against challenge infection with Schistosoma mansoni in mouse. Parasite Immunol. 2010;32:252–2. doi: 10.1111/j.1365-3024.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Ahmad G, Torben W, Siddiqui AA. Schistosoma mansoni antigen Sm-p80: prophylactic efficacy of a vaccine formulated in human approved plasmid vector and adjuvant (VR 1020 and alum) Acta Trop. 2011;118:142–51. doi: 10.1016/j.actatropica.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun. 2008;76:942–51. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villinger F, Brar SS, Mayne A, Chikkala N, Ansari AA. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–54. [PubMed] [Google Scholar]
- 33.Wu H, Zhu B, Shimoishi Y, Murata Y, Nakamura Y. (-)-Epigallocatechin-3-gallate induces up-regulation of Th1 and Th2 cytokine genes in Jurkat T cells. Arch Biochem Biophys. 2009;483:99–105. doi: 10.1016/j.abb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Black CL, Muok EM, Mwinzi PN, et al. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis. 2010;202:399–405. doi: 10.1086/653828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith QK, Liang Y, Onguru DO, Mwinzi PN, Ganley-Leal LM. CD23-bound IgE augments and dominates recall responses through human naive B cells. J Immunol. 2011;186:1060–7. doi: 10.4049/jimmunol.1002709. [DOI] [PubMed] [Google Scholar]
- 36.Bergquist R. Prospects of vaccination against schistosomiasis. Scand J Infect Dis Suppl. 1990;76:60–71. [PubMed] [Google Scholar]
- 37.Todd CW, Colley DG. Practical and ethical issues in the development of a vaccine against schistosomiasis mansoni. Am J Trop Med Hyg. 2002;66:348–58. doi: 10.4269/ajtmh.2002.66.348. [DOI] [PubMed] [Google Scholar]
- 38.Damian RT, de la Rosa MA, Murfin DJ, Rawlings CA, Weina PJ, Xue YP. Further development of the baboon as a model for acute schistosomiasis. Mem Inst Oswaldo Cruz. 1992;87(suppl 4):261–9. doi: 10.1590/s0074-02761992000800041. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997;15:903–8. doi: 10.1016/s0264-410x(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 40.Tangye SG, Ferguson A, Avery DT, Ma CS, Hodgkin PD. Isotype switching by human B cells is division-associated and regulated by cytokines. J Immunol. 2002;169:4298–306. doi: 10.4049/jimmunol.169.8.4298. [DOI] [PubMed] [Google Scholar]
- 41.Avery DT, Bryant VL, Ma CS, de Waal MR, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008;181:1767–79. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 42.Garraud O, Perraut R, Riveau G, Nutman TB. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 2003;19:300–4. doi: 10.1016/s1471-4922(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 43.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–9. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 44.Mattos AM, Almeida CS, Franken KL, et al. Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol. 2010;22:775–82. doi: 10.1093/intimm/dxq429. [DOI] [PubMed] [Google Scholar]
- 45.Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun. 2004;72:5089–96. doi: 10.1128/IAI.72.9.5089-5096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol. 2010;134:178–87. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun. 2006;74:2169–76. doi: 10.1128/IAI.74.4.2169-2176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira-Prado R, Caldas IR, Teixeira-Carvalho A, et al. CD4 and CD8 distribution profile in individuals infected by Schistosoma mansoni. Scand J Immunol. 2009;69:521–8. doi: 10.1111/j.1365-3083.2009.02247.x. [DOI] [PubMed] [Google Scholar]
- 49.Karanja DM, Hightower AW, Colley DG, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360:592–6. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]