Abstract
Patients with advanced solid tumors refractory to and progressing after conventional therapies were treated with three different regimens of low-dose cyclophosphamide (CP) in combination with oncolytic adenovirus. CP was given with oral metronomic dosing (50 mg/day, N = 21), intravenously (single 1,000 mg dose, N = 7) or both (N = 7). Virus was injected intratumorally. Controls (N = 8) received virus without CP. Treatments were well tolerated and safe regardless of schedule. Antibody formation and virus replication were not affected by CP. Metronomic CP (oral and oral + intravenous schedules) decreased regulatory T cells (Tregs) without compromising induction of antitumor or antiviral T-cell responses. Oncolytic adenovirus given together with metronomic CP increased cytotoxic T cells and induced Th1 type immunity on a systemic level in most patients. All CP regimens resulted in higher rates of disease control than virus only (all P < 0.0001) and the best progression-free (PFS) and overall survival (OS) was seen in the oral + intravenous group. One year PFS and OS were 53 and 42% (P = 0.0016 and P < 0.02 versus virus only), respectively, both which are unusually high for chemotherapy refractory patients. We conclude that low-dose CP results in immunological effects appealing for oncolytic virotherapy. While these first-in-human data suggest good safety, intriguing efficacy and extended survival, the results should be confirmed in a randomized trial.
Introduction
New approaches are needed for treatment of metastatic solid tumors. One strategy is oncolytic viruses, which selectively replicate in and kill tumor cells.1,2,3,4 Adenoviruses are quite immunogenic,5 which might be a key aspect for eliciting antitumor immunity as suggested by preclinical6 and clinical data.7 However, in spite of encouraging data showing that immunotherapy (including oncolytic viruses) has the ability to elicit antitumor immunity,8,9,10 human data has demonstrated that breaking immune suppression acquired by tumors is also required11 for immunotherapy to give meaningful clinical benefits. One of the key suppressive components present in advanced tumors is regulatory T cells (Tregs).10
Tregs were first identified by Gershon and colleagues in the early 70s' and dubbed “suppressive cells” for their ability to suppress the activity of T lymphocytes.12 Tregs represent 2–3% of the human T cells (about 10% of CD4+ cells) and promote peripheral immune tolerance by suppressing self-antigen-reactive T cells, hence preventing autoimmune diseases, but since tumors emerge from normal tissues, Tregs are effective also in reducing antitumor immune responses.10 Although initially identified as CD4+ T cells expressing CD2513 and forkhead box P3 (Foxp3),14 recent studies have demonstrated that CD127 expression inversely correlates with Foxp3 and the suppressive function of human CD4+ Treg cells.15 Hence, Tregs are now identified as CD4+CD25+CD127−Foxp3high.
Several decades after their first identification it became clear that Treg-mediated immunosuppression is one of the crucial tumor immune-evasion mechanisms and may be a key obstacle for successful tumor immunotherapy.16 Recent data demonstrate that tumors actively prevent the induction of tumor-associated antigen-specific immunity through induction of Treg trafficking, differentiation, and expansion.10 In fact, an elevated frequency of Tregs in peripheral blood has been demonstrated in several tumor types, including nonsmall cell lung cancer,17 breast cancer,17,18 colorectal cancer,19 esophageal cancer,17 gastric cancer,17 hepatocellular carcinoma,17,20 leukemia,17 lung cancer,21 lymphoma,21 and melanoma.22 It is clear that modulation of Treg trafficking, signaling, and differentiation is becoming of key importance for cancer therapy.
Cyclophosphamide (CP) is an alkylating agent that mediates DNA crosslinking and is used to treat various tumors. High doses are required for direct effects on tumor cells which results in immunosuppression. In striking contrast, low doses of CP improve antitumor immune responses in various animal tumor models,23 in patients with metastatic melanoma24 and the approach is popular in cancer vaccine trials.25 A particularly attractive schedule is daily oral (metronomic) administration which is easy, safe, well-tolerated and effective in downregulating both the activity and the number of Tregs as demonstrated in humans previously.26,27 Another antitumor mechanism ascribed to metronomic CP is an antivascular effect.28
Despite these appealing characteristics, single agent metronomic low-dose CP is usually not very effective in controlling advanced solid tumors. Only a few positive randomized trials have been reported, and therefore the approach is not very widely used in contemporary oncology.27,28,29,30 In this study, we hypothesized that it would be feasible to combine low-dose CP with oncolytic adenovirus treatment for potentially synergistic immunological and clinical effects.
Results
Low-dose CP enhances the efficacy of oncolytic adenovirus in a syngeneic model of pancreatic cancer in immunocompetent Syrian hamsters
We have shown previously that oncolytic adenoviruses armed with granulocyte macrophage colony-stimulating factor (GMCSF) can inhibit tumor growth in immunocompetent Syrian hamsters.7,31 Unlike other rodent models, this model is semi-permissive for human adenovirus and sensitive to human GMCSF.7,31 It has also been shown that low-dose CP can32,33 give antitumor activity by reducing the number and activity of Tregs.26,27 To test whether CP would enhance the efficacy of the oncolytic adenovirus treatment, Syrian hamsters bearing a syngeneic pancreatic tumor were treated with low-dose CP in combination with Ad5-D24-GMCSF, which is an p16-Rb pathway selective adenovirus with an unmodified Ad5 capsid expressing GMCSF driven by the adenoviral E3 promoter.7
Interestingly, we found that the group of animals treated with the combination of virus and CP showed a significant reduction in tumor size compared with untreated animals or treated with Ad5-D24-GMCSF only (Supplementary Figure S1a). To exclude that the observed phenomenon was restricted to this particular virus, we repeated the experiment with Ad5/3-D24-GMCSF, which is a similar p16-Rb selective virus with GMCSF but features a 5/3 chimeric capsid which enhances transduction of human tumors.32,33 Because it is difficult to estimate dose correlation between hamsters and humans, we changed also the CP dose slightly. Also in this experiment, we observed a significant reduction of tumor growth in animals treated with Ad5/3-D24-GMCSF with CP versus no treatment (Supplementary Figure S1b). Thus, we concluded that low-dose CP can enhance the potency of an oncolytic adenovirus coding for GMCSF.
Study design and treatment protocol
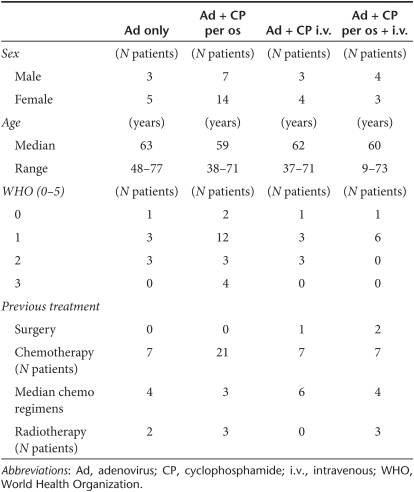
Patients with advanced metastatic tumors progressing after conventional therapies (Table 1 and Supplementary Table S1) were treated with a single round of oncolytic adenovirus in combination with CP. Four cohorts of patients were treated as follows: (i) oncolytic adenovirus only; (ii) oncolytic adenovirus and metronomic CP (50 mg/day) starting 1 week before virus; (iii) oncolytic adenovirus with a single intravenous 1,000 mg dose of CP given 1 hour prior to virus as a 30-minute infusion; (iv) oncolytic adenovirus combined with both metronomic (50 mg/day) and intravenous (1,000 mg except 500 mg for U157) CP (Supplementary Figure S2). A single round of virus was injected intratumorally in ultrasound guidance as reported.7,34,35
Table 1. Summary of the patients at baseline.
Safety of low-dose CP given concurrently with oncolytic adenovirus
Treatments were well tolerated in all cohorts. Only two symptomatic grade 3 events, both of which occurred in the same patient, and no grade 4–5 events were reported. Most patients experienced grade 1–2 flu-like symptoms, including fever, chills, fatigue and injection site pain, without correlation to CP (Supplementary Table S2). Thus, patients experienced adverse events typical of oncolytic adenovirus treatment,7,32,34 and CP did not seem to affect the safety profile.
Low-dose CP reduces Tregs in cancer patients treated with oncolytic adenovirus
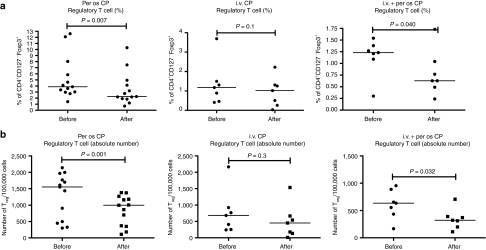
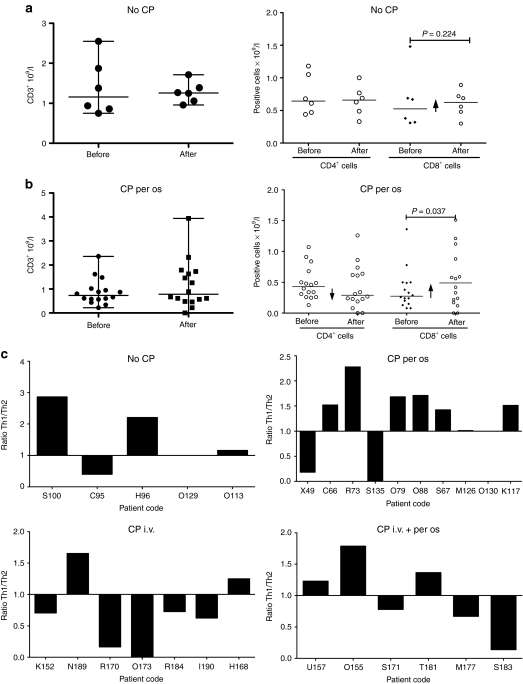
It has been previously described that metronomic administration of CP reduces the number of Tregs in cancer patients without compromising other immune components.27,36 However, the effect on Tregs of CP in combination with oncolytic adenovirus has not been studied before in humans. Peripheral blood mononuclear cells were analyzed for percentage and absolute number of Tregs before and 1 month after treatment (Figure 1 and Supplementary Figures S3–S5). We observed a significant reduction in Tregs in patients treated with oral metronomic low-dose CP together with the virus (Figure 1a,b). The second cohort of patients, treated with a single intravenous injection of CP 1 hour before virus administration, showed a nonsignificant trend for a decrease in Tregs (Figure 1a,b). In patients treated with the combination of metronomic oral and intravenous CP, the number and the percentage of circulating Tregs was significantly lower than before the treatment (Figure 1a,b). The conclusion of these analyses was that metronomic administration of low-dose CP in combination with oncolytic adenovirus reduces the number of circulating Tregs and in particular the regimens that included the metronomic component seemed effective in this regard.
Figure 1.
Low-dose cyclophosphamide in combination with oncolytic adenoviruses significantly reduces circulating regulatory T cells (Tregs) in cancer patients. Patients treated with one of three different cyclophosphamide (CP) regimes were monitored for the frequencies of circulating Treg. Peripheral blood mononuclear cells from patients were collected before and after the treatment and stained for CD4, CD127 and Foxp3. (a) percentage of Treg in the total CD4+. (b) Total number of Tregs. The values for each patient can be found in Supplementary Figures S3–S5. i.v., intravenous.
CP administration did not influence virus replication or the development of anti-adenovirus neutralizing antibodies
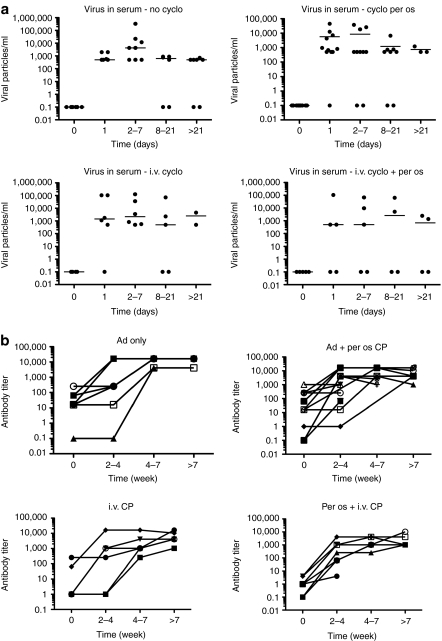
It has been proposed that persistence, re-emergence and titer increases after day 1 indicate replication of oncolytic adenoviruses.1,2,3,4,7,35 Given that CP treatment might influence this through immunological or direct mechanisms, we analyzed the presence of the virus in serum after treatment. No differences between groups were detected and signs of emphatic virus replication were seen regardless of CP (Figure 2a).
Figure 2.
Cyclophosphamide (CP) administration did not influence the presence of the virus in circulation nor the neutralizing antibody response. Serum from patients treated with oncolytic adenovirus in combination with one of the three different regimens of CP was collected and analyzed for (a) presence of the virus, and (b) neutralizing antibodies for adenovirus. i.v., intravenous.
High-dose CP is known to be immunosuppressive and can thwart the development of neutralizing antibodies.37 However, as expected for low-dose CP, antibodies were rapidly induced in most patients without differences between cohorts (Figure 2b).
Administration of CP in combination with oncolytic adenovirus did not impair antitumor and anti-adenovirus T-cell responses
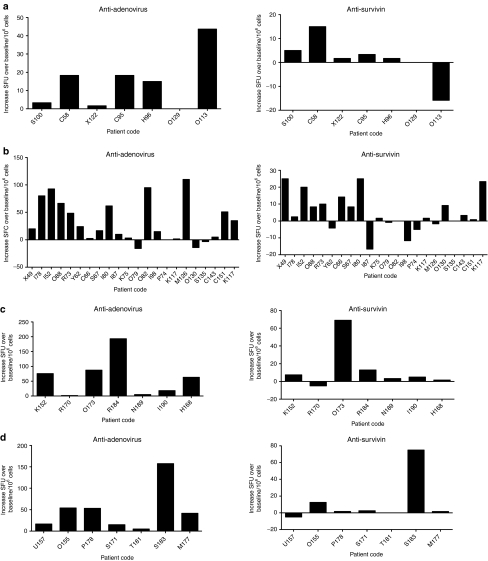
We and others have demonstrated previously that oncolytic adenoviruses can induce an adenovirus-specific as well as a tumor-specific T-cell–mediated immune response, both of which might be relevant for the antitumor effect.6,7,32 To elucidate the effect of CP on this phenomenon, peripheral blood mononuclear cells were harvested and analyzed by ELISPOT. Interestingly, we did not observe any significant differences attributable to CP in any cohorts (Figure 3). One-way analysis of variance was performed to compare the groups, with regard to anti-adenovirus immunity but no differences were seen. The same was observed for antitumor reactive T cells, although oral CP showed a trend for increased antitumor response (P = 0.051). As surrogate of tumor-associated antigen survivin was chosen given its high expression in nearly all the known tumors.38 These results are well in line with previous data27 suggesting that despite reducing Tregs, low-dose CP does not affect the antitumor capacity of cytotoxic CD8+ T cells.
Figure 3.
Administration of low-dose cyclophosphamide (CP) in combination with oncolytic adenoviruses did not impair antitumor and anti-adenovirus T-cell response. Interferon-γ (IFN-γ) ELISPOT analysis was performed on patients before and after treatment. (a) Adenovirus only (Ad); (b) Ad + metronomic CP per os; (c) Ad + CP single intravenous administration; (d) Ad + metronomic CP per os + single intravenous (i.v.) administration. SFU, spot forming units.
To clarify this further we performed a more detailed analysis of lymphocyte subsets potentially relevant for cancer immunotherapy, including CD3+ T cells and their subpopulations: CD4+ helper (Th) and CD8+ effector T cells (Figure 4). Patients treated with CP and virus showed a slight reduction in the CD4+ population (which includes Tregs) while an increase in CD8+ was observed (Figure 4b). In patients treated with virus only (Figure 4a), CD4+ cells did not change while CD8+ cells (including antiviral and antitumor cells) increased slightly as shown previously.7 However, the increase in CD8+ was significant only in the patients treated with metronomic CP.
Figure 4.
Metronomic administration of low-dose cyclophosphamide (CP) in combination with oncolytic adenoviruses promotes a Th1 immune response profile. Total number of T cell was analyzed in (a) patients treated with oncolytic adenovirus only and (b) patients treated with oral CP together with the virus. (c) Cytokines profile was analyzed in patients treated with CP per os and adenovirus before and after the treatment. i.v., intravenous.
In addition to flowcytometry analysis, cytokine profile was analyzed before and after the treatment and it showed a trend of increase of interferon-γ, tumor necrosis factor-α and interleukin-2 (IL-2) suggesting an ongoing Th1 type immune response (Figure 4c).
Treatment efficacy
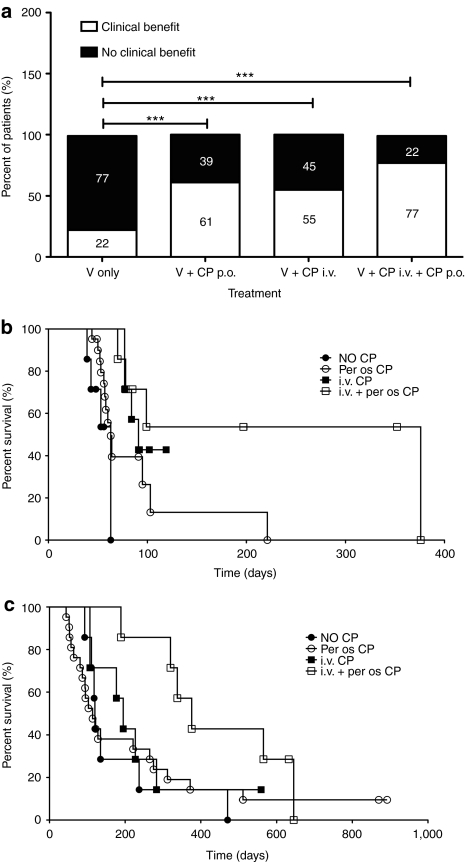
When both radiological data and tumor markers are taken into account, 64% of efficacy evaluations suggested possible clinical benefits (Supplementary Table S1). This number includes all SD or better and may therefore suggest successful disease control, since all patients had progressing tumors before treatment. In the control patients treated with virus only, 22% of evaluations resulted in SD or better (Figure 5a). In the oral CP group, disease control was seen in 61% of the patients (Fisher's exact test, P < 0.0001 versus virus only), while the numbers were 55% (P < 0.0001 versus virus only) and 77% (P < 0.0001 versus virus only) in the intravenous and oral + intravenous cohorts, respectively.
Figure 5.
High rates of disease control and extended survival in patients treated with low-dose cyclophosphamide (CP) and oncolytic adenovirus. (a) Possible indicators of treatment benefits (complete response, partial response, minor response, stable disease in imaging or tumor markers) were evaluated in patients treated with oncolytic adenovirus with or without concomitant administration of CP and reported as percentage within that cohort. ***Two-sided Fisher's exact P < 0.0001 (b) progression-free survival; (c) overall survival of the patients. i.v, intravenous; p.o., per os; V, virus.
Median progression-free survival (PFS) times (Figure 5b) were 63, 63, 91, and 376 days for virus only, oral CP, intravenous CP and oral + intravenous CP group, respectively. 53% of patients in the latter group were progression free at 1 year (P = 0.0016 versus virus only).
With regard to overall survival (OS) (Figure 5c), median times were 120, 114, 195, and 376 for virus only, oral CP, intravenous CP, and oral + intravenous CP group, respectively and 42% of patients were alive at 1 year in the combination group (P < 0.02 versus virus only).
Discussion
Tolerance to self-antigens is a crucial aspect of animal biology and its malfunction can lead to autoimmune conditions. With regard to peripheral tolerance (i.e., lymphocytes that have exited primary lymphoid organs) the main regulatory cells are Tregs. Most tumors express a plethora of mutated self-antigens or unnaturally expressed normal antigens, which should result in recognition by the immune system. Nevertheless, the tumors are present and must therefore have developed mechanisms for dampening immunity. It is now recognized that Tregs are one of the most important suppressor cells tumors utilize for self-preservation.9,10,39
Most cancer immunotherapy studies have thus far been designed to increase antitumor immunity through supplementing active elements such as dendritic cells, tumor-associated antigen-specific T cells or cytokines. However, although antitumor immune response has often been demonstrated, it has only seldom translated into clinical benefit because of the strong immunosuppressive mechanisms exhibited by tumors. One way to tackle this problem is to modulate the immunosuppressive mechanisms at the tumor site. The clinical potential of this was powerfully demonstrated by the landmark randomized phase 3 trial with ipilimumab, which downregulates immunosuppressive CTLA4 circuits.11
In our study, we used three different regimens of CP to selectively reduce Tregs in cancer patients with refractory solid tumors resistant to conventional therapies and being treated with oncolytic adenoviruses. We found that both regimens that included a prolonged exposure to CP promoted Tregs reduction. In most patient treated with the intravenous bolus CP, Tregs were also reduced, but the overall result was not statistically significant. This is in line with the hypothesis that this schedule exerts its effect chiefly at the tumor microenvironment.40,41 As Tregs were downregulated in 89% of all patients treated with CP (Supplementary Figures S3–S5), the approach may be applicable across different tumor and patient types. This is not completely surprising since the effect of the drug is on nonmalignant cells (Tregs), in which there may be less variation between individuals than in tumor cells.
Our data are well in accordance with what was previously shown by Ghiringhelli and colleagues.27 Although it is risky comparing two studies different in a variety of ways, the reduction in Tregs seemed even more prominent in their work. One reason could be differences in CP schedule. They used 100 mg/day for 2 weeks and then 2 weeks off, while we used a continuous schedule or a combination of a “loading dose” followed by continuous dosing.
Another aspect that might influence the degree of Treg downregulation by CP includes the effects that adenovirus might have on Tregs. However, antagonistic effects seem unlikely given that binding of toll-like receptor 9 (one of the main ways adenovirus is recognized by the immune system) has been reported to reduce immune suppressive signals.42 Instead, a more likely explanation for adenovirus diluting the effect of CP on Foxp3+ cells is that the immune activation caused by the virus increases the expression of Foxp3 in non-Treg cells. This does not indicate induction of Tregs by adenovirus. Instead, Foxp3 induction is part of the normal differentiation of T cells, including CD8+ cytotoxic T cells.25,43 If this is true, the CP-mediated reduction in Foxp3+ cells, despite the opposite effect of adenovirus, becomes an even more powerful indicator of CP-mediated downregulation of Tregs.
Evolution of lymphocyte subpopulations during treatment was analyzed and we observed a nonsignificant decrease in the frequency of overall CD4+ cells. Since this population includes Tregs, which were significantly decreased, the data suggests that CD4+ helper cells, potentially important for antitumor immune responses, were not affected, and this is in accordance with previous reports.17,27 Importantly, we saw a significant increase in CD8+ cells, which includes cytotoxic antitumor T cells (Figure 4a,b). Also, serum cytokine profiles suggested a Th1 immune response in most patients although clearer data might be obtainable at the tumor level (Figure 4c).
When we performed ELISPOT and T-cell analysis to assess the functionality of T cells, we did not observe differences the way T cells harvested from the patients responded to adenovirus- and tumor-specific stimuli, which is in accord to previous reports.27,36 As an example of a tumor-associated antigen, we chose survivin which is expressed on nearly all malignancies.38 However, since survivin is not a very immunogenic protein, even stronger reactivity could probably be observed against other antigens. The main result in our study was that induction of effector T cells was not compromised by CP.
In 1987 Berd and colleagues showed a significant reduction in “suppressive activity” following a single intravenous 300 mg/m2 CP administration, while no change in CD4+ and CD8+ cells was observed. Interestingly, a similar study was performed by Audia and colleagues 20 years later with three different regimen of CP (250, 500, and 750 mg/m2 intravenously), but this time T-cell numbers (instead of activity) were studied and no effect on Tregs was found.17 These results were in accord with our intravenous CP cohort who received 1,000 mg (circa 600 mg/m2), and no significant decrease of Tregs was seen. Taken together, these data suggest that CP in any form can reduce the activity of Tregs, while metronomic CP can in addition reduce the number and proportion of Tregs, and may thus be more potent that intravenous CP. Importantly, all available reports suggest that this can be achieved without affecting potentially useful CD8+ cells. Finally, it is critical to note that these conclusions are based on human data.
Previous animal studies suggest that intravenous high-dose CP can reduce formation of neutralizing antibodies. We used low-dose CP and thus it is logical that no effect was seen given the strong immunogenicity of adenovirus. Low-dose CP has been proposed to induce Th2 to Th1 switch and our cytokine data seemed to support this hypothesis.40,41 However, since adenovirus alone has a potent effect in the same direction, there were no significant differences between groups.
Interestingly, our findings provide preliminary evidence that CP treatment might enhance the efficacy of oncolytic adenoviruses (Figure 5). Higher clinical benefit rates were seen in all CP groups in comparison to the virus-only control group. Also, better PFS and OS was seen, in particular with the oral + intravenous schedule. Moreover, median OS in all cohorts seems to compare well with the 30–115 days reported for palliative care patients with advanced and chemotherapy refractory disease.39,44 It is crucial to keep in mind that larger patient cohorts and randomization would be required to reliably assess treatment benefits which were not a main endpoint in this study. This first-in-man report suggest that such studies may be feasible from a safety perspective and provide initial suggestions of potentially useful schedules for further testing in clinical trials.
In summary, metronomic schedules of CP reduce Tregs in cancer patients treated with oncolytic adenoviruses without compromising cytotoxic T-cell responses. Further studies should explore the relevance of these findings at the tumor, where the actions of both cell types is most critical. Repeated biopsies would therefore be important. Also, the promising efficacy seen in some groups would be an appealing subject for a randomized trial. In the meanwhile, a phase 1–2 study is ongoing with the aim of more in-depth characterization of the immunological findings reported here.
Materials and Methods
Adenoviruses. Safety of the oncolytic adenoviruses utilized in this work has been previously reported: Ad5-D24-GMCSF,7 ICOVIR-7,34 Ad5/3-D24-GMCSF,32 and Ad5-RGD-D24-GMCSF.45
Cell lines. Hamster pancreatic carcinoma derived cell line HaP-T1 was kindly provided by Dr Hernandez-Alcoceba (Pamplona, Spain).
Animals. All animal protocols were reviewed and approved by the Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland. Syrian hamsters (Mesocricetus auratus) were obtained from Taconic (Ejby, Denmark) at 4–5 weeks of age and quarantined for at least 1 week prior to the study. HaP-T1 cells were implanted subcutaneously on day zero and tumor growth was followed every 2 days. The experiment was terminated when tumor size reached the limit predefined by animal regulations. Since intravenous injection (hamsters lack a tail vein) and controlled dose oral administration to hamsters are challenging, CP was administered intraperitoneally at the dose of 30 mg and 50 mg per animal. Viruses were administered intratumorally at the dose of 1 × 108 viral particle/tumor. In the experiments described in Supplementary Figure S1, groups consist of four animal bearing four tumors each.
Patients. Patients with advanced metastatic tumors progressing after conventional therapies (Table 1 and Supplementary Table S1) were treated with a single round of oncolytic adenovirus in combination with CP. Controls received virus only. Inclusion criteria were refractory solid tumor, World Health Organization performance score 3 or less, no major organ function deficiencies, and written informed consent. Exclusion criteria were known brain metastases or glioma, organ transplant, HIV, severe cardiovascular, metabolic or pulmonary disease, platelets <75 × 106/ml, aspartate aminotransferase or alanine aminotransferase >3 × normal, elevated bilirubin. Patients were treated within a Finnish Medicines Agency FIMEA regulated Advanced Therapy Access Program (ISRCTN10141600) according to Good Clinical Practice and the Declaration of Helsinki. Virus was produced by Oncos Therapeutics, Helsinki, Finland.
Treatment protocol. The main endpoints of this controlled, nonrandomized, retrospective study were safety and immunological response in patients receiving oncolytic adenovirus and CP. Secondary endpoints included virological parameters, efficacy and survival. Four cohorts of patients were identified: (i) oncolytic adenovirus only; (ii) oncolytic adenovirus and metronomic CP (50 mg/day) starting 1 week before virus; (iii) oncolytic adenovirus with a single intravenous 1,000 mg dose of CP given 1 hour prior to virus as a 30-minute infusion; (iv) oncolytic adenovirus combined with both metronomic (50 mg/day) and intravenous (1,000 mg except 500 mg for U157) CP (Supplementary Figure S2). A single round of virus was injected intratumorally in ultrasound guidance as reported.7,34,35 Patients were followed ad infinitum and adverse events occurring within 1 month of treatment were recorded according to CTCEA 3.0 (Supplementary Table S2).
Evaluation of efficacy. Tumor size was assessed by contrast-enhanced computer tomography scanning. After treatment imaging was done at circa 2 months. Response Evaluation Criteria in Solid Tumors (RECIST1.1) criteria were applied to overall disease, including injected and noninjected lesions. Partial response indicates more than 30% decrease, while progressive disease is more than 20% increase in sum of tumor diameters, and stable disease indicates neither response nor progression. In addition to standard RECIST1.1 criteria, minor response was used to indicate 10–29% reduction. Tumor markers, when elevated, were measured from blood and scored with the same percentages.
Quantitation of virus and antibodies. The number of the viral particles in serum was analyzed by Quantitative Real-Time PCR LightCycler (Roche, Mannheim, Germany) as described.7,34,35,46 Neutralizing antibody titer was assessed as described.47
T-cell studies. Interferon-γ ELISPOT analysis was performed as described.7,32 Briefly, peripheral blood mononuclear cells were isolated by Percoll gradient according to standard protocols. Cells were immediately frozen in CTL-CryoABC serum-free media (Cellular Technology, Cleveland, OH) for further ELISPOT and flow cytometry analysis. ELISPOT was performed according to MABtech manufacturer instructions (h-IFNγ ELISPOT PRO 10 plate kit, code 3420-2APT-10). 200,000 living cells were counted and plated in each well. For adenovirus ELISPOT, cells were stimulated with the HAdV-5 Penton peptide pool (Proimmune, Oxford, UK), which consists of 140 peptides, each 15 amino acids long and overlapping by 11 amino acids.
Survivin was chosen to represent a classic pan-carcinoma antigen, as most tumors have been reported Survivin positive,38 and we did not have access to pretreatment tumor samples for determination of the optimal antigen for each patient. BIRC5 PONAB peptide pool (Proimmune) was used. It consists of a pool consists of 33 peptides, each 15 amino acids long and overlapping by 11 amino acids. The plates were read with an AID-ELISPOT reader machine (BioReader 3000; Bio-Sys, Karben, Germany). The results were calculated as spot forming counts as a mean of a triplicate count from the specific antigen stimulation minus the negative control. The ratio of before and after treatment values were calculated for each individual.
Flow cytometry analysis was performed on FacsCalibur (Becton Dickinson, Palo Alto, CA) with human CD4 PE-Cy5-labeled (eBioscience, San Diego, CA), human CD127 FITC-labeled (eBioscience) and intracellular Foxp3 PE-labeled (eBioscience) antibodies. Tregs cells were defined as CD4 positive, CD127 negative and Foxp3 high.15
Cytokine analysis. Human interferon-γ, IL-2, tumor necrosis factor-α, IL-4, IL-5, IL-10 were assayed using the BD cytokine multiplex bead array system (BD Biosciences, Palo Alto, CA), and analyzed using BD FacsArray (BD Biosciences) according to the manufacturer's instructions. Th1/Th2 ratio was calculated as follows: the sum of the fold increase over baseline was calculated for each Th1 (interferon-γ, IL-2, tumor necrosis factor-α)and Th2 (IL-4, IL-5, IL-10) cytokine and the ratio of these sums was plotted.
Statistical analysis. Statistical tests were performed with SPSS 15.0 (SPSS, Chicago, IL) and GraphPad Prism version 5.00 for Mac (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Metronomic administration of low-dose cyclophosphamide enhances oncolytic adenovirus efficacy in immunocompetent syngeneic Syrian hamsters. Figure S2. Schematic representation of the treatment schedule. Figure S3. PBMCs from patients treated with oncolytic adenovirus in combination with low-dose CP per os were collected before and after the treatment and stained for CD4, CD127 and Foxp3. Figure S4. PBMCs from patients treated with oncolytic adenovirus in combination with intravenous CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3. Figure S5. PBMCs from patients treated with the combination of oncolytic adenovirus, intravenous CP and low-dose per os CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3. Table S1. Tumor type treated and summary of responses to treatment. Table S2. Summary of adverse effects.
Acknowledgments
This work was supported by the University of Helsinki, Marie Curie FP7-IRG-2008, and K. Albin Johansson Foundation. We thank Saila Eksymä-Sillman, Marina Rosliakova, and other personnel at International Comprehensive Cancer Center Docrates, and at Eira Hospital, Helsinki, Finland, for patient care. A.H. is K. Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute. A.H. is shareholder in Oncos Therapeutics Ltd.
Supplementary Material
Metronomic administration of low-dose cyclophosphamide enhances oncolytic adenovirus efficacy in immunocompetent syngeneic Syrian hamsters.
Schematic representation of the treatment schedule.
PBMCs from patients treated with oncolytic adenovirus in combination with low-dose CP per os were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
PBMCs from patients treated with oncolytic adenovirus in combination with intravenous CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
PBMCs from patients treated with the combination of oncolytic adenovirus, intravenous CP and low-dose per os CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
Tumor type treated and summary of responses to treatment.
Summary of adverse effects.
REFERENCES
- Alemany R, Balagué C., and, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J. Live viruses in cancer treatment. Oncology (Williston Park, NY) 2002;16:1483–92; discussion 1495. [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE., and, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn D, Martuza RL., and, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK.et al. (2007Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors Mol Ther 15378–385. [DOI] [PubMed] [Google Scholar]
- Tuve S, Liu Y, Tragoolpua K, Jacobs JD, Yumul RC, Li ZY.et al. (2009In situ adenovirus vaccination engages T effector cells against cancer Vaccine 274225–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M.et al. (2010Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients Cancer Res 704297–4309. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A., and, Kroemer G. The anticancer immune response: indispensable for therapeutic success. J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB., and, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB.et al. (2010Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RK., and, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M., and, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Hori S, Nomura T., and, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S.et al. (2006CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells J Exp Med 2031701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P.et al. (2007Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes Clin Exp Immunol 150523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G.et al. (2002Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma J Immunol 1692756–2761. [DOI] [PubMed] [Google Scholar]
- Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S.et al. (2002Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta Cancer Res 625267–5272. [PubMed] [Google Scholar]
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF., and, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E., and, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- Viguier M, Lemaître F, Verola O, Cho MS, Gorochov G, Dubertret L.et al. (2004Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells J Immunol 1731444–1453. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M, Starzinski-Powitz A, Pfizenmaier K., and, Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977;145:455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berd D., and, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- Bass KK., and, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1–12. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C.et al. (2004CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative Eur J Immunol 34336–344. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F.et al. (2007Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients Cancer Immunol Immunother 56641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord R, Nair S, Schache A, Spicer J, Somaihah N, Khoo V.et al. (2007Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study J Urol 1772136–40; discussion 2140. [DOI] [PubMed] [Google Scholar]
- Penel N, Clisant S, Dansin E, Desauw C, Dégardin M, Mortier L.et al. (2010Megestrol acetate versus metronomic cyclophosphamide in patients having exhausted all effective therapies under standard care Br J Cancer 1021207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S.et al. (2006Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients J Clin Oncol 243623–3628. [DOI] [PubMed] [Google Scholar]
- Diaconu I, Cerullo V, Escutenaire S, Kanerva A, Bauerschmitz GJ, Hernandez-Alcoceba R.et al. (2010Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir J Gene Med 12435–445. [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I.et al. (2010Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF Mol Ther 181874–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG.et al. (2003Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus Mol Ther 8449–458. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors Clin Cancer Res 163035–3043. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Nokisalmi P, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors Gene Ther 17892–904. [DOI] [PubMed] [Google Scholar]
- Berd D., and, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47:3317–3321. [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ., and, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Viganò A, Dorgan M, Buckingham J, Bruera E., and, Suarez-Almazor ME. Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med. 2000;14:363–374. doi: 10.1191/026921600701536192. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Krüger JA., and, Reisfeld RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65:5027–5030. doi: 10.1158/0008-5472.CAN-05-0646. [DOI] [PubMed] [Google Scholar]
- Bhatti R, Ray P., and, Bell N. Immunomodulatory effect of cyclophosphamide on host humoral immunity in Dunning's R-3327 adenocarcinoma of the prostate. Urol Res. 1991;19:15–18. doi: 10.1007/BF00294015. [DOI] [PubMed] [Google Scholar]
- Pasare C., and, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Carson BD., and, Ziegler SF. Impaired T cell receptor signaling in Foxp3+ CD4 T cells. Ann N Y Acad Sci. 2007;1103:167–178. doi: 10.1196/annals.1394.022. [DOI] [PubMed] [Google Scholar]
- Llobera J, Esteva M, Rifà J, Benito E, Terrasa J, Rojas C.et al. (2000Terminal cancer. duration and prediction of survival time Eur J Cancer 362036–2043. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L.et al. (2011Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors Int J Cancerepub ahead of print). [DOI] [PubMed]
- Pesonen S, Helin H, Nokisalmi P, Escutenaire S, Ribacka C, Sarkioja M.et al. (2010Oncolytic adenovirus treatment of a patient with refractory neuroblastoma Acta Oncol 49117–119. [DOI] [PubMed] [Google Scholar]
- Särkioja M, Pesonen S, Raki M, Hakkarainen T, Salo J, Ahonen MT.et al. (2008Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies Gene Ther 15921–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metronomic administration of low-dose cyclophosphamide enhances oncolytic adenovirus efficacy in immunocompetent syngeneic Syrian hamsters.
Schematic representation of the treatment schedule.
PBMCs from patients treated with oncolytic adenovirus in combination with low-dose CP per os were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
PBMCs from patients treated with oncolytic adenovirus in combination with intravenous CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
PBMCs from patients treated with the combination of oncolytic adenovirus, intravenous CP and low-dose per os CP were collected before and after the treatment and stained for CD4, CD127 and Foxp3.
Tumor type treated and summary of responses to treatment.
Summary of adverse effects.