Abstract
Ventricular enlargement is one of the most consistent abnormal structural brain findings in schizophrenia and has been used to infer brain shrinkage. However, whether ventricular enlargement is related to local overlying cortex and/or adjacent subcortical structures or whether it is related to brain volume change globally has not been assessed. We systematically assessed interrelations of ventricular volumes with gray and white matter volumes of 40 Brodmann areas (BAs), the thalamus and its medial dorsal nucleus and pulvinar, the internal capsule, caudate and putamen. We acquired structural MRI ( patients with schizophrenia (n = 64) and healthy controls (n = 56)) and diffusion tensor fractional anisotropy (FA) (untreated schizophrenia n = 19, controls n = 32). Volumes were assessed by manual tracing of central structures and a semi-automated parcellation of BAs. Patients with schizophrenia had increased ventricular size associated with decreased cortical gray matter volumes widely across the brain; a similar but less pronounced pattern was seen in normal controls; local correlations (e.g. temporal horn with temporal lobe volume) were not appreciably higher than non-local correlations (e.g. temporal horn with prefrontal volume). White matter regions adjacent to the ventricles similarly did not reveal strong regional relationships. FA and center of mass of the anterior limb of the internal capsule also appeared differentially influenced by ventricular volume but findings were similarly not regional. Taken together, these findings indicate that ventricular enlargement is globally interrelated with gray matter volume diminution but not directly correlated with volume loss in the immediately adjacent caudate, putamen, or internal capsule.
Electronic supplementary material
The online version of this article (doi:10.1007/s00406-011-0202-x) contains supplementary material, which is available to authorized users.
Keywords: Cerebral spinal fluid, Sulcal enlargement, Myelin, Fronto-thalamic connectivity
Introduction
Ventricular enlargement is one of the earliest [1] and most consistent findings in schizophrenia [2]. The ventricles of patients with schizophrenia are approximately 130% the size of normal controls, an absolute volume difference in the order of 1.5 cm³ [3]. This has been interpreted as an indicator of brain shrinkage after the onset of the brain process associated with schizophrenia since the skull in an adult or adolescent is unlikely to contract. Thus, in schizophrenia, the cranial vault would have increased fluid volume if there were less brain tissue. If ventricular enlargement was primarily due to shrinkage of tissue surrounding the ventricle (caudate, thalamus, white matter), we would expect that it would appear at some delay after illness onset and be associated with relatively large local volume change. If, alternately, ventricular enlargement was due to diffuse cortical shrinkage, a very small percentage loss (1%) of whole cortical volume could produce a ventricular enlargement of 1.5 cm3. While some studies suggest that ventricular enlargement is present by the time of first psychotic episodes of schizophrenia, high-risk studies tend to find a reduction in cortical gray matter volume that is more prominent than ventricular enlargement [4, 5]. In fact, first episode patients may show little ventricular enlargement, [2] which appears later in the illness [6]. If ventricular enlargement is present at illness onset, it seems compatible with a developmental abnormality and potentially regional. If it is more prominent in older individuals ill for a long time but initially relatively well as adults, a progressive and diffuse cortical gray matter loss and concomitant ventricular enlargement seem more consistent.
While ventricular enlargement might result in part from decreased cortical volume in the frontal and temporal lobes where gray matter decreases have been found as reviewed earlier, structures immediately adjacent to the anterior horn of the ventricles, the caudate[7], internal capsule [8, 9], and thalamus [10] have also been found to be smaller in patients. The order of magnitude of reductions in these areas is 0.2–0.5 cm3 each. Cumulative volume loss in these structures is 30–50% less than the total enlargement of the ventricles, but could make a contribution to size increase. However, progressive cortical shrinkage over time has been estimated at approximately 37 cm3 in patients in comparison with healthy subjects in a review of six studies [6] and thus might fully explain ventricular enlargement.
Few studies have examined neuroanatomical correlates of ventricular enlargement in schizophrenia. Voxel-based morphometry revealed increased ventricular/brain ratio associated with volume decrease in the thalamus and striatum as well as the anterior temporal lobe [11], suggesting a regional relationship. Post-mortem results found temporal horn volume correlations with temporal lobe gyral volume [12]. Cortical shrinkage associated with ventricular enlargement might mechanically press on or pull apart adjacent structures contributing to their volume loss. For example, the interthalamic adhesion is more often missing in subjects with larger third ventricles [13]. If this is a more general mechanism, anterior horn size might be more closely correlated with adjacent structure volumes than with more distant cortical volumes. To our knowledge, no previous study on schizophrenia has systematically assessed the correlations between subregions of the ventricular system and both regional cortical and subcortical volumes. We hypothesized that there may be higher correlations between anterior ventricle size and adjacent frontal gray matter or the white matter tracts between the thalamus and prefrontal cortex running through the anterior limb of the internal capsule. Because the white matter medial to Brodmann area 22, an area of prominent gray matter shrinkage, lies adjacent to the ventricular space, we hypothesized that both gray and white matter in this area might be especially associated with temporal ventricular enlargement.
Methods
Subjects
Sample one Patients with schizophrenia (n = 64) were recruited from outpatient and inpatient departments at Mount Sinai Hospital, the Bronx VA Medical Center, and Pilgrim State Psychiatric Hospital, New York. Normal comparison subjects (n = 56) were recruited through word of mouth and advertisements. Healthy comparison subjects were screened with a modified version of the Comprehensive Assessment of Symptom History CASH [14] to exclude any history of an Axis I psychiatric disorder. All participants were screened by medical history, physical examination, and laboratory testing. Individuals with a history of substance abuse/dependence, neurological disorders, head trauma, clinical dementia, or a positive urine test for drugs of abuse on the day of the scan were excluded. After a complete description of the study, all participants provided written informed consent. Sample two 19 unmedicated or previously never-medicated patients and 32 healthy controls were used for the assessment of fractional anisotropy (FA) to volume correlations.
All patients met criteria for either schizophrenia (n = 79) or schizoaffective disorder (n = 4) as determined by the CASH [14]. Patients were either never previously medicated (n = 52) or had been neuroleptic free for at least 2 weeks (n = 31). One hundred and forty-seven subjects were right-handed, 15 were left-handed, and seven were ambidextrous (two undetermined) based on the Edinburgh Handedness Inventory [15]. Subjects (1 patient and 1 control) with whole lateral ventricle volumes more than 3 standard deviations above their group mean were considered outliers and eliminated. Forty-four of the patients were evaluated with the 18-item Brief Psychiatric Rating Scale (BPRS [16]) (mean score = 52.9, SD = 10.08, range = 30–83). There were no significant age or sex differences between patients with schizophrenia and normal volunteers neither in the subset of individuals assessed separately for FA analysis (See Table 1). Volumetric data on gray matter but not ventricular size from a smaller subset of patients were reported previously [17] [10, 18, 19].
Table 1.
Demographic and main volumetric data of the subjects included in the study
| Patients | Sample 1 | Patients | Sample 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Controls | Statistic | P value | Controls | Statistic | P value | |||
| n | 64 | 56 | 19 | 32 | ||||
| Age (SD) | 33.7 (17.4) | 36.8 (11.9) | t 118 = 1.15 | 0.25 | 32.1 (10.95) | 31.1 (8.26) | t 49 = 0.95 | 0.72 |
| Gender (F:M) | 17:47 | 21:35 | χ2 = 1.65 | 0.20 | 7:12 | 12:20 | χ2 < 0.00 | 0.96 |
| Handedness (R:L:A) | 55:5:3 | 51:4:1 | χ2 = 0.43 | 0.51 | 14:2:3 | 27:4 | χ2 = 1.44 | 0.23 |
| Education (years) | 12.8 (2.5) | 16.27 (2.5) | t 83 = 6.27 | <0.05 | 13 (1.79) | 18.5 (10.5) | t 30 = 1.25 | 0.22 |
| Age at onset | 24.4 (7) | NAa | ||||||
| Duration of illness (years) | 19.1 (16) | NAa | ||||||
| Total volume (cm3) | 1,191,000 (90,000) | 1,220,000 (115,000) | t 120 = 1.44 | 0.15 | 1,204,000 (125,000) | 1,172,000 (106,000) | t 49 = −0.95 | 0.34 |
| Cortical gray matter | 356 (40) | 384 (34) | t 120 = 4.19 | <0.001 | 434 (58) | 432 (38) | t 49 = −0.17 | 0.87 |
| Cortical white matter | 325 (47) | 326 (45) | t 120 = 0.05 | 0.96 | 297 (39) | 290 (36) | t 49 = −0.58 | 0.56 |
| Lateral ventricles | 22 (12) | 19 (8) | t 120 = −1.23 | 0.22 | 23 (17) | 15 (11) | t 23 = −1.35 | 0.19 |
aNot determined in this sample
Image acquisition and processing for two separate non-overlapping samples
Sample one The Signa 5x system (1.5T GE Medical Systems, Milwaukee, WI) with a 3D-SPGR sequence (TR = 24 ms, TE = 5 ms, flip angle = 40°, field of view 23 cm, slice thickness 1.2 mm, total slices 128, NEX = 1, matrix size 256 × 256, in plane voxel size = 0.9 × 0.9 × 1.2 mm) was used for MRI acquisition. Anatomical SPGR MRI images were resectioned to standard Talairach–Tournoux position. We used the FSL program FAST with bias field correction of MRIs followed by k-means clustering and local Markov analysis at each voxel for the segmentation into the white and gray tissue types [20]. Sample two Anatomical images were acquired with a 3.0 T Siemens Allegra (typical scanning time 50 min), using a magnetization prepared rapid gradient echo (MP-RAGE) sequence with the following parameters: voxel size = 0.82 × 0.82 × 0.82 mm, field of view = 210 mm, TR = 2500 ms, TE time = 4.38 ms, T1 = 1100 ms, flip angle = 8°. Diffusion tensor weighted images were obtained for all subjects using a sequence of 28 3-mm-thick slices in 12 gradient directions. Preprocessing included conversion of all image data to Analyze format, motion correction (MCFLIRT [21]); Brain Extraction Tool [22]; spatial smoothing with Gaussian profile filter of full-width-half-maximum (FWHM) 5 mm; high-pass temporal filtering with Gaussian-weighted running line detrending (cutoff = 70 s). The FA for area was determined by calculating the mean FA for every voxel in the anisotropy image, which matched a voxel identified as white matter on the MRI as we have done with FDG images [23–25]. All analyses were carried out only with subject images acquired on one scanner.
Anatomical analyses of the images
The Brodmann areas (Bas) of the cerebral cortex were assessed in native space, based on the Perry coronal atlas of the brain [26], as described in detail elsewhere [27–30], with image segmentation into gray and white matter voxels as in earlier reports [31]. Note that white matter adjacent and medial to each gray matter area is counted white matter associated with the area for the purposes of this analysis, even though it may contain white matter from adjacent or even distant cortical areas (Fig. 1, top row). The striatum (caudate nucleus and putamen), whole thalamus, mediodorsal nucleus (MDN), pulvinar, anterior limb of the internal capsule (ALIC) and the subdivisions of the lateral ventricles (anterior, lateral, and temporal horns) were traced manually in the axial plane as previously reported [7, 32, 33] (Fig. 1, bottom row left, A) and the volume obtained from application of the trapezoidal rule. The thalamic nuclei included in this study were traced following the guidelines carefully described elsewhere [10, 18]. The intraclass tracing between two independent tracers was 0.92 for the caudate nucleus, 0.98 for the putamen, 0.90 for the whole thalamus, 0.80 for the MDN, 0.91 for the pulvinar, and 0.82 for the ALIC, and for ventricles were: 0.86 for the anterior, 0.93 for the lateral, and 0.87 for the temporal horns. We calculated the xyz coordinates of the center of mass of the caudate nucleus, putamen, thalamus, MDN, pulvinar, and ALIC based on the tracings and expressed this location relative to the midline anterior commissure coordinates and corrected it for brain size (Fig. 1c).
Fig. 1.
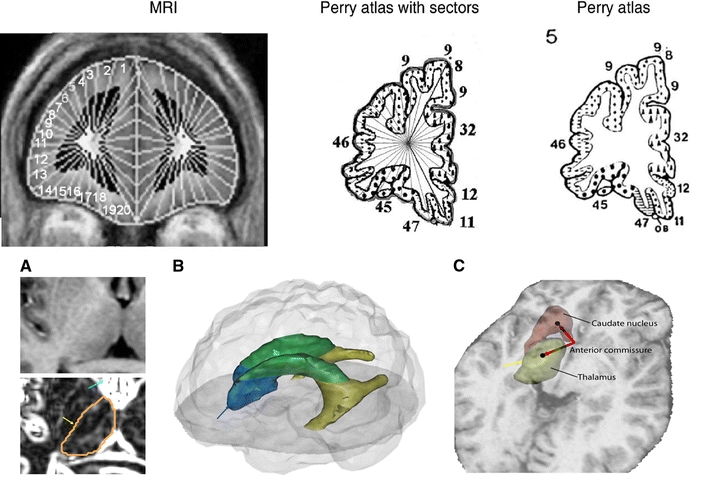
Anatomical methods: stereotaxic and Manual tracings. Upper panel Left Coronal MRI showing application of 20 lateral and 10 medial sectors. Center and Right Perry atlas with same sector algorithm applied (see “Methods”). Lower Panel a Axial view of an MRI slice before (above) and after (below) applying the Sobel filter. This method allows distinguishing the boundaries of the different subcortical structures, especially the thalamus, whose tracing is shown. Yellow arrow indicates lateral edge of thalamus and blue arrow enhanced anterior horn of ventricle. b Three-dimensional model merged from the manual tracings of the lateral ventricles. The anterior horn is depicted in blue (note blue arrow indicating edge in a), the lateral horn is in green, and the temporal horn appears in yellow. c Location of the centers of mass of the caudate nucleus and thalamus, and vectors showing their position regarding to the anterior commissure in the midline of the brain (yellow arrow for orientation to a)
Data analysis
We carried out t-tests comparing healthy participants and patients for each structure, averaging the right and left hemisphere values. We calculated the bivariate Pearson product–moment correlation matrices between absolute and relative ventricular volumes and other volumes with significance level of P < 0.05. Relative tissue volumes were computed as the ratio of region of interest volume in mm3 to total brain volume with the latter being the sum of the absolute gray and white matter volumes of 33 coronal brain slices. Finally, correlations of ventricular sizes with FA were analyzed in the subset of subjects in whom DTI was acquired (sample two). Kullback’s χ2 test for correlational matrices was employed to test group differences in correlation matrices [34], and Fisher’s z transformation was used to compare individual correlations. Mixed factorial repeated measures ANOVAs were employed to examine group differences in ventricular volume. Since earlier investigators have reported tables of correlations among structure volumes [35–37], we present the full analysis set (38 BAs identified by Perry), for comparisons with past and future exploratory analyses. From a Pub-Med search on schizophrenia and BAs by number, we observe for the BA and number of publication matches, respectively (6–17, 8–11, 9–35, 10–36, 11–18, 12–12, 44–6, 45–5, 46–16, 47–3, 32–12). It would not be appropriate to produce automated data on all 38 Brodmann’s areas in the right and left hemispheres and then adopt a Bonferroni corrected probability criterion that disconfirmed earlier reports or failed to find consistency with earlier single regional results. For these reasons, we present the exploratory analysis at a P value equal or less than 0.05. Lastly, we used factor analysis to reduce the dimensionality and examine whether a factor exists which loads the volume of a specific portion of the ventricles and a specific gray matter regional volume confirming the regional effects hypothesis, or whether all ventricle subregions load together with all cortical regions confirming a global shrinkage hypothesis.
Results
Brodmann areas and whole brain volume
Volumes of many of the BAs of the frontal, temporal, and parietal lobes, including BAs 1, 4, 9, 10, 11, 12, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 32, 36, 40, 41, 43, 44, and 46, were significantly smaller in patients than in normal controls (Supplementary Table 1, see Electronic Materials and Fig. 2). Whole brain volume did not differ between both groups (Table 1).
Fig. 2.
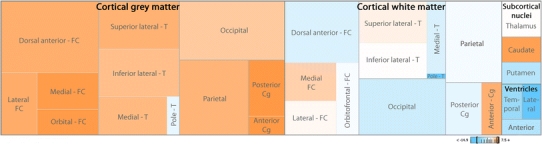
Relative volume proportions in the human brain in controls compared with schizophrenics. The dimension of the boxes represents the percentage of every cortical or subcortical subdivision in the brain of the control sample. Colors indicate whether relative volumes of these subdivisions are bigger in controls (orange), in patients (blue), or do not differ (white). Note how, in general, the relative volume of cortical gray matter and caudate nucleus is bigger in controls, but most aspects of cortical white matter, ventricles, and putamen are bigger in schizophrenics. The values in the color legend (−14.9 and 7.5) are expressed in percentage. Cg cingulate cortex, FC frontal cortex, T temporal cortex
Subcortical structures
The absolute volume of the right and left caudate together and separately was found to be smaller in patients with schizophrenia (left 4210, SD = 668, right 4210, SD = 701 mm3) than normal controls (left 4570, SD = 0.666; right 4660, SD = 740 mm3; t = 2.95, df = 118, P = 0.0038; t = 3.43, P = 0.00084, respectively). The right thalamus was smaller in patients with schizophrenia (0.637, SD = 0.110) than normals (0.688, SD = 0.128, t = 2.32, P = 0.022, diagnosis × hemisphere interaction, ns). Putamen absolute value was not different between groups, and putamen relative volume was significantly greater in patients. Relative volumes of the thalamus, MDN, and pulvinar did not differ significantly (Supplementary Table 1 in Electronic Supplementary Material). The ALIC was smaller in patients with schizophrenia, and this was most marked in the left hemisphere (diagnosis × hemisphere interaction, F = 4.02, df = 118, P = 0.047). The centers of mass of subcortical structures were displaced, generally posteriorly and laterally with respect to the anterior commissure (Supplementary Fig. 1, Supplementary Table 2 in Electronic Supplementary Material).
Ventricular volume
Patients with schizophrenia had larger mean relative ventricular volumes for anterior, lateral, and temporal horns but this effect was only at a trend level (main effect of group F = 3.33, df = 1,118, P = 0.070). Repeated measures ANOVA confirmed greater lateral than anterior or temporal enlargement in schizophrenia (group by ventricular region interaction, F = 3.45, df = 2, 236, P = 0.033, Huynh–Feldt df = 1.46, 172, P = 0.048), and follow-up ANOVA confirmed this (lateral ventricle, main effect of diagnosis, F = 4.01, df = 1,118, P = 0.047 and diagnosis by age F = 4.23, df = 1,118, P = 0.041). Ventricular enlargement in comparison with normal controls was significantly greater in older patients (>29 years old, mean age 51.6, SD = 10.5) than younger ones (<29 years old, mean age 20.1, SD = 4.3) (repeated measures ANOVA, group by age group interaction, F = 5.24, df = 1, 118, P = 0.023). Relative ventricular volume of the left hemisphere was greater than the right across both groups (F = 17.3, df = 1,118), but no significant interaction with diagnosis was found.
Ventricular volume was also enlarged when volume in mm3 uncorrected for brain volume was examined with a similar statistical pattern. Older patients had total ventricular volume (right + left) of 29710 mm3 (SD = 17590), which was 9,600 mm3 greater than older normal controls (20110 SD = 9940, t = 2.87, P = 0.005). Of the 38 BAs tested, 36 showed normals with significantly greater mm3 volume than patients with schizophrenia (BAs 34 and 38 were exceptions). Total gray matter absolute volumes were 436,000 mm3 for normals and 402,000 mm3 for older patients, a difference of 34,100 mm3 or 3.54 times as great as the ventricular enlargement. Thus, the ventricular enlargement in older patients with schizophrenia is only 28.2% of gray matter loss. Total sulcal cerebrospinal fluid (CSF) space was 123,064 in older normals and 144,706 mm3 in older patients with schizophrenia, a difference of 21642 mm3, which is 63.7% of the gray matter loss. Thus, taken together, CSF increase accounts for 91.9% of the gray matter decrease (third ventricle not included in measurement).
Correlations between ventricular enlargement and gray matter volumes of interest
We examined whether left temporal horn ventricular enlargement, common in patients with schizophrenia, was associated with local cortical volume loss. We expected a significant negative correlation (larger ventricles associated with greater gray matter loss) and for the negative correlation to be greater for the adjacent ventricular region than for a distant one. For example, in patients, the correlations for relative ventricular volume of the left temporal horn and gray matter volume of left lateral temporal lobe (BA 20 r = −0.41, BA 21 r = −41, BA 22 r = −0.45) are all far above statistical significance but actually the same or lower than the correlations between left anterior horn and left lateral temporal lobe (BA 20 r = −0.39, BA 21 r = −0.45, BA22 r = −0.51, for r > 0.38, n = 63, P < 0.002). Similarly for the posterior temporal and cingulate regions near the left temporal horn (BA 23, 29, 30, 31), the correlations between left relative gray matter volume and left temporal horn volume were −0.54, −0.47, −0.53, −0.42 and highly significant, but correlations with the distant left anterior horn were also significant but not significantly lower (−0.48, −0.42, −0.38, −0.41, respectively, Fisher’s z-tests, 1-tailed, not significant between any corresponding pair). Thus, support for a regional hypothesis was not obtained. Correlation matrices between bilateral ventricular volumes, both as a whole and regionally, and other volumes of interest were significantly different in patients as compared to controls (df = 231, Kullback’s χ2 = 345.119, P < 0.001). Patients showed relatively stronger negative correlations (Table 2) for all ventricular regions except for the anterior horns and BAs 20, 21, and 38 (inferior temporal region and temporal pole). Volumes of the superior lateral temporal (BAs 22, 41, and 42) and medial temporal regions (BAs 27, 28, 34, 35, and 36) were more strongly correlated with the lateral horn volume than with the temporal horn volume in patients with schizophrenia, and this difference did not extend to normal controls, where correlations were not significant. Gray matter volume in cortical areas is more strongly correlated with ventricular size in patients than healthy subjects in 60 of the 72 regional correlations of Table 2. Factor analysis in patients with schizophrenia was used with volumes for ventricle (anterior, lateral, and temporal horn), subcortical gray (caudate, putamen, thalamus) and cortical gray (BA of the prefrontal, cingulate, and temporal regions). The three-factor solution showed a cortical gray factor, a ventricle factor, and a subcortical gray factor and did not confirm a suggestion of a temporal lobe gray/ventricle factor or frontal/anterior horn factor (Table 3 in Electronic Supplementary Material).
Table 2.
Relative volume correlations between ventricles and gray matter structures and ALI
| ALIC | Caudate | Putamen | Whole thalamus | MDN | Pulvinar | Dorsal anterior prefrontal | Medial prefrontal | Orbito-frontal | Lateral prefrontal | Inferior lateral temporal | Superior lateral temporal | Temporal pole | Medial temporal | Anterior cingulate | Posterior cingulate | Occipital | Parietal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||||||||||||
| Whole lateral ventricles | −0.19 | −0.22 | −0.27* | −0.29* | −0.00 | −0.03† | −0.45* | −0.56* | −0.34* | −0.35* | −0.15† | −0.29* | 0.08† | −0.13 | −0.44* | −0.35* | −0.21 | −0.38* |
| Anterior horns | −0.19 | −0.35* | −0.32* | −0.17 | 0.08 | −0.02 | −0.48* | −0.50* | −0.37* | −0.46* | −0.29* | −0.41* | −0.15 | −0.29* | −0.41* | −0.30* | −0.29* | −0.38* |
| Lateral horns | −0.20 | −0.19 | −0.24 | −0.31* | −0.06 | −0.08† | −0.43* | −0.57* | −0.28* | −0.25 | −0.10 † | −0.30*† | 0.10 † | −0.08 † | −0.44* | −0.37* | −0.17 | −0.35* |
| Temporal horns | −0.12 | −0.12 | −0.18 | −0.24 | 0.03 | 0.03 | −0.32* | −0.41* | −0.30* | −0.31* | −0.07† | −0.12 | 0.17† | −0.06 | −0.32* | −0.24 | −0.15 | −0.29* |
| Patients | ||||||||||||||||||
| Whole lateral ventricles | −0.21 | −0.27* | −0.30* | −0.34* | −0.17 | −0.39*† | −0.52* | −0.56* | −0.28* | −0.47* | −0.55*† | −0.57* | −0.36*† | −0.41* | −0.54* | −0.61* | −0.38* | −0.52* |
| Anterior horns | −0.22 | −0.26* | −0.22 | −0.18 | −0.06 | −0.28* | −0.45* | −0.47* | −0.14 | −0.32* | −0.49* | −0.48* | −0.34* | −0.31* | −0.49* | −0.47* | −0.37* | −0.46* |
| Lateral horns | −0.22 | −0.37* | −0.37* | −0.36* | −0.22 | −0.44*† | −0.54* | −0.60* | −0.30* | −0.51* | −0.55* † | −0.61*† | −0.35* † | −0.44* † | −0.56* | −0.62* | −0.34* | −0.50* |
| Temporal horns | −0.13 | 0.01 | −0.13 | −0.31* | −0.11 | −0.27* | −0.39* | −0.39* | −0.26* | −0.37* | −0.43*† | −0.40* | −0.29*† | −0.31* | −0.39* | −0.49* | −0.36* | −0.45* |
Correlations between lateral ventricles and gray matter regions and ALIC relative volumes in controls and patients with schizophrenia. Asterisks denote significant Pearson correlation coefficients within the group (P < 0.05). Daggers indicate significantly different correlations between groups for a particular region of interest (P < 0.05, two-sided). Numbers in italic represent significant results corrected for multiple comparisons by controlling the false discovery rate (q = 0.05)
Correlations between ventricular enlargement and white matter volumes
The hypothesis that temporal lobe white matter volume would be associated with temporal horn enlargement was not supported. Instead, data were more consistent with generalized gray matter shrinkage being associated with whole ventricle enlargement. In patients with schizophrenia, the correlation between the left temporal horn volume and left temporal white matter volume for BA area 22 was r = 0.19, (positive correlation indicating larger ventricles with larger white matter and non-significant), and other white matter correlations were similar. Regarding between-group comparison, there were no statistically significant group differences between the correlation matrices of white matter and ventricle volumes (df = 78, Kullback’s χ2 = 12.756, P > 0.995). Moreover, no significant group differences were detected in Fisher’s z-tests for individual arrays of ventricle to white matter volume correlations. Both controls and patients showed negative correlations of whole ventricular volumes with white matter volume in orbitofrontal and medial prefrontal areas (r = −0.29 and r = −0.34 in controls vs. r = −0.46 and r = −0.16 in patients, respectively). Anterior prefrontal, dorsolateral prefrontal, temporal, occipital, and parietal regions showed positive correlations with lateral ventricle volumes in both study groups.
CSF volume
CSF space associated with left temporal BA area 22 was equally well correlated with left anterior horn (r = 0.41), lateral horn (r = 0.39), and temporal horn (r = 0.39, all P < 0.002). Left BA 22 relative gray matter volume was correlated −0.60 (P = <0.001) with CSF volume for left area 22, but this correlation is inflated by the fact that the fixed BA area 22 is segmented into gray, white, and CSF. Brodmann left area 22 CSF was correlated with adjacent left area 20 gray matter volume r = −0.32, area 21 r = −0.48, area 23 −0.46, but more distant correlations were higher (left BA 17 r = −0.55, and BA 8 r = −0.69). Thus, we were not able to demonstrate any regionality for CSF in sulci of the brain for the most clearly regionalized hypothesis.
Correlations between ventricular enlargement and fractional anisotropy in white matter
Significant positive correlations were found ipsilaterally between FA in the left ALIC and volumes of ventricular regions in the control group (lateral horn r = 0.41, temporal horn, r = 0.35, whole ventricle r = 0.42, all P < 0.05). In contrast, no significant correlations with FA in ALIC and ventricular volumes were discovered in schizophrenic patients (Fig. 2 in Electronic supplementary material). No significant correlations between bilateral ventricle volumes and averaged FA in prefrontal, temporal, parietal, occipital, anterior or posterior cingulate areas were found in either of the groups. Regional correlations with FA in white matter adjacent to BAs 19, 22, 23, 24, 25, 28, 31, 32, 34, 39, 40, 44, 46 were examined in both groups in search for a local effect. No significant correlations were found among controls for any part of the ventricles adding left and right volumes together. In the schizophrenic group, significant correlations of anterior horn volumes and FA in white matter adjacent to left BAs 44 and 46 were observed (P < 0.05). Also, temporal horns volumes were correlated with right BAs 19 and 22 (P < 0.05). However, when comparing intra-group correlations for each BA and the different regions of the ventricles, i.e. anterior horns versus temporal horns, none of the resulting z-tests reached statistical significance.
Medication history
There were no significant effects of medication status (never previously medicated vs. currently unmedicated) on ventricular size at the time of the MRI nor were there interactions with laterality or age.
Discussion
The major findings of the present study are enlargement of the three major portions of the ventricular system in schizophrenia, the lack of a regional effect of ventricular enlargement on adjacent white or gray matter, an association of ventricular enlargement with central gray decreases and displacement, and the more marked ventricular enlargement in older patients.
Our findings are consistent with the widely reported ventricular enlargement in older (over age 29) patients with schizophrenia. This enlargement in patients was found throughout the anterior, lateral and temporal horns but age by diagnosis effects were only significant for the lateral ventricles analyzed separately (perhaps explaining why we observed a significant age by diagnosis interaction but this was not observed elsewhere [38]). However, these local increases were not regionally associated with volume decreases in the overlying Brodmann areas. In contrast, decreased volumes in both the caudate and putamen were associated with enlargement of the adjacent region of the ventricle. The volume increase in the ventricles was only 29% of gray matter loss in schizophrenia compared to controls, while CSF increase in sulcal space was equal to 63% of gray matter loss. Since both automated and hand-tracing of the ventricles may be more reliable than sulcal measures, ventricular size remains an advantageous indicator of cortical loss. It is of interest that for high-level cognitive skills requiring integration of many brain areas, ventricular size was the best correlate of performance [39]. Our finding of widespread cortical gray matter decrease in schizophrenia, somewhat more prominent frontotemporally, is consistent with other recent studies (e.g. [40]). While the correlation patterns did not support a regional relationship between gray or white matter volume and adjacent ventricular volume, we found that larger ventricles in schizophrenia are associated with a relative loss of gray matter in lateral temporal, cingulate and occipital cortices, and shrinkage in some regions of the thalamus, namely the pulvinar nucleus. Thus, these results corroborate previous evidence pointing to an interrelation between general ventricular enlargement in schizophrenia and volume deficit in specific gray matter regions, especially in the temporal lobes and certain thalamic subnuclei. It is interesting that Gaser et al. [11] found similar correlations using a deformation-based morphometry approach in a sample of schizophrenic patients.
Correlations between temporal lobe volume and temporal horn enlargement in post-mortem data showed strongly negative values similar to ours but correlations to other brain regions (also similarly significant in our data) were not evaluated [12]. We also noted that the center of mass (see supplementary material) of the whole thalamus and its nuclei as well as the caudate appear relatively displaced in schizophrenia as compared to healthy controls. A ventricular expansion could ‘push’ subcortical nuclei outward, especially those lying close to the walls of the ventricles and potentially compress the anterior limb of the internal capsule, altering its size or anisotropy. While larger ventricles did push the thalamus and internal capsule, in patients with schizophrenia this was not associated with local FA change. Thus, local ventricular enlargement appears primarily related to diffuse cortical volume loss, and it is difficult to demonstrate a localized effect on adjacent white matter volume or its anisotropy. While brain tumors and compression of white matter tracts in hydrocephalus have been associated with increased FA [41, 42], this effect was missing in our patients with schizophrenia.
Limitations of our study include lack of longitudinal data, few white matter tracts volumetrically evaluated, and a limited and thick section set of anisotropy data. A longitudinal analysis beginning in the prodromal stage would have allowed us to assess the correlates of ventricular enlargement over time, but since much enlargement appeared after age 29, a very long follow-up would have been required to address developmental issues. The limited number of selected white matter regions and the lack of volumetric assessment of many major ones make difficult to rule out an involvement of brain regions other than those analyzed. However, our strategy of examining white matter under each Brodmann area has the advantage of evaluating clearly adjacent position and extending into areas contiguous with the ventricles. We recognize that deeper white matter at the apex of the triangle may not be closely associated with the gray matter at the base, but clear tract dividing lines cannot be drawn on MRI images. Thus, our current method is not inappropriate for testing the hypotheses in this report of local ventricular deformation effects. Multiple correlations between FA in the aforementioned areas and ventricular size were obtained in a relatively small sample, making our analyses susceptible to a type II error. Larger samples of higher resolution anisotropy data will assist in this process. Lastly, it may be that associations between specific ventricular areas and particular symptoms or neuropsychological deficits may be present (e.g. [43]) and that we have missed these regional ventricular effects.
Cortical thickness loss through aging may be a common determinant of ventricular size in both healthy controls and patients with schizophrenia. In schizophrenia, however, ventricular enlargement seems to be more strongly associated with temporal cortex volume reduction. Gray matter volume in cortical areas is more strongly correlated with ventricular size in patients than healthy subjects in 60 of the 72 regional correlations of Table 2. Normal decline in temporal lobe volume is greater later in life, while frontal lobe volume declines more early in life (e.g. Sowell’s study [44] frontal age vs. volume curves in superior frontal region decline in first few decades in comparison with curves in superior temporal regions), and this is possibly consistent with a more marked effect of age in patients with schizophrenia. Further, the posterior portion of the temporal ventricle may be the most markedly enlarged [45]. The temporal lobe peaks later in females [46]. This is particularly intriguing because schizophrenia usually manifests during adolescence or early adulthood, with a later onset in females. We speculate that the same neural maturation process responsible for the normal temporal cortex aging change might be critical in the pathophysiology of schizophrenia with some morphometric changes appearing early [47] and others late [48] [19] in its chronic course.
Ventricular enlargement may be a result of both brain development and neurodegeneration across life span, and the salience of both aspects may help explain the pervasive finding of ventricular enlargement in schizophrenia. Our percentage increases in ventricular size show patients at 127% of controls, similar to the left and right enlargements of 130 and 120% found in 18 studies by Wright and coworkers [3], but our temporal horn enlargement of 111% was somewhat smaller than the 126% found in 13 studies. Our findings suggest that ventricular enlargement in schizophrenia is not apparently linked to volume reduction in the surrounding structures. White matter abnormalities may not contribute greatly to ventricular enlargement and may not be closely related to the effect of vetriculomegaly in schizophrenia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1. Centers of mass of the thalamus in patients and controls. Spheres represent thalamic centers of mass in controls (green) and patients (red). Notice that thalamic centers of mass of patients appear further displaced dorsally and posteriorly in relation to controls (TIFF 328 kb)
Fig. 2. Differences in ALIC FA in small versus large lateral ventricles subgroups of controls (upper row) and patients (bottom). Left and right areas of interest of middle ALIC slices are displayed for both groups. T-tests comparing small and large ventricles groups within controls show significantly higher FA in left and right ALIC in the subgroup with larger ventricles. Comparison between both schizophrenia subgroups demonstrates no differences in the left ALIC and differences comprising a reduced number of pixels in the right side (TIFF 1851 kb)
Acknowledgments
All patients signed written informed consent approved by the IRB.
Conflict of interest
No author had any conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Johnstone EC, et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2(7992):924–926. doi: 10.1016/S0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 2.Narr KL, et al. Regional specificity of cerebrospinal fluid abnormalities in first episode schizophrenia. Psychiatry Res. 2006;146(1):21–33. doi: 10.1016/j.pscychresns.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie SM, et al. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34(2):330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood SJ, et al. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34(2):322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34(2):354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchsbaum MS, et al. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res. 2003;64(1):53–62. doi: 10.1016/S0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- 8.Brickman AM et al (2006) Internal capsule size in good and poor outcome schizophrenia. J Neuropsychiatry Clin Neurosci 18:364–376 [DOI] [PubMed]
- 9.Wobrock T, et al. Internal capsule size associated with outcome in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009;259(5):278–283. doi: 10.1007/s00406-008-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemether EM, et al. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry. 2003;60(10):983–991. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- 11.Gaser C, et al. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161(1):154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- 12.Chance SA, Esiri MM, Crow TJ. Ventricular enlargement in schizophrenia: a primary change in the temporal lobe? Schizophr Res. 2003;62(1–2):123–131. doi: 10.1016/S0920-9964(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, et al. Association between absence of the adhesio interthalamica and amygdala volume in schizophrenia. Psychiatry Res. 2008;162(2):101–111. doi: 10.1016/j.pscychresns.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen N, Flaum M, Arndt S (1992) The comprehensive assessment of symptoms and history (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 49:615–623 [DOI] [PubMed]
- 15.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 16.Overall J, Gorham D. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 17.Hazlett EA et al (2008) Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr Res 101:111–123 [DOI] [PMC free article] [PubMed]
- 18.Mitelman SA, et al. Correlations between volumes of the pulvinar, centromedian, and mediodorsal nuclei and cortical Brodmann’s areas in schizophrenia. Neurosci Lett. 2006;392(1–2):16–21. doi: 10.1016/j.neulet.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Mitelman SA, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. NeuroImage. 2007;37(2):449–462. doi: 10.1016/j.neuroimage.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz M, et al. Correlational patterns of cerebral glucose metabolism in never-medicated schizophrenics. Neuropsychobiology. 1996;33:1–11. doi: 10.1159/000119241. [DOI] [PubMed] [Google Scholar]
- 24.Buchsbaum MS, et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr Res. 2002;54(1–2):141–150. doi: 10.1016/S0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- 25.Mitelman SA, et al. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophr Res. 2005;75(2–3):265–281. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Perry R, Oakley A, Perry E (1991) Coronal brain map and dissection guide: localization of Brodman areas in coronal sections
- 27.Stein DJ, et al. Greater metabolic rate decreases in hippocampal formation and proisocortex than in neocortex in Alzheimer’s disease. Neuropsychobiology. 1998;37(1):10–19. doi: 10.1159/000026471. [DOI] [PubMed] [Google Scholar]
- 28.Mitelman S, et al. MRI assessment of gray and white matter distribution in Brodmann areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- 29.Hazlett EA, et al. Regional glucose metabolism within cortical Brodmann areas in healthy individuals and autistic patients. Neuropsychobiology. 2004;49(3):115–125. doi: 10.1159/000076719. [DOI] [PubMed] [Google Scholar]
- 30.Mitelman SA, et al. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27(4):753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Buchsbaum MS et al (2007) Relative glucose metabolic rate higher in White Matter in Schizophrenia. Am J Psychiatry 164:1072–1081 [DOI] [PubMed]
- 32.Byne W, et al. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry. 2001;58(2):133–140. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- 33.Brickman AM, et al. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Brain Res Cogn Brain Res. 2003;17(1):106–116. doi: 10.1016/S0926-6410(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 34.Kullback S. On testing correlation matrices. Appl Stat. 1967;16:80–85. doi: 10.2307/2985240. [DOI] [Google Scholar]
- 35.Woodruff PW, et al. Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med. 1997;27(6):1257–1266. doi: 10.1017/S0033291797005229. [DOI] [PubMed] [Google Scholar]
- 36.Portas C, et al. Volumetric evaluation of the Thalamus in Schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry. 1998;43:649–659. doi: 10.1016/S0006-3223(97)00339-9. [DOI] [PubMed] [Google Scholar]
- 37.Bullmore ET, et al. Does dysplasia cause anatomical dysconnectivity in schizophrenia? Schizophr Res. 1998;30(2):127–135. doi: 10.1016/S0920-9964(97)00141-2. [DOI] [PubMed] [Google Scholar]
- 38.Okugawa G, Tamagaki C, Agartz I. Frontal and temporal volume size of grey and white matter in patients with schizophrenia: an MRI parcellation study. Eur Arch Psychiatry Clin Neurosci. 2007;257(5):304–307. doi: 10.1007/s00406-007-0721-7. [DOI] [PubMed] [Google Scholar]
- 39.Spalletta G, et al. Cortico-subcortical underpinnings of narrative processing impairment in schizophrenia. Psychiatry Res. 2010;182(1):77–80. doi: 10.1016/j.pscychresns.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Schultz CC et al (2010) Complex pattern of cortical thinning in schizophrenia: Results from an automated surface based analysis of cortical thickness. Psychiatry Res 182:134–140 [DOI] [PubMed]
- 41.Wieshmann UC, et al. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry. 2000;68(4):501–503. doi: 10.1136/jnnp.68.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assaf Y, et al. Diffusion tensor imaging in hydrocephalus: initial experience. Ajnr. 2006;27(8):1717–1724. [PMC free article] [PubMed] [Google Scholar]
- 43.Spalletta G et al (2010) Cortico-subcortical underpinnings of narrative processing impairment in schizophrenia. Psychiatry Res 182(1):77–80 [DOI] [PubMed]
- 44.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 45.Meduri M et al (2010) Morphometrical and morphological analysis of lateral ventricles in schizophrenia patients versus healthy controls. Psychiatry Res 183(1):52–58 [DOI] [PubMed]
- 46.Sowell ER, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex (New York) 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz CC et al (2010) Psychopathological correlates of the entorhinal cortical shape in schizophrenia. Eur Arch Psychiatry Clin Neurosci 260(4):351–358 [DOI] [PubMed]
- 48.Mitelman SA, et al. Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophr Res. 2009;113(2–3):241–245. doi: 10.1016/j.schres.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Centers of mass of the thalamus in patients and controls. Spheres represent thalamic centers of mass in controls (green) and patients (red). Notice that thalamic centers of mass of patients appear further displaced dorsally and posteriorly in relation to controls (TIFF 328 kb)
Fig. 2. Differences in ALIC FA in small versus large lateral ventricles subgroups of controls (upper row) and patients (bottom). Left and right areas of interest of middle ALIC slices are displayed for both groups. T-tests comparing small and large ventricles groups within controls show significantly higher FA in left and right ALIC in the subgroup with larger ventricles. Comparison between both schizophrenia subgroups demonstrates no differences in the left ALIC and differences comprising a reduced number of pixels in the right side (TIFF 1851 kb)


