Abstract
Previously, we generated a cancer-specific gene therapy system using adenovirus vectors (Adv) conjugated to polyethylene glycol (Adv-PEG). Here, we developed a novel Adv that targets both tumor tissues and tumor vasculatures after systemic administration by conjugating CGKRK tumor vasculature homing peptide to the end of a 20-kDa PEG chain (Adv-PEGCGKRK). In a primary tumor model, systemic administration of Adv-PEGCGKRK resulted in ~500- and 100-fold higher transgene expression in tumor than that of unmodified Adv and Adv-PEG, respectively. In contrast, the transgene expression of Adv-PEGCGKRK in liver was about 400-fold lower than that of unmodified Adv, and was almost the same as that of Adv-PEG. We also demonstrated that transgene expression with Adv-PEGCGKRK was enhanced in tumor vessels. Systemic administration of Adv-PEGCGKRK expressing the herpes simplex virus thymidine kinase (HSVtk) gene (Adv-PEGCGKRK-HSVtk) showed superior antitumor effects against primary tumors and metastases with negligible side effects by both direct cytotoxic effects and inhibition of tumor angiogenesis. These results indicate that Adv-PEGCGKRK has potential as a prototype Adv with suitable efficacy and safety for systemic cancer gene therapy against both primary tumors and metastases.
Introduction
The adenovirus vector (Adv) is the most used vectors in cancer gene therapy.1,2 In several clinical trials, intratumoral injection of Adv resulted in therapeutic efficacy against primary tumors.3,4,5,6 However, it is difficult to treat metastases with Adv because of low accumulation and poor transgene expression in tumors after systemic administration.7,8 Moreover, systemic administration of Adv leads to accumulation and transgene expression in the liver, which may cause considerable toxicity.9 For this reason, clinical application of systemically administered Adv as gene therapeutic vectors has been limited.
Covalent conjugation of polyethylene glycol (PEG) to the Adv surface, called “PEGylation,” is a promising strategy to overcome the limitations of Adv.2,10,11,12,13,14 PEGylation can prolong the plasma half-life, and alter the tissue distribution of the conjugates compared with the native form.15,16 The extended circulating lifetime in blood induces the enhanced permeability and retention effect, which is based on the leaky nature of the tumor blood vessels, resulting in increased delivery of the conjugates to tumor tissue.17 Previously, we showed that systemic administration of Adv PEGylated with 20-kDa PEG at a 45% modification ratio (Adv-PEG) resulted in higher tumor-selective transgene expression than unmodified Adv.14 In addition, we showed that Adv-PEG, expressing the herpes simplex virus thymidine kinase (HSVtk) gene as the therapeutic gene, induced strong antitumor effects against metastases after systemic administration.14
Attaching targeting ligands to the ends of PEG chains is considered as a promising approach for achieving tissue-specific gene transfer using PEGylated Adv.18,19,20,21,22,23,24,25 However, there are no reports that demonstrate efficient tumor-specific transgene expression and anticancer efficacy against primary and metastatic cancer through systemic administration of PEGylated Adv-containing targeting ligands in vivo.
Tumor angiogenesis is widely recognized to be crucial for the progression and metastasis of tumors.26,27 Angiogenesis and tumor vasculature have received increased attention as targets for potential anticancer therapies. For example, in vivo screening of peptide-phage libraries has proven useful for the discovery of novel peptide ligands that selectively home to tumor vessels.28,29 One such ligand, the CGKRK peptide, accumulates both on the surface of tumor vessels and within tumor tissues after intravenous injection, suggesting that CGKRK peptide has potential as an active targeting ligand.30,31
We here report a novel vector system that can be systemically administered and that targets both tumor tissues and tumor vasculatures. We constructed a targeting Adv that has the CGKRK peptide at the end of the 20-kDa PEG chain conjugated with Adv (Adv-PEGCGKRK).
Results
Construction of Adv-PEGCGKRK
To conjugate the CGKRK peptide with Adv-PEG, we used a heterobifunctional-activated PEG with NHS and maleimide. We constructed Adv-PEG by using 50-fold molar heterobifunctional-activated PEG and 150-fold molar monofunctional-activated PEG for the modification of adenoviral lysine residues. The modification ratio of Adv by PEG was ~45% in all experiments, which was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described (data not shown).14 In addition, fluorescamine assay32 also showed that ~45% of free amines on capsid of Advs were modified by PEG (data not shown). To construct Adv-PEGCGKRK, cysteine of the CGKRK peptide was reacted with maleimide of the heterobifunctional PEG chain conjugated with Adv-PEG, resulting in attachment of the CGKRK peptide to the end of the PEG chain. We could not measure the number of CGKRK peptide on Adv-PEGCGKRK.
Transgene expression of Adv-PEGCGKRK
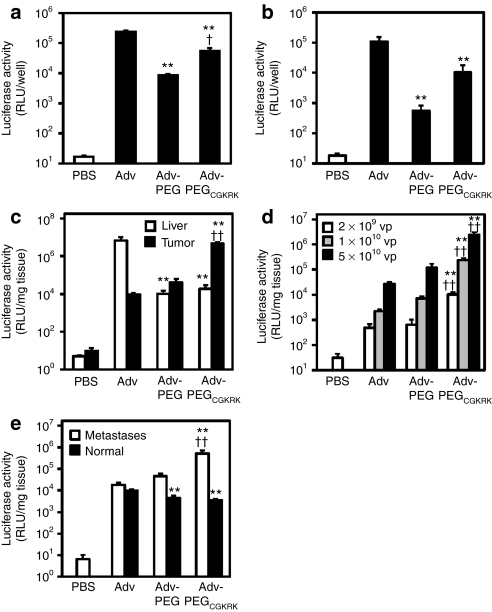
It has been reported that CGKRK peptides bind to and accumulate within certain tumor cells such as MDA-MB-435S and tumor endothelial cells in vitro and in vivo.30 We selected MDA-MB-435S cells for in vitro evaluation of transgene expression of Adv-PEGCGKRK encoding the luciferase gene. B16BL6 cells were selected for in vitro and in vivo evaluation of transgene expression of Adv-PEGCGKRK because it is known that sufficient gene expression by Adv is difficult to obtain in these cells33 due to a lack of Ad primary receptor expression. Luciferase expression from Adv-PEG was lower than that of unmodified Adv in MDA-MB-435S cells (Figure 1a) and B16BL6 cells (Figure 1b). In contrast, luciferase expression from Adv-PEGCGKRK was 7-fold and 20-fold higher than the expression from Adv-PEG in MDA-MB-435S and B16BL6 cells, respectively (Figure 1a,b). Next, we evaluated in vivo transgene expression of Adv-PEGCGKRK in tumor and liver tissue after intravenous injection into B16BL6 primary tumor model mice (Figure 1c). Luciferase expression from Adv-PEG in tumor tissue was fourfold higher than that from unmodified Adv and in liver tissue >600-fold lower than that from unmodified Adv (Figure 1c). Furthermore, luciferase expression in tumor tissue with administration of Adv-PEGCGKRK was 480-fold and 110-fold higher than those with administration of unmodified Adv and Adv-PEG, respectively (Figure 1c). In liver tissue, luciferase expression after Adv-PEGCGKRK administration was 370-fold less than that after unmodified Adv administration, and was almost the same as that after Adv-PEG administration (Figure 1c). In addition, we examined the dose dependency of in vivo transgene expression of each Adv (Figure 1d). In tumor tissue, luciferase expression at 1 × 1010 virus particles (vp) and 2 × 109 vp Adv-PEGCGKRK was 100-fold and 20-fold, respectively, higher than that with unmodified Adv at the same doses (Figure 1d). Furthermore, luciferase expression of Adv-PEGCGKRK at 1 × 1010 vp was the same as that of Adv-PEG at 5 × 1010 vp, and ninefold higher than that of unmodified Adv at 5 × 1010 vp (Figure 1d). These results indicate that administration of Adv-PEGCGKRK results in significant tumor-specific transgene expression compared with Adv-PEG and unmodified Adv.
Figure 1.
Transgene expression of Adv-PEGCGKRK. (a) MDA-MB-435S cells or (b) B16BL6 cells (2 × 104 cells/well) were transduced with the indicated adenovirus vectors (Advs). After culturing for 24 hours, luciferase expression was measured (n = 3). (c) In vivo transgene expression in a primary tumor model. B16BL6-bearing mice were intravenously administered with 5 × 1010 virus particles (vp) of the indicated Advs. After 48 hours, the luciferase expression in tumors and livers was measured (n = 4). (d) Gene transduction at various doses in a primary tumor model. B16BL6-bearing mice were intravenously administered with several doses of the indicated Advs. After 48 hours, the luciferase expression in tumors was measured (n = 4). (e) In vivo transgene expression in a pulmonary metastasis model. B16BL6 cells were injected into C57BL6 mice via the tail vein. After 12 days, 5 × 1010 vp of the indicated Advs were intravenously injected into metastatic mice or normal mice. Forty-eight hours later, the luciferase expression in lungs was measured (n = 5). All data are represented as the means ± (a,b) SD or (c–e) SEM (**P < 0.01 versus value for unmodified Adv-treated group by analysis of variance (ANOVA); †P < 0.05, ††P < 0.01 versus value for Adv-PEG-treated group by ANOVA). PEG, polyethylene glycol; RLU, relative light units.
Next, we evaluated the transgene expression of Adv-PEGCGKRK at 5 × 1010 vp in an established pulmonary metastasis mouse model (Figure 1e).34 In normal mice, the luciferase expression in lung of both Adv-PEG and Adv-PEGCGKRK were several-fold lower than that of unmodified Adv (Figure 1e). In contrast, in B16BL6-metastatic mice, luciferase expression in lung with Adv-PEGCGKRK was 27-fold and 12-fold higher than those with unmodified Adv and Adv-PEG, respectively (Figure 1e). These results suggest that Adv-PEGCGKRK not only reduces expression in liver, but also that it actively targets both primary tumor and metastases.
Blood clearance, tissue distribution, and localization of Adv-PEGCGKRK
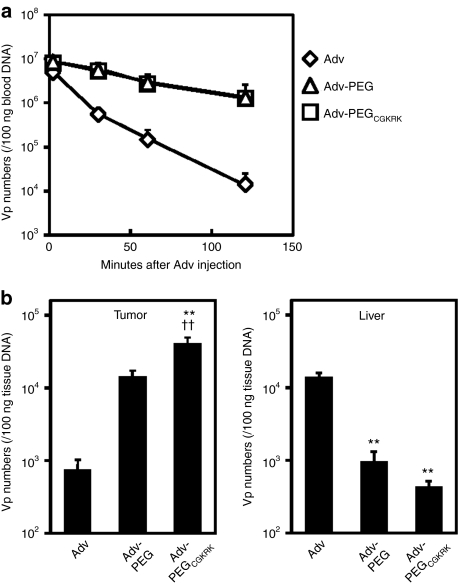
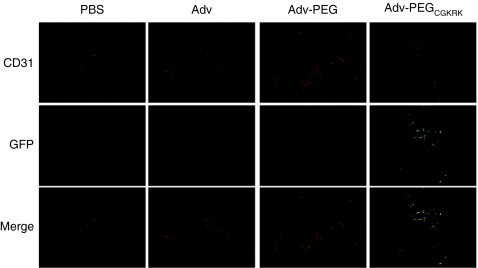
We assessed blood clearance by using normal C57BL6 mice (Figure 2a) and tissue distribution by using B16BL6-bearing mice (Figure 2b) of each Adv after intravenous administration into mice by using real-time quantitative PCR. The plasma level of Adv-PEGCGKRK was higher than the plasma level of unmodified Adv and was almost the same as that of Adv-PEG (Figure 2a). At 6 hours after intravenous administration into B16BL6-bearing mice, the amount of Adv-PEGCGKRK in tumor tissue was 60-fold and 3-fold higher than that of unmodified Adv and Adv-PEG, respectively (Figure 2b). Moreover, the amount of Adv-PEGCGKRK in liver tissue was 15-fold less than that of unmodified Adv, and almost the same as that of Adv-PEG (Figure 2b). These results indicate that systemic administration of Adv-PEGCGKRK enabled tumor targeting due to active targeting and the enhanced permeability and retention effect, and decreased distribution in the liver. Next, we investigated the localization of the transgene expression of Adv-PEGCGKRK in tumor tissues. B16Bl6-bearing mice were intravenously administered with 5 × 1010 vp of Advs encoding the green fluorescent protein (GFP) gene and we visualized the localization by using histological procedures (Figure 3). GFP expression could not be detected after intravenous administration of unmodified Adv or Adv-PEG (Figure 3). In contrast, intravenous administration of Adv-PEGCGKRK resulted in strong GFP expression in endothelial cells and colocalization with CD31+ endothelial cells (Figure 3). These results indicate that transgene expression of Adv-PEGCGKRK is localized at tumor endothelial cells.
Figure 2.
Blood clearance and tissue distribution of adenovirus vectors (Advs). (a) Circulation time of Adv-PEGCGKRK. Normal C57BL6 mice were intravenously administered with 5 × 1010 virus particles (vp) of the indicated Advs. Blood samples were collected at 2, 30, 60, and 120 minutes after intravenous administration of each Adv. The total amount of DNA in the blood at various time points was determined using real-time quantitative PCR. (b) Tissue distribution of Adv-PEGCGKRK. Once the tumor diameter was ~8 mm, B16BL6-bearing mice were intravenously administered with 5 × 1010 vp of the indicated Advs. Six hours after the administration, the number of viral genomes in tumors and livers was measured by real-time quantitative PCR. Data are presented as means ± SEM (n = 5; **P < 0.01 versus value for unmodified Adv-treated group by analysis of variance (ANOVA); ††P < 0.01 versus value for Adv-PEG-treated group by ANOVA).
Figure 3.
Tumor tissue and vascular localization of intravenously injected Adv-PEGCGKRK. Once the tumor diameter was ~8 mm, B16BL6-bearing mice were intravenously administered with 5 × 1010 virus particles (vp) of the indicated adenovirus vectors- (Advs) expressing green fluorescent protein (GFP). The mice were sacrificed 2 days later and GFP expression was visualized in tumor tissue sections with fluorescein (green). Blood vessels were stained with rat anti-mouse CD31 (red; Alexa568). Original magnification: ×200.
Therapeutic effect of Adv-PEGCGKRK
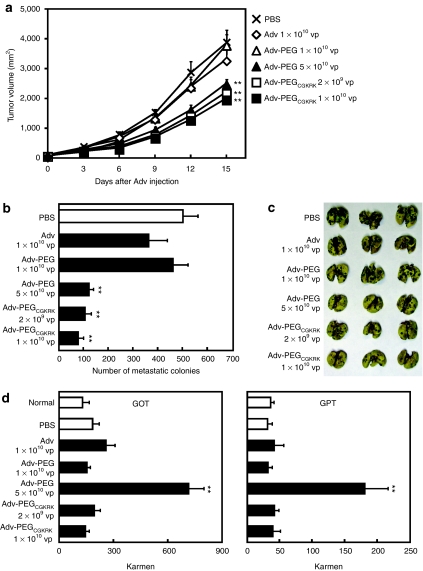
To evaluate the therapeutic potential of Adv-PEGCGKRK, we intravenously administered each Adv encoding the HSVtk gene to B16BL6-bearing mice (Figure 4a). Administration of 5 × 1010 vp unmodified Adv induced a marked reduction in body weight, and within 7 days of administration all treated mice had died from toxicity via HSVtk expression in the liver (data not shown). Administration of 1 × 1010 vp unmodified Adv did not inhibit tumor growth (Figure 4a). Administration of 5 × 1010 vp Adv-PEG reduced tumor volumes by 35% compared with that of phosphate-buffered saline (PBS) (Figure 4a). However, a dose of 1 × 1010 vp, Adv-PEG did not show any antitumor effects (Figure 4a). In contrast, Adv-PEGCGKRK showed strong antitumor effects with administration of 1 × 1010 and 2 × 109 vp Adv-PEGCGKRK reducing tumor volume by 48 and 42%, respectively (Figure 4a). Next, we evaluated the therapeutic effect of Adv-PEGCGKRK against metastases (Figure 4b,c). Consistent with the results of the primary tumor experiment, systemic administration of unmodified Adv lacked therapeutic efficacy (Figure 4b,c). Administration of 5 × 1010 vp Adv-PEG reduced the number of metastatic colonies, but 1 × 1010 vp Adv-PEG did not (Figure 4b,c). In contrast, compared with untreated mice, administration of 1 × 1010 and 2 × 109 vp Adv-PEGCGKRK significantly suppressed metastases in lung (Figure 4b,c). Furthermore, to evaluate the side effects of intravenous administration of Adv-PEGCGKRK, we measured the blood levels of glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) on day 7 after Advs administration (Figure 4d). Administration of 5 × 1010 vp Adv-PEG induced a substantial elevation in the levels of GOT and GPT compared with that of normal mice (Figure 4d). In contrast, administration of 1 × 1010 and 2 × 109 vp Adv-PEGCGKRK did not induce any elevation of GOT or GPT levels (Figure 4d), although 5 × 1010 vp Adv-PEGCGKRK induced a elevation in the levels of GOT and GPT as similar as 5 × 1010 vp Adv-PEG (data not shown). Taken together, these data suggest that Adv-PEG did not induce the therapeutic effect without severe side effects and intravenous administration of Adv-PEGCGKRK could be therapeutically effective at therapeutic doses 5–25-fold lower than that of Adv-PEG.
Figure 4.
Therapeutic effect of Adv-PEGCGKRK against primary tumors and metastases. (a) Therapeutic effect of intravenously injected Adv-PEGCGKRK against primary tumors. Once the tumor diameter was ~8 mm, phosphate-buffered saline (PBS) or the indicated adenovirus vectors (Advs) encoding the herpes simplex virus thymidine kinase (HSVtk) gene was intravenously administered at the indicated doses to B16BL6-bearing mice. The mice then received daily intraperitoneal administrations of ganciclovir (GCV) (50 mg/kg) for 10 days. Tumor sizes were calculated every 3 days. (b,c) Therapeutic effect of intravenously injected Adv-PEGCGKRK against metastases. B16BL6 cells were injected intravenously into C57BL/6 mice via the tail vein. PBS or the indicated Advs encoding the HSVtk gene at the indicated doses was injected intravenously on day 7 after the B16BL6 administration. The mice then received daily intraperitoneal administrations of GCV (50 mg/kg) for 7 days. On day 14 after the B16BL6 administration, therapeutic effects were assessed by (b) counting the number of metastatic colonies in the lungs and by (c) examining photographs of the lungs. (d) Side effects were assessed using glutamic-oxaloacetic transaminase (GOT)/glutamic-pyruvic transaminase (GPT) activity in serum. Data are presented as means ± SEM [n = 6; **P < 0.01 versus value for phosphate-buffered saline (PBS)-treated group by analysis of variance (ANOVA)].
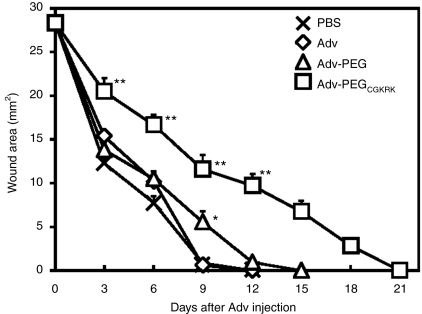
Delayed wound healing by systemic administration of Adv-PEGCGKRK
Angiogenesis is essential not only for tumor growth but also for wound repair.35 To investigate whether the therapeutic effect of Adv-PEGCGKRK is related to damage of neovascular vessels, we examined the wound repair effects of Adv-PEGCGKRK after injury in mice. Wounds were created on the abdominal area of mice and 1 × 1010 vp of each Adv encoding the HSVtk gene were intravenously injected into the mice 1 day after injury (Figure 5). The wound was completely closed by day 9 after PBS administration (Figure 5). No obvious differences in the wound-healing process were observed in the mice injected with unmodified Adv or Adv-PEG compared with that of the mice injected with PBS (Figure 5). In contrast, the wound areas of the mice injected with Adv-PEGCGKRK were still recovering until day 21 after administration (Figure 5), indicating that the wound-healing process was markedly delayed by Adv-PEGCGKRK. These findings suggest that intravenous administration of Adv-PEGCGKRK might destroy neovascular vessels, thereby producing a therapeutic synergy.
Figure 5.
Inhibition of wound healing by Adv-PEGCGKRK. Wounds of 6-mm diameter were created on the abdominal area of BALB/c mice and phosphate-buffered saline (PBS) or 1 × 1010 virus particles (vp) of the indicated adenovirus vectors (Advs) encoding the herpes simplex virus thymidine kinase (HSVtk) gene were intravenously injected into mice 1 day after injury. The mice then received daily intraperitoneal administrations of ganciclovir (GCV) (50 mg/kg) for 10 days. Data are represented as means ± SEM [n = 6; *P < 0.05, **P < 0.01 versus value for PBS-treated group by analysis of variance (ANOVA)].
Discussion
Various molecular weights of PEGs have been used in the PEGylation of Adv because PEGs with different molecular weights result in different body distribution patterns and retention times in blood.11,12,36,37 Previously, we examined the correlation between PEG modification and gene expression patterns, and we showed that PEGylation with 20-kDa PEG at 45% modification ratio was optimal for tumor-selective gene expression after systemic administration.14 Therefore, we used 20-kDa PEG at 45% in this study, with the CGKRK peptide attached to the end of the PEG chain. Although the modification ratio of the CGKRK peptide was not evaluated, the peptide was used in excess quantity. A quantitative analysis of the modification ratio is needed in a future study.
Compared with Adv-PEG, the transgene expression of Adv-PEGCGKRK was higher in tumor tissue and the same in liver tissue (Figure 1c–e). We found that after intravenous administration, Adv-PEGCGKRK was present in plasma at the same level as Adv-PEG, but accumulated to higher levels in tumor tissue (Figure 2). In addition, we confirmed that transgene expression of Adv-PEGCGKRK occurs at tumor endothelial cells mainly (Figure 3). Taken together, our findings suggest that PEGylation results in greater suppression of the transition of Adv-PEG and Adv-PEGCGKRK into the liver, thereby prolonging the circulating lifetime. Adv-PEGCGKRK's long-retention time in the blood increases the chances of contact with tumor vasculature, leading to increased gene transduction in tumor endothelial cells. In addition, the tumor endothelium-targeting ligand CGKRK peptide may actively lead Adv-PEGCGKRK to tumor tissue, resulting in increased accumulation and gene transduction in tumor cells.
In both primary and metastatic tumor models, intravenous administration of Adv-PEGCGKRK showed remarkable therapeutic effect with negligible side effects (Figure 4). Administration of unmodified Adv lacked a therapeutic window and administration of Adv-PEG only induced a therapeutic effect at 5 × 1010 vp and was associated with substantial side effects. In contrast, a therapeutic effect comparable with 5 × 1010 vp Adv-PEG was induced by 2 × 109 vp Adv-PEGCGKRK with negligible side effects. However, transgene expression with 2 × 109 vp of Adv-PEGCGKRK was lower than that with 5 × 1010 vp Adv-PEG (Figure 1d). In addition, we demonstrated that Adv-PEGCGKRK-HSVtk inhibits wound healing, indicating that Adv-PEGCGKRK-HSVtk might destroy angiogenic vessels (Figure 5). Thus, we speculated that the therapeutic effects of Adv-PEGCGKRK might be caused not only by a direct antitumor effect, but also by an antiangiogenesis effect via transgene expression in tumor endothelial cells. In general, it is known that destroying one vascular endothelial cell in tumor tissue vessels results in the death of many more tumor cells because a single vessel supports the survival of many tumor cells through the provision of oxygen and nutrients.38 Therefore, the remarkable therapeutic antitumor effect of Adv-PEGCGKRK might be considered to be a combination of a direct tumor killing effect and an antiangiogenesis effect.
One of the hurdles confronting Adv-mediated gene transfer is that infection with Adv is dependent on the presence of the coxackie-adenovirus receptor on the target cells.39 Many tumor cells that are targets for gene therapy, such as melanoma cells, express little or no coxsackie-adenovirus receptor, making it difficult to achieve sufficient gene expression and therapeutic effect.39,40 We demonstrated here that Adv-PEGCGKRK induces a high level of transgene expression in coxackie-adenovirus receptor-negative B16BL6 cells in vivo compared with unmodified Adv and Adv-PEG. In addition, because angiogenesis is a known hallmark of most tumors41 and the CGKRK peptide targets several tumor types30, it is possible that Adv-PEGCGKRK might be effective across a range of tumors. Adv-PEGCGKRK has the potential to be a wide-ranging tumor-targeting vector that overcomes the problem of conventional Adv.
In this study, we showed that Adv-PEG conjugated with the targeting ligand CGKRK peptide targeted both tumor tissues and tumor vasculatures after systemic administration. We propose that Adv-PEGCGKRK has potential for use as a prototype vector with suitable efficacy and safety for systemic cancer gene therapy against primary tumors and metastases.
Materials and Methods
Mice and cell lines. Five-week-old female C57BL/6 mice and BALB/c mice were purchased from SLC (Hamamatsu, Japan). All of the animal experimental procedures were performed in accordance with the Osaka University guidelines for the welfare of animals. B16BL6 (mouse melanoma) cells were obtained from RIKEN Cell Bank (Tsukuba, Japan). MDA-MB-435S (human breast carcinoma) cells were obtained from American Type Culture Collection (Manassas, VA). The B16BL6 cells were cultured in Minimal Essential Medium (Sigma-Aldrich, St Louis, MO) containing 7.5% fetal bovine serum and antibiotics. The MDA-MB-435S cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich) containing 10% fetal bovine serum and antibiotics.
Plasmids and vectors. An improved in vitro ligation method14,33,42,43 was used to construct E1/E3-deleted adenovirus type 5 expressing firefly luciferase, GFP, or HSVtk, all under control of the cytomegalovirus promoter. Each Adv was generated using established methods.10 The vps and biological titer were determined as described.10,44 The ratio of the particle-to-biological titer was between 10 and 30.
Preparation of Adv-PEGCGKRK. A heterobifunctional PEG (SUNBRIGHT MA-200TS; molecular weight, 20,000; NOF, Tokyo, Japan), and methoxy-PEG-succinimidyl propionate, a monofunctional PEG (mPEG-SPA; molecular weight, 20,000; Nektar, San Carlos, CA), were used for PEGylation. To confirm the attachment of SUNBRIGHT MA-200TS to Adv, we used two-step procedure. To construct Adv-PEG, Adv was incubated with SUNBRIGHT MA-200TS at 50-fold molar excess to viral lysine residues at 37 °C for 45 minutes and then incubated with mPEG-SPA at 150-fold molar excess to viral lysine residues at 37 °C for 45 minutes. The excess NHS group of free PEG was blocked by incubation with 6-aminocaproic acid (Sigma-Aldrich) at 37 °C for 30 minutes. Adv-PEGCGKRK was prepared by adding CGKRK peptide at fivefold molar excess to SUNBRIGHT MA-200TS to Adv-PEG and incubating at room temperature for 1 hour. Unreacted maleimide was blocked by incubation with 2-mercaptoethanol at room temperature for 1 hour. To purify Adv-PEG and Adv-PEGCGKRK, free peptides and free PEG were removed by dialysis using 1,000-kDa molecular weight cutoff membranes (Spectrum Laboratories, Rancho Dominguez, CA). The concentration of Adv was measured by picogreen assay (Invitrogen, Carlsbad, CA). The modification ratio of Adv-PEG and Adv-PEGCGKRK was 45%, which was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis as described.10
In vitro gene transduction. MDA-MB-435S and B16BL6 cells (2 × 104 cells/well) were seeded into separate 48-well plates. The following day, the cells were transduced with 104 vp/cell each Adv, encoding the luciferase gene. After 24 hours luciferase activity was determined using the luciferase assay system (Promega, Madison, WI) and a Lumat LB 9507 luminometer (EG&G Berthold, Bad Wildbad, Germany) in accordance with the manufacturers' instructions. Luciferase activity was calculated as relative light units/well.
In vivo gene transduction. C57BL/6 mice were intradermally inoculated in the flank with 3 × 105 B16BL6 cells. After the tumor diameter had reached ~8 mm, the mice were intravenously administered 5 × 1010 vp each Adv encoding the luciferase gene. The tumors and livers were harvested 48 hours after administration and luciferase activity was measured. To evaluate transgene expression in a pulmonary metastasis model in vivo, 3 × 105 B16BL6 cells were injected into the tail veins of C57BL/6 mice. After 12 days, the mice were intravenously administered 5 × 1010 vp each Adv encoding the luciferase gene. The lungs were harvested 48 hours after injection and luciferase activity was measured.
Blood clearance of Adv-PEGCGKRK. Blood clearance was determined using real-time quantitative PCR, as described.14 In brief, blood samples were collected at 2, 30, 60, and 120 minutes after intravenous administration of 5 × 1010 vp each Adv in normal C57BL6 mice. Total DNA, including Adv DNA, from whole blood was extracted. The number of viral genomes was counted using real-time quantitative PCR. Standard samples were generated using known amounts of viral DNA with control whole blood DNA.
Tissue distribution of Adv-PEGCGKRK. Once the tumor diameter was ~8 mm, B16BL6-bearing mice were intravenously administered 5 × 1010 vp each Adv. The tumors and livers were harvested 6 hours after administration and the DNA was extracted. The number of viral genomes in each sample was counted using real-time quantitative PCR as described.10
Localization of Adv-PEGCGKRK. Once the tumor diameter was ~8 mm, B16BL6-bearing mice were intravenously administered 5 × 1010 vp each Adv encoding the GFP gene. Two days later, the tumors were collected and fixed in 4% paraformaldehyde. Frozen sections (6 µm) were prepared and subjected to double-label immunohistochemical staining with rat anti-mouse CD31 antibody (BD Biosciences, San Diego, CA) and goat anti-rat IgG Alexa 568 antibody (Invitrogen).
Antitumor effect of Adv-PEGCGKRK against primary tumors. Once the tumor diameter was ~8 mm, B16BL6-bearing mice were intravenously administered PBS or Advs encoding the HSVtk gene. After Adv injection, the mice received daily intraperitoneal administrations of ganciclovir (50 mg/kg) for 10 days. Every 3 days, the major and minor axes of the tumor were measured with microcalipers; tumor volume was calculated using the following formula: (tumor volume; mm3) = (major axis; mm) × (minor axis; mm)2 × 0.5. The mice were euthanized when tumor volume exceeded 4,000 mm3.
Antitumor effect of Adv-PEGCGKRK against metastases. Into the tail vein of C57BL/6 mice, we injected 3 × 105 B16BL6 cells. After 7 days, the mice were intravenously administered PBS or Advs encoding the HSVtk gene. The mice then received daily intraperitoneal administrations of ganciclovir (50 mg/kg) for 7 days. On day 7 after Adv administration, serum activities of GOT and GPT, used as indicators of hepatotoxicity, were measured using the Transaminase CII test (Wako Pure Chemical, Osaka, Japan) according to the manufacturer's instructions. On the same day, the lungs were harvested and fixed in Bouin's solution for 24 hours; photographs of lung sections were taken, and the numbers of metastatic colonies were counted.
Wound healing by Adv-PEGCGKRK. Six-mm diameter wounds were created on the abdominal area of BALB/c mice by using sterile disposable biopsy punch (Natsume Seisakusho, Tokyo, Japan) and PBS or 1 × 1010 vp each Adv encoding the HSVtk gene were intravenously injected into the mice 1 day after injury. After Adv administration, ganciclovir was intraperitoneally administered to the mice (50 mg/kg) daily for 10 days. Every 3 days, the major and minor axes of the wound were measured with microcalipers by a single investigator who was blinded to the treatment group; wound area was calculated using the following formula: (wound area; mm2) = (major axis; mm) × (minor axis; mm) × 3.14 × 0.25.
Statistical analysis. All results are expressed as mean ± SD or SEM. Differences were compared using Bonferroni's method after analysis of variance.
Acknowledgments
This study was supported in part by grants from the Ministry of Health, Labor, and Welfare in Japan; by the Research on Health Sciences focusing on Drug Innovation from the Japan Health Sciences Foundation; and by the Global COE Program “In Silico Medicine” at Osaka University. The authors declared no conflict of interest.
REFERENCES
- Mizuguchi H., and, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034–1044. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- Eto Y, Yoshioka Y, Mukai Y, Okada N., and, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354:3–8. doi: 10.1016/j.ijpharm.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Horowitz J. Adenovirus-mediated p53 gene therapy: overview of preclinical studies and potential clinical applications. Curr Opin Mol Ther. 1999;1:500–509. [PubMed] [Google Scholar]
- Vattemi E., and, Claudio PP. Adenoviral gene therapy in head and neck cancer. Drug News Perspect. 2006;19:329–337. doi: 10.1358/dnp.2006.19.6.1015352. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther. 2006;6:823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- Kim S, Peng Z., and, Kaneda Y. Current status of gene therapy in Asia. Mol Ther. 2008;16:237–243. doi: 10.1038/sj.mt.6300336. [DOI] [PubMed] [Google Scholar]
- Alemany R, Suzuki K., and, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81 Pt 11:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y., and, Hayakawa T. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and alphav integrin-binding ablation. J Virol. 2003;77:13062–13072. doi: 10.1128/JVI.77.24.13062-13072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y.et al. (2007Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production J Immunol 1781767–1773. [DOI] [PubMed] [Google Scholar]
- Gao JQ, Eto Y, Yoshioka Y, Sekiguchi F, Kurachi S, Morishige T.et al. (2007Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration J Control Release 122102–110. [DOI] [PubMed] [Google Scholar]
- Wortmann A, Vöhringer S, Engler T, Corjon S, Schirmbeck R, Reimann J.et al. (2008Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies Mol Ther 16154–162. [DOI] [PubMed] [Google Scholar]
- Hofherr SE, Shashkova EV, Weaver EA, Khare R., and, Barry MA. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther. 2008;16:1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- Kreppel F., and, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- Yao X, Yoshioka Y, Morishige T, Eto Y, Watanabe H, Okada Y.et al. (2009Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis Gene Ther 161395–1404. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Tsutsumi Y, Nakagawa S., and, Mayumi T. Recent progress on tumor missile therapy and tumor vascular targeting therapy as a new approach. Curr Vasc Pharmacol. 2004;2:259–270. doi: 10.2174/1570161043385682. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Mantovani G, Wang X, Haddleton DM., and, Brayden DJ. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin Drug Deliv. 2008;5:371–383. doi: 10.1517/17425247.5.4.371. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Khaled G, Fang J., and, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R.et al. (2003Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates Mol Ther 899–107. [DOI] [PubMed] [Google Scholar]
- Ogawara K, Rots MG, Kok RJ, Moorlag HE, Van Loenen AM, Meijer DK.et al. (2004A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo Hum Gene Ther 15433–443. [DOI] [PubMed] [Google Scholar]
- Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M.et al. (2005PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability J Gene Med 7604–612. [DOI] [PubMed] [Google Scholar]
- Menezes KM, Mok HS., and, Barry MA. Increased transduction of skeletal muscle cells by fibroblast growth factor-modified adenoviral vectors. Hum Gene Ther. 2006;17:314–320. doi: 10.1089/hum.2006.17.314. [DOI] [PubMed] [Google Scholar]
- Ogawara K, Kuldo JM, Oosterhuis K, Kroesen BJ, Rots MG, Trautwein C.et al. (2006Functional inhibition of NF-kappaB signal transduction in alphavbeta3 integrin expressing endothelial cells by using RGD-PEG-modified adenovirus with a mutant IkappaB gene Arthritis Res Ther 8R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Park HJ, Kim PH, Lee J, Hyung W, Yang J.et al. (2007Retargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cells J Control Release 123164–171. [DOI] [PubMed] [Google Scholar]
- Park JW, Mok H., and, Park TG. Epidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirus. Biochem Biophys Res Commun. 2008;366:769–774. doi: 10.1016/j.bbrc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Morrison J, Briggs SS, Green N, Fisher K, Subr V, Ulbrich K.et al. (2008Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor Mol Ther 16244–251. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Ellis LM., and, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Zurita AJ, Arap W., and, Pasqualini R. Mapping tumor vascular diversity by screening phage display libraries. J Control Release. 2003;91:183–186. doi: 10.1016/s0168-3659(03)00236-0. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Pasqualini R., and, Arap W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16:80–88. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JA, Giraudo E, Singh M, Zhang L, Inoue M, Porkka K.et al. (2003Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma Cancer Cell 4383–391. [DOI] [PubMed] [Google Scholar]
- Mäkelä AR, Matilainen H, White DJ, Ruoslahti E., and, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H, Palmer DJ, Ng P., and, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Asavatanabodee R, Eto Y, Watanabe H, Morishige T, Yao X.et al. (2008Tat conjugation of adenovirus vector broadens tropism and enhances transduction efficiency Life Sci 83747–755. [DOI] [PubMed] [Google Scholar]
- Mu Y, Kamada H, Kodaira H, Sato K, Tsutsumi Y, Maeda M.et al. (1999Bioconjugation of laminin-related peptide YIGSR with polyvinyl pyrrolidone increases its antimetastatic effect due to a longer plasma half-life Biochem Biophys Res Commun 264763–767. [DOI] [PubMed] [Google Scholar]
- Hanahan D., and, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Kaneda Y, Tsutsumi Y, Yoshioka Y, Kamada H, Yamamoto Y, Kodaira H.et al. (2004The use of PVP as a polymeric carrier to improve the plasma half-life of drugs Biomaterials 253259–3266. [DOI] [PubMed] [Google Scholar]
- Doronin K, Shashkova EV, May SM, Hofherr SE., and, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., and, Deisseroth A. Tumor vascular targeting therapy with viral vectors. Blood. 2006;107:3027–3033. doi: 10.1182/blood-2005-10-4114. [DOI] [PubMed] [Google Scholar]
- Mathis JM, Stewart PL, Zhu ZB., and, Curiel DT. Advanced generation adenoviral virotherapy agents embody enhanced potency based upon CAR-independent tropism. Clin Cancer Res. 2006;12:2651–2656. doi: 10.1158/1078-0432.CCR-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP.et al. (1999Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy Cancer Res 59325–330. [PubMed] [Google Scholar]
- Neri D., and, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H., and, Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H., and, Kay MA. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum Gene Ther. 1999;10:2013–2017. doi: 10.1089/10430349950017374. [DOI] [PubMed] [Google Scholar]
- Yao X, Yoshioka Y, Eto Y, Morishige T, Okada Y, Mizuguchi H.et al. (2007TERT promoter-driven adenovirus vector for cancer gene therapy via systemic injection Biochem Biophys Res Commun 362419–424. [DOI] [PubMed] [Google Scholar]