Abstract
Since the first demonstration of in vivo gene transfer into myocardium there have been a series of advancements that have driven the evolution of cardiac gene delivery from an experimental tool into a therapy currently at the threshold of becoming a viable clinical option. Innovative methods have been established to address practical challenges related to tissue-type specificity, choice of delivery vehicle, potency of the delivered material, and delivery route. Most importantly for therapeutic purposes, these strategies are being thoroughly tested to ensure safety of the delivery system and the delivered genetic material. This review focuses on the development of recombinant adeno-associated virus (rAAV) as one of the most valuable cardiac gene transfer agents available today. Various forms of rAAV have been used to deliver “pre-event” cardiac protection and to temper the severity of hypertrophy, cardiac ischemia, or infarct size. Adeno-associated virus (AAV) vectors have also been functional delivery tools for cardiac gene expression knockdown studies and successfully improving the cardiac aspects of several metabolic and neuromuscular diseases. Viral capsid manipulations along with the development of tissue-specific and regulated promoters have greatly increased the utility of rAAV-mediated gene transfer. Important clinical studies are currently underway to evaluate AAV-based cardiac gene delivery in humans.
Introduction
A number of strategies for cardiac gene transfer have been described since the earliest report of naked DNA delivery to the heart.1 Each approach has specific attributes and limitations which when understood can be adapted and used for particular applications. Recent clinical trials have demonstrated success using the parvovirus, recombinant adeno-associated virus (rAAV) as a vector for gene transfer to myocardium. This review will cover the advances in rAAV research that have aided its development into a safe, clinically relevant vehicle for cardiac gene therapy applications. Examples of treatments in animal models for a variety of inherited and acquired diseases affecting the heart are provided, current data from rAAV-mediated cardiac gene therapy trials is discussed and future directions for the field are proposed.
Wild-Type and Recombinant AAV Biology
Wild-type adeno-associated virus (wtAAV) was first discovered as a 20 nm, icosahedral contaminant in adenovirus preparations.2 It is a nonpathogenic, nonenveloped, DNA virus containing a linear single-stranded genome of 4.6–4.8 kb that requires coinfection with a helper virus for viral replication.2,3 In a productive infection, the wtAAV DNA becomes uncoated, converted to duplex form and is integrated into host cellular DNA during the latent phase in a site-specific manner.4 The wtAAV genome consists of two open reading frames flanked by 145 base-pair inverted terminal repeats (ITRs). The viral genes encode alternatively spliced capsid (Cap) proteins and multifunctional replication (Rep) proteins.5
To generate recombinant AAV (rAAV) for gene delivery, a plasmid vector is designed containing the control region and complementary DNA of interest flanked by wtAAV ITRs. The size of the promoter elements and transgene are ideally between 4.1 and 4.9 kb for efficient packaging although studies have shown that it is possible to package up to 5.2 kb with decreased efficiency.3 In a transfection based production scheme, the vector plasmid is co-transfected into mammalian cells with helper plasmids containing the rep and cap genes and the essential adenoviral gene products which provide helper functions in trans.6 The ITRs supply all cis acting sequences necessary for viral packaging. Once the helper genes are expressed, the vector genome is rescued from the plasmid DNA and replication occurs. In the presence of rAAV capsids the single-stranded vector DNA becomes encapsidated to generate mature viral particles. These particles containing the transgene of interest can then be purified from a cell lysate using either anion exchange, affinity chromatography, or density gradient centrifugation techniques.5 Once a titer is determined the transgene containing rAAV may be frozen or immediately used for experiments. In contrast to the ability of wtAAV to integrate into a host genome, the rAAV used for gene delivery applications has been shown to largely persist as an episome for long periods of time following transduction.7 This in addition to the lack of rep and cap genes or helper virus proteins in rAAV stocks further increases the general safety and appeal of rAAV as a vehicle for gene transfer.
Natural and Modified AAV Capsids for Cardiac Gene Transfer
The efficiency of AAV receptor-mediated cellular entry is controlled by the capsid proteins. The AAV cap gene encodes 3 capsid proteins (VP1, VP2, and VP3) and displays considerable sequence diversity between serotypes. These differences alter receptor-binding sites on the surface of the capsid and lead to the observed variations in tissue transduction. One way in which investigators can restrict the location of transgene expression to the heart is to identify a naturally cardiotropic AAV capsid and design a pseudotyped rAAV that is optimized for delivery to the heart. Initial pseudotype experiments in a variety of animal models and using many different injection routes compared various combinations of the AAV capsids 1–5 to establish their respective natural tropisms or affinity for particular tissues. When considered together, the results suggested that rAAV2/1 yielded the highest level of transgene expression in the heart.8,9,10,11 The next general wave of vector comparisons was made between rAAV2/1 and more recently described pseudotypes: rAAV2/6, rAAV2/8, rAAV2/9, and rAAV2/10.12,13,14,15,16 For a compilation of these studies and the models they were performed in please see (Table 1). Taken together, various groups have demonstrated great success with all of these pseudotypes with rAAV2/9 appearing to be the most naturally cardiotropic.17,18,19,20,21,22 It is important to note that although extremely high levels of cardiac expression are observed, other organs (including liver, skeletal muscle, and pancreas) also display robust levels of expression.17 Additional research in nonhuman primates will be required to confirm preliminary capsid comparison studies in rodent, porcine, canine models as well as the initial primate experiments.18
Table 1. Compilation of the different types of rAAV that have been assessed in their ability to confer cardiac gene expression including the species and delivery routes that have been used as well as the duration of expression (this is the final time point of the experiment and not an indication of termination or significant decrease in expression).
In addition to evaluating natural capsids rescued from primate or human tissue, investigators have also manipulated the virus in a variety of ways to enhance the specificity of cardiac transduction. DNA shuffling is a method used to introduce permutations of genetic variations using in vitro recombination and has been employed to design cardiotropic rAAV. The technique generates chimeric AAV capsids by shuffling known serotype capsid sequences and then performing direct in vivo biopanning to identify novel cardiotropic mutant capsids. This type of screen retrieved a unique capsid (M41) based upon its high transduction frequency in muscle and low frequency in liver in both mice and hamsters.23 The efficiency of rAAVM41 transduction in the heart was comparable to that of rAAV2/9 but transduction of nonmuscle tissues was greatly reduced. Use of such cardiac-specific chimeras enhances the overall safety of cardiac gene transfer through minimization of off-target effects that could be introduced when performing delivery via the systemic circulation. Another key benefit displayed by the M41 variant is the reduced sequestration of virus in the liver. The decrease in the vector genomes lost in the liver results in a higher effective dose of vector in the heart, essentially providing greater overall efficacy.
Another technique to modify viral tropisms is to integrate peptides for specific cardiovascular targets into the capsid. This has been particularly useful for developing therapies for atherosclerosis, a narrowing of the coronary arteries due to fatty plaque accumulation that is the single leading cause of death in the United States today.24 Investigators found that vascular transduction was increased by isolating human venous endothelial cell-targeting peptides by phage display and genetically incorporating them into AAV capsids.25 In another study, two plaque-targeting peptides, CAPGPSKSC (CAP) and CNHRYMQMC (CNH) were inserted into the AAV2 capsid. In mice, this retargeting resulted in substantially higher levels of vector in the brachiocephalic artery (the site of advanced atherosclerotic plaques) and in the aorta.26
Methods to Increase rAAV Transgene Expression
Although AAV is a single-stranded DNA virus, both wtAAV gene and rAAV-mediated transgene expression require the conversion of single-stranded DNA to a double-stranded form before transcription.27 This process is partially responsible for the delayed onset of transgene expression as compared to other gene delivery vehicles.28,29,30 By mutating one of the ITRs it is possible to create vectors that exclusively package hairpin-like double-stranded AAV (dsAAV) DNA genomes which result in greater and more rapid transduction of tissues following intravenous administration in mice.27,31
The dsAAV strategy has been employed to develop a gene-based therapy for hypertension. Approximately 74.5 million adults in the United States have been diagnosed with high-blood pressure. If left untreated, hypertension can lead to damage to the heart, coronary arteries, and kidneys as well as increase ones chances of having a stoke, vision loss, or suffering other serious consequences.32 Investigators have found that dsAAV-mediated gene delivery of adrenomedullin successfully lowered blood pressure in spontaneously hypertensive rats.33 Of note, one disadvantage to using dsAAV is that the control elements plus transgene are limited to a length of ~2.2 kb. An alternative method to accelerate the onset of expression following rAAV-mediated gene transfer is to inhibit topoisomerase using camptothecine which leads to the rapid conversion of single-stranded rAAV genomes to the transcriptionally active double-stranded form.34 Clearly, elegant transgene expression enhancement techniques such as these are powerful tools that could be implemented in many gene transfer applications.
rAAV Delivery Routes
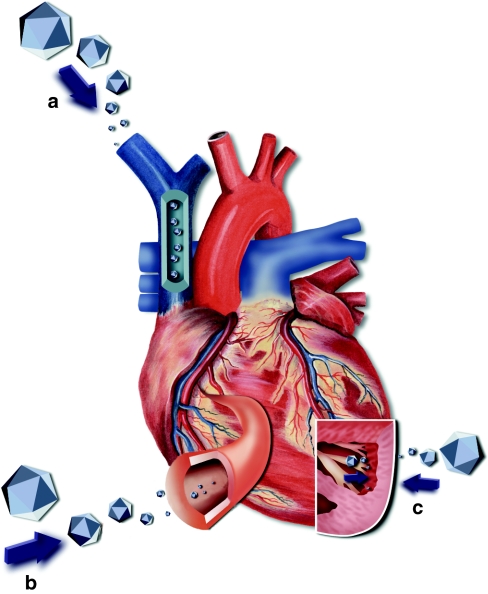
There are a variety of routes that may be employed to physically introduce rAAV to the heart (Figure 1). The optimal choice largely depends upon whether the targeted area is a defined location within the heart (such as an infarct) or the entire heart itself. Options for global cardiac delivery include an assortment of intravenous or intra-arterial routes as well as multiple intramuscular cardiac injections. Injections of rAAV into the coronary artery have been shown to achieve effective and uniform gene transfer throughout the myocardium as demonstrated in rats, pigs, and hamsters.35,36,37,38,39 Successful rAAV-mediated gene delivery to the whole heart has also been demonstrated following aortic occlusion in mice and pressurized venous infusion into dogs, rats, and pigs.40,41,42 In genetic diseases where the heart is only one component of the required treatment, systemic intravenous administration has provided efficient cardiac transduction when the optimal AAV serotype is used.43,44
Figure 1.
Recombinant adeno-associated virus (rAAV) delivery routes. (a) Systemic venous delivery—through the superior vena cava. (b) Vascular delivery—through the coronary artery. (c) Intramyocardial delivery—showing entry from the inside though endocardium and from the outside through epicardium (both ultimately target the heart muscle directly).
Transgene delivery to a precise location within the heart can be achieved through direct intramyocardial injections from the outside of the heart with a needle or via the endocardial surface using a catheter as demonstrated in hamster and swine models.39,45 Prenatal administration has been demonstrated in mice using in utero-intraperitoneal injections of the luciferase gene. Whole-body imaging analysis of these animals revealed low level, but positive cardiac expression.46 In general, each of the outlined routes of delivery can be combined with the most advantageous AAV capsid and incorporated into gene therapy treatments to successfully provide local, regional, or global transduction of the heart.
Tissue Restricted and Inducible Promoters
When using an intravenous delivery route even the most cardiotropic capsid choices may result in low-level transduction of noncardiac tissue. Many investigators have demonstrated the utility of cardiac promoters and their ability to augment specificity for either all striated muscle (useful for diseases that affect both the heart and skeletal muscle) or cardiac tissue alone in mice and rats.47,48,49 Promoters that have shown the most cardiac-specific expression include: cardiac myosin light chain and cardiac myosin heavy chain.47,50,51
Another cardiac selective promoter was designed by ligation of a 316 bp fragment of the mouse α-cardiac actin gene enhancer containing 2 myocyte enhancing factor-2 sequences and 2 MyoD (a myogenic regulatory factor) enhancer sequences attached to the elongation factor 1α promoter. The promoter was used to drive expression of a therapeutic transgene (the calcium (Ca2+)-sensing S100A1) in a rat model of heart failure (HF) and resulted in improved contractile function and left ventricular (LV) remodeling. Importantly, no S100A1 expression was observed in the other tissues analyzed.52
Promoters have also been developed which function as switches that are controlled under specific conditions. One such example is a hypoxia response element concatemer combined with either a minimal simian virus 40 promoter or a cardiac-specific promoter that becomes induced under ischemic conditions.53,54,55 Other useful promoters are those that manipulate transgene expression pharmacologically such as the tet-on or tet-off systems which are controlled by the antibiotic tetracycline or analogs such as doxycycline.56,57 In general, the use of tissue-specific or regulatable promoters adds another layer of control over the location, timing and amount of transgene expression and can further increase the safety of virtually any gene delivery system.
Gene Therapy for Heart Failure (HF) and Hypertrophy
HF is a chronic, progressive condition in which the heart is unable to pump enough blood to meet the body's demand. In the United States, ~5 million people suffer from HF and it contributes to ~300,000 deaths each year.58 Several approaches have been developed to ameliorate HF using rAAV-mediated gene transfer. One strategy is through manipulation of the sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) pump. SERCA2a controls the transport of Ca2+ to the sarcoplasmic reticulum during relaxation in the cardiac cycle. Intracoronary rAAV2/1-mediated gene transfer of SERCA2a into a swine model of volume-overload HF resulted in positive LV inotropic effects and LV reverse-remodeling through increased of calcium availability.59 This SERCA overexpression strategy is the basis of the “Calcium Up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease” or CUPID, the SERCA2a Gene Therapy in LAVD Patients and the “AAV6-CMV-Serca2a GENe Therapy Trial in HF” or AGENT-HF clinical trials which are discussed in greater detail later in this review.60
Another approach to augment Ca2+ release is through reduction of phospholamban—an inhibitor of the SERCA2a pump. Competitive inhibition by delivery of a pseudophosphorylated form of phospholamban improved LV systolic function and contractility through enhanced calcium release in cardiomyopathic hamsters and in a rat infarction model.36,61 Additionally, rAAV-mediated delivery of RNA interference and small hairpin RNA have also shown signs of enhanced contractility and calcium handling in vivtro in cardiomyocytes as well as improved cardiac function in a rat model of HF and through direct knockdown of phospholamban expression.62,63,64
Other groups have reversed cardiac dysfunction in models of HF through the inhibition of protein phosphatase 1, a phosphatase that becomes activated in HF and plays a key role in depressed cardiac function. Studies designed to investigate whether protein phosphatase 1 could be repressed by inhibitor-2 (INH-2), an endogenous protein phosphatase 1 inhibitor, showed that rAAV2-mediated INH-2 delivery through the coronary arterial route increased survival in the cardiomyopathic hamster model of HF by 3 months.65
Sick euthyroid syndrome can accompany cardiac HF and hypertrophy and lead to lower serum triiodothyronine (T3) levels, decreased expression of the thyroid hormone receptor isoforms TRα1 and TRβ1 and ultimately lower SERCA expression.66,67 SERCA expression increases when T3 occupied receptors bind to the thyroid response elements located in the SERCA promoter. A study in mice took advantage of this association by showing rAAV2-mediated overexpression of the thyroid receptor isoforms α1 and β1 improved contractile function in pressure overload-induced cardiac hypertrophy through increased SERCA expression.66
In HF, the upregulation of G protein–coupled receptor kinase 2 contributes to dysfunctional β-adrenergic receptor (β-AR) signaling and ultimately impaired cardiac function.68 The β-AR kinase inhibitor (β-ARKct) can inhibit the activation of G protein–coupled receptor kinase 2 and improve β-AR signaling. The current findings are that rAAV2/6-mediated delivery of β-ARKct results in sustained improvement of global cardiac function and a reversal of negative remodeling in a rat model of HF. Interestingly, β-ARKct overexpression improved outcomes to a greater degree than did administration of a β-blocker alone.69 In sum, a theme that emerges from all of these investigations is that multiple pathways can be approached to manage the many varied aspects of HF. Careful selection of a therapeutic approach that is best suited to address the specific pathobiologies of HF patients will enable future treatments to be tailored to individual needs.
Cardio-Protective Gene Transfer
Ischemic heart disease is the lack of blood flow and oxygen to parts of the heart that is caused by a narrowing of the coronary arteries and can ultimately lead to heart muscle damage.70 Many investigators have worked to develop rAAV gene therapy as a preventative treatment for ischemic heart disease. One strategy involving rAAV2-mediated delivery of transforming growth factor β1 suggested that the hearts of treated rats were protected from ischemia-reperfusion injury via an antioxidant mechanism through reduced activation of nicotinamide adenine dinucleotide phosphate oxidase and nuclear factor κ-light-chain-enhancer of activated B-cells (NF-κB).71 Another approach promoted neovascularization in ischemic mouse hearts following rAAV2-mediated transfer of the stress inducible enzyme heme oxygenase-1 (HO-1) through coinduction of vascular endothelial growth factor (VEGF) and stromal-cell derived factor 1 (SDF-1).72
HO-1 gene transfer has also protected tissue from ischemia-reperfusion injury. rAAV2-mediated transfer of the human HO-1 gene (hHO-1) to rats 8 weeks before acute coronary artery occlusion led to a dramatic reduction (>75%) in LV myocardial infarction size.73 This was accompanied by decreases in myocardial lipid peroxidation, proapoptotic Bcl-2-associated X protein (Bax) and proinflammatory interleukin-1β protein abundance as well as an increase in the level of antiapoptotic Bcl-2 protein. The investigators concluded that the hHO-1 transgene exerts its cardioprotective effects by reducing oxidative stress, inflammation, and apoptotic cell death.73 Similarly, other groups have shown that rAAV-mediated gene delivery of the nuclear factor-κB (NF-κB) inhibitor—iκBα, the extracellular superoxide dismutase (Ec-SOD), and the inducible heat-shock protein 70 (HSP70i) are each able to limit infarct size when predelivered to rodent models of ischemia-reperfusion injury.74,75,76,77,78
An early in vitro study showed that rAAV mediated transfer of the human VEGF gene into rat cardiomyocytes increased the concentration of VEGF protein expression both in cells as well as in the culture medium of those myocytes in a dose-dependent manner.79 Subsequent in vivo studies have demonstrated improvement in rodent and dog models of ischemia and infarction following rAAV-mediated VEGF, angiogenin, and human growth hormone delivery through increased angiogenesis.80,81,82,83 Future studies will be necessary to evaluate the long-term effects and ensure the safety of each of these approaches to temper the severity and improve outcomes for patients with ischemic heart disease.
Gene transfer can also be a useful method to provide immunoprotection to a heart that is to be transplanted or to a particular area of the heart that is susceptible to injury. Investigators have found that rAAV2-mediated delivery of a dominant-negative suppressor of cytokine signaling (SOCS1) transgene in mice increased resistance to acute cardiac injury caused by enteroviral infection. These results imply that inhibition of SOCS in the heart augments the host-cell antiviral system, thus preventing viral-mediated end-organ damage during the early stages of infection.84 Additional rodent studies that demonstrate protective gene transfer include the prevention of heart allograft rejection and prolonged allograft survival in cardiac transplantation models through rAAV-mediated delivery of HO-1 or the immunosuppressive CTLA4Ig gene.85,86 Given the existing deficiencies in current management options, a host of additional gene transfer opportunities exist in the realm of cardio-protective gene transfer.
Treatment of Metabolic or Neuromuscular Diseases that have Cardiac Involvement
Extensive studies have been conducted to investigate the use of rAAV-mediated gene delivery to treat rare genetic disorders resulting in metabolic alterations, such as Pompe disease, Fabry disease, and mucopolysaccharidosis type VII as well as others. Pompe disease is caused by a deficiency in the acid α-glucosidase enzyme and has an estimated incidence of 1 in 40,000.87 The build-up of glycogen in the lysosomes of severely affected patients leads to an enlarged heart, abnormal electrocardiogram readings and if left untreated it can result in cardiorespiratory failure within the first year of life. Both intramuscular and systemic delivery of acid α-glucosidase using rAAV2 or rAAV2/1 (respectively) have demonstrated clearance of glycogen from the lysosomes of cardiomyocytes and improved cardiac function in a model of Pompe disease as measured by enzyme detection assays and periodic acid Schiff staining as well as magnetic resonance imaging and electrocardiogram analysis.43,88,89
A deficiency in the lysosomal enzyme, α-galactosidase A (GLA) results in Fabry disease. The estimated prevalence of Fabry disease in the general population is ~1 in 117,000.90 With age their vital organs become increasingly affected and Fabry patients can experience complications such as heart disease and stroke. A single injection of rAAV2-GLA into the quadriceps muscle of a mouse model of the disease provided structural improvement of cardiac hypertrophy by echocardiographic examination 25 weeks after administration.91 Similarly, other groups have demonstrated restoration of α-galactosidase A to wild-type levels in the hearts of Fabry mice following a single intravenous injection of rAAV2-GLA to either the hepatic portal vein or tail vein.92,93
Mucopolysaccharidosis type VII is a lysosomal storage disease caused by deficiency of the acid hydrolase β-glucuronidase. It is estimated to occur in 1 in 250,000 newborns and can cause valvular heart disease and aortic regurgitation.94,95 Investigators working to find a treatment for mucopolysaccharidosis type VII found that one intrahepatic administration of rAAV2-β-glucuronidase was sufficient to reduce the lysosomal storage deficiency of this disease within the heart via the therapeutic effect of the secreted protein, much like enzyme replacement therapy.96
Very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a condition that blocks the conversion of very long-chain fatty acids to energy, particularly during periods of fasting. It occurs in ~1 in 30,000 individuals in the United States and the most rare but severe, early onset form typically presents in the first months of life with hypertrophic or dilated cardiomyopathy, pericardial effusion, and arrhythmias, as well as hypotonia, hepatomegaly, and intermittent hypoglycemia.97 Studies have shown that a single IV administration of rAAV2/8-VLCAD provided long-term cardiac expression and corrected the biochemical phenotype in VLCAD-deficient mice.98
Other examples of genetic diseases affecting the heart that may be treatable using a gene therapy approach are those which are primarily neuromuscular disorders. Several groups have demonstrated successful treatment of the cardiac phenotype in animal models such as the dystrophic hamster (Bio 14.6, Bio TO-2) which has a defect in the Δ-sarcoglycan (SGCD) gene. This was achieved by delivery of rAAV2 or dsAAV2/8-SGCD through either intramural, intraperitoneal, intravenous administration, direct infusion of the vector into the coronary artery ex vivo in a heterotopically transplanted heart and by transcoronary rAAV2-mediated transfer of a dominant-negative form of apoptosis signal-regulating kinase 1.35,45,99,100,101
The mdx mouse model of Duchene muscular dystrophy has also been utilized as a tool to test cardiac gene delivery methods. Investigations have shown that rAAV2 delivery of the microdystrophin gene to the newborn mdx mouse cardiac cavity yields efficient expression throughout the myocardium, restores the critical dystrophin-glycoprotein complex and improves sarcolemmal integrity in the heart for up to at least 10 months postadministration.102 Additional studies showed that systemic administration of rAAV2/6 carrying microdystrophin provided restoration of cardiac geometry and prevented dobutamine-induced cardiac failure.103 Once rAAV-mediated treatments are confirmed to be safe, diseases such as these that result from single gene defects will likely be among the first to be primarily treated using gene therapy approaches.
Gene Transfer to Influence Cardiac Electrophysiology
Several investigations have utilized rAAV as a delivery tool to manipulate cardiac electrophysiology. One study showed that rAAV2-mediated delivery of the voltage dependent, 4-aminopyridine-sensitive outward potassium current (Kv1.5) gene provided long-term normalization of the action potential duration in a mouse model for long-QT syndrome.104 Another study corrected the characteristically shortened PR interval in a mouse model of Pompe disease following rAAV2/9-mediated delivery of the acid α glucosidase (acid α-glucosidase) gene necessary for glycogen hydrolysis in lysosomes.18 Others have shown that rAAV2/9 transfer of the microdystrophin gene is able to ameliorate the electrocardiographic abnormalities in the mdx mouse model of Duchenne muscular dystrophy.105 In principle, a number of other channel genes could be accommodated into rAAV vectors for gain of function studies. Additionally, arrhythmias could be alleviated through rAAV-mediated gene-specific knockdown of dominant mutations. Finally, there is the potential to establish ectopic automatic pacemaker activity, which would be especially useful for the treatment of complete congenital heart block or other situations which require long-term restoration of intrinsic pacemaker activity.
rAAV as a Tool to Understand In Vivo Processes
In addition to being used as a therapeutic delivery system, rAAV can also be a valuable tool for increasing our knowledge of in vivo biological processes. The protective anti-inflammatory and antiapoptotic effects of smooth muscle were revealed following rAAV2-mediated intracoronary infusion of angiopoietin 1 and 2 into rat-cardiac allografts.106 Further investigations also uncovered the diverse effects of platelet-derived growth factor (PDGF) ligands in cardiac allograft vasculopathy and fibrosis following administration of rAAV2 carrying PDGF-A, B, C and D. This work led to the suggestion that a targeted therapy using monoclonal antibodies to block the active sites of PDGF-A, -C, and -D may be beneficial to heart transplant survival.107
A novel target for regenerative therapy was discovered in a study that showed proliferation of immature rat cardiomyocytes could be stimulated through the NOTCH1 signaling pathway following rAAV2/8 delivery of the activated form of NOTCH1.108 Other investigations revealed the mechanisms by which hypoxia/reperfusion induces signals for remodeling through blockade of hypoxia-reoxygenation-mediated collagen type I expression and matrix metalloproteinase activity following rAAV2-mediated overexpression of transforming growth factor β1 in mouse cardiomyocytes.109
To gain a better understanding of the role of angiotensin-converting enzyme type 2 (ACE2) in the regulation of cardiac structure and function, a study was conducted which showed induction of fibrosis in the myocardium of stroke-prone spontaneously hypertensive rats following administration of rAAV2/6-ACE.110 Finally, the ability of hypoxia-induced mitogenic factor (HIMF) to provoke the vascular and hemodynamic changes observed in pulmonary hypertension was established following HIMF gene transfer using rAAV2 to transduce rat lungs.111 Beyond gene transfer studies, increased use of rAAV vectors to introduce stable, long-term expression of gene-specific silencing sequences could yield vast amounts of information regarding cardiac development and cardiovascular disease progression.
Transduction of Cells Prior to Cell Therapy
Overall, rAAV has not been the most efficient vehicle option for stem cell transduction. One key investigation showed that lentiviral vectors achieved significantly higher transduction efficiencies in cardiosphere-derived resident cardiac stem cells than any of nine rAAV serotypes tested.112 Despite this, other researchers have found that mesenchymal stem cells can be genetically manipulated through rAAV-mediated delivery of tumor necrosis factor receptor (TNFR). Transfer of these modified mesenchymal stem cells improved LV function in a rat model of myocardial infarction through antiapoptotic and anti-inflammatory mechanisms.113 Other studies established that the modification of tyrosine on the AAV2 capsid increases viral transduction of stem cells.114,115 Novel approaches to direct the molecular evolution of AAV capsids will allow for the selection of additional unique structures with affinities for specific stem cell types.23,116
Clinical Trials
A careful assessment of data from preclinical biodistribution and toxicity studies to evaluate the safety profile of a gene transfer treatment is absolutely necessary prior to introduction of that approach to the clinic. One of the foremost aims of biodistribution studies is to assess the potential spread of vector sequences beyond the intended site of delivery. The chosen route of delivery greatly impacts this behavior. A study evaluating the biodistribution of rAAV2 vector DNA following myocardial delivery in rats showed that vector expression in extra cardiac tissues such as liver, kidney, and testes was present 6 months postadministration.117 Other detailed studies in mice and rabbits were designed to track the biodistribution of rAAV2/1 vectors delivered to striated muscle. Those results indicated transient distribution to blood at high doses; however, no toxicities were observed.118 It is of the utmost importance that studies such as these be performed with a well-characterized vector stock. Additionally, they must be conducted using a good laboratory practice design under an established protocol with well-defined endpoints.
A cellular immune response to transgenes encoding foreign proteins is another essential consideration for rAAV-based cardiac gene therapy. One investigation demonstrated this significance through a comparison between the delivery of empty rAAV2 capsids and rAAV2-mediated delivery of the human TNF receptor II immunoglobulin G-Fc fusion protein (rAAV2-TNFRII-Fc) following direct injections into baboon hearts.119 The results showed that the baboons which had received rAAV2-TNFRII-Fc developed myocardial infiltrates including CD8+ cells whereas those receiving empty rAAV2 capsids did not. This study highlights the importance of investigations into transgene as well as delivery vehicle safety in a large animal model where the response to transgene expression may be more relevant to human clinical studies.
A clinical trial to evaluate intracoronary administration of rAAV2/1-SERCA2a in patients with HF was recently performed.120 The initial study evaluated the potential for calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID).121 As a phase I study, the focus was on safety and this was demonstrated in the open label portion of the trial. No significant changes were observed in exams of major organs, blood pressure, heart rate, or body temperature following rAAV2/1-SERCA2a administration. Of those adverse events that were observed only two events of fatigue, one of a fever, and one of muscle spasms were determined to have been possibly related to rAAV2/1-SERCA2a delivery. Significantly, the two patients in the trial with pre-existing neutralizing antibodies (Nab) to the AAV1 capsid failed to improve while several of those without pre-existing Nab did demonstrate improvement as determined by several endpoints over a 6-month period. Recent evaluation of patients enrolled in the phase II double-blind, placebo controlled portion of the trial has showed a favorable safety signal and demonstrated early indications of efficacy. These included a decreased frequency of cardiovascular events per patient from each of the three dose cohorts.122 Importantly, no increases in adverse events, disease-related events, laboratory abnormalities, or arrhythmias were observed in any of the treated patients compared to those receiving placebo.60
Two other cardiac gene therapy trials are being conducted which employ rAAV6 as the gene delivery vehicle for SERCA2a. The SERCA2a Gene Therapy in LAVD Patients is a currently enrolling phase I/II trial in the United Kingdom that is targeting patients with advanced HF that have received an LV assist device.60 The AGENT-HF trial is a currently enrolling phase II trial in France that is focused on determining the effects of SERCA2a delivery on cardiac remodeling.60 Although larger studies will be required to truly establish rAAV-SERCA2a as a proven treatment modality for advanced HF, these highly promising initial results substantiate the utility of rAAV as a clinically relevant vehicle for cardiac gene delivery.
Conclusions
Years of investigation using a number of gene delivery systems have provided a firm foundation of data recognizing rAAV as a preferred vehicle to achieve stable cardiac gene transfer. The high efficiency of transduction by newly characterized AAV capsids, both natural and those derived from directed evolution is an exciting advance in the field that opens the door to additional clinical opportunities.23,123 The field is slowly but steadily designing an ideal cardiac gene delivery system which will combine the utilization of a safe and highly cardiotropic capsid with an optimized delivery route and a tissue-specific and/or regulatable promoter. The most attractive transgene for each application would be those in which the expressed protein remains safe even after many years of sustained expression. While great advances have been made in all of these areas, the incorporation of optimized capsid, promoter, route and transgene into one gene delivery system is still under construction.
An important consideration for advancing rAAV gene delivery in the clinical arena will continue to be the presence of preexisting antibodies or memory B-cells to AAV capsids which may diminish efficacy.121 Solutions to the challenges presented by pre-existing immunity are currently being evaluated. One simple option to minimize this effect is to choose vectors in which the presence of neutralizing antibodies to those capsids in the sera of healthy humans is rare and/or minimal.87
Another remaining challenge to moving rAAV-mediated therapies to the forefront of biological medicine is large scale clinical-grade production of the virus. While there have been several advances in the area of saleable rAAV vector manufacturing, these methods have yet to be widely implemented.124,125,126 Although the manufacturing of biologics will always be more costly than that of pharmaceuticals, the durable therapy provided by such treatments could ultimately result in a more commercially viable product. These factors are certainly a strong driving force behind the clinical development of therapeutics that could benefit large patient populations.124,126
The ability to provide highly specific treatment for the spectrum of inherited and acquired heart disease makes cardiac gene therapy an important clinical approach that fulfills the principles of molecular medicine. During the next phase of rAAV vector development for cardiac gene transfer, we should continue to see useful experimental tools develop into viable clinical opportunities.
REFERENCES
- Lin H, Parmacek MS, Morle G, Bolling S., and, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Atchison RW, Casto BC., and, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Dong JY, Fan PD., and, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA.et al. (1990Site-specific integration by adeno-associated virus Proc Natl Acad Sci USA 872211–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR., and, Berns KI.ed). (2005Adeno-associated Viral Vectors for Gene Therapy Elsevier B.V.: Amsterdam, the Netherlands [Google Scholar]
- Samulski RJ, Chang LS., and, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp BC, Clark KR, Klemanski DL, Pacak CA., and, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77:3495–3504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard S, MacKenzie TC, Radu AP, Hayashi S, Peranteau WH, Chirmule N.et al. (2003Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice J Gene Med 5941–950. [DOI] [PubMed] [Google Scholar]
- Du L, Kido M, Lee DV, Rabinowitz JE, Samulski RJ, Jamieson SW.et al. (2004Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors Mol Ther 10604–608. [DOI] [PubMed] [Google Scholar]
- Su H, Huang Y, Takagawa J, Barcena A, Arakawa-Hoyt J, Ye J.et al. (2006AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect Gene Ther 131495–1502. [DOI] [PubMed] [Google Scholar]
- Su H, Yeghiazarians Y, Lee A, Huang Y, Arakawa-Hoyt J, Ye J.et al. (2008AAV serotype 1 mediates more efficient gene transfer to pig myocardium than AAV serotype 2 and plasmid J Gene Med 1033–41. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG.et al. (2004Systemic delivery of genes to striated muscles using adeno-associated viral vectors Nat Med 10828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A.et al. (2005Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II Mol Ther 1157–65. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J.et al. (2005Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart Nat Biotechnol 23321–328. [DOI] [PubMed] [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F.et al. (2007Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo Gene Ther 14989–997. [DOI] [PubMed] [Google Scholar]
- Tarantal AF., and, Lee CC. Long-term luciferase expression monitored by bioluminescence imaging after adeno-associated virus-mediated fetal gene delivery in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2010;21:143–148. doi: 10.1089/hum.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA.et al. (2006Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8 Mol Ther 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE.et al. (2006Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo Circ Res 99e3–e9. [DOI] [PubMed] [Google Scholar]
- Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L.et al. (2007Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy J Thromb Haemost 516–24. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Miyagi N, Rao VP, Ricci D, Du Z, Byrne GW, Bailey KR.et al. (2008Efficient and durable gene transfer to transplanted heart using adeno-associated virus 9 vector J Heart Lung Transplant 27554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G.et al. (2008Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat Hum Gene Ther 191359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jiang J, Drouin LM, Agbandje-McKenna M, Chen C, Qiao C.et al. (2009A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection Proc Natl Acad Sci USA 1063946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHA 2011Cardiovascular Disease StatisticsRetrieved 2 April 2011. < . < http://www.americanheart.org/presenter.jhtml?identifier=4478 >.
- White SJ, Nicklin SA, Büning H, Brosnan MJ, Leike K, Papadakis ED.et al. (2004Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors Circulation 109513–519. [DOI] [PubMed] [Google Scholar]
- White K, Büning H, Kritz A, Janicki H, McVey J, Perabo L.et al. (2008Engineering adeno-associated virus 2 vectors for targeted gene delivery to atherosclerotic lesions Gene Ther 15443–451. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J., and, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Hauck B, Zhao W, High K., and, Xiao W. Intracellular viral processing, not single-stranded DNA accumulation, is crucial for recombinant adeno-associated virus transduction. J Virol. 2004;78:13678–13686. doi: 10.1128/JVI.78.24.13678-13686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li W, Yang Z, Qing K, Tan M, Hansen J.et al. (2004Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells Hum Gene Ther 151207–1218. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P., and, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- AHA 2011About High Blood PressureRetrieved 2 April 2011. < . < http://www.heart.org/HEARTORG/ Conditions/HighBloodPressure/AboutHighBloodPressure/About-High-Blood-Pressure_ DCM_002050_Article.jsp >
- Wei X, Zhao C, Jiang J, Li J, Xiao X., and, Wang DW. Adrenomedullin gene delivery alleviates hypertension and its secondary injuries of cardiovascular system. Hum Gene Ther. 2005;16:372–380. doi: 10.1089/hum.2005.16.372. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Xu Y, Yang Z, Toufektsian MC, Berr SS., and, French BA. Topoisomerase inhibition accelerates gene expression after adeno-associated virus-mediated gene transfer to the mammalian heart. Mol Ther. 2007;15:764–771. doi: 10.1038/sj.mt.6300071. [DOI] [PubMed] [Google Scholar]
- Li J, Wang D, Qian S, Chen Z, Zhu T., and, Xiao X. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 2003;10:1807–1813. doi: 10.1038/sj.gt.3302078. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y.et al. (2002Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery Nat Med 8864–871. [DOI] [PubMed] [Google Scholar]
- Asfour B, Baba HA, Scheld HH, Hruban RH, Hammel D., and, Byrne BJ. Uniform long-term gene expression using adeno-associated virus (AAV) by ex vivo recirculation in rat-cardiac isografts. Thorac Cardiovasc Surg. 2002;50:347–350. doi: 10.1055/s-2002-35745. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, McKirnan MD.et al. (2005Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus J Gene Med 7316–324. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Xiao X, Samulski RJ, Li J, Ojamaa K, Klein IL.et al. (1996Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector Ann Thorac Surg 621669–1676. [DOI] [PubMed] [Google Scholar]
- Champion HC, Georgakopoulos D, Haldar S, Wang L, Wang Y., and, Kass DA. Robust adenoviral and adeno-associated viral gene transfer to the in vivo murine heart: application to study of phospholamban physiology. Circulation. 2003;108:2790–2797. doi: 10.1161/01.CIR.0000096487.88897.9B. [DOI] [PubMed] [Google Scholar]
- Raake PW, Hinkel R, Müller S, Delker S, Kreuzpointner R, Kupatt C.et al. (2008Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors Gene Ther 1512–17. [DOI] [PubMed] [Google Scholar]
- Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW.et al. (2005Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector Circulation 1121780–1788. [DOI] [PubMed] [Google Scholar]
- Mah C, Cresawn KO, Fraites TJ, Jr, Pacak CA, Lewis MA, Zolotukhin I.et al. (2005Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors Gene Ther 121405–1409. [DOI] [PubMed] [Google Scholar]
- Müller OJ, Schinkel S, Kleinschmidt JA, Katus HA., and, Bekeredjian R. Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther. 2008;15:1558–1565. doi: 10.1038/gt.2008.111. [DOI] [PubMed] [Google Scholar]
- Kawada T, Sakamoto A, Nakazawa M, Urabe M, Masuda F, Hemmi C.et al. (2001Morphological and physiological restorations of hereditary form of dilated cardiomyopathy by somatic gene therapy Biochem Biophys Res Commun 284431–435. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Gruber CA, Cao Y, Hardy J, Contag CH., and, Gaensler KM. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther. 2001;3:284–292. doi: 10.1006/mthe.2001.0267. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Sakai Y, Thattaliyath BD, Mah CS., and, Byrne BJ. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther. 2008;6:13. doi: 10.1186/1479-0556-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Zhou X, Polce DR, El-Sawy T, Patel SR, Thakker GD.et al. (1999Exogenous control of cardiac gene therapy: evidence of regulated myocardial transgene expression after adenovirus and adeno-associated virus transfer of expression cassettes containing corticosteroid response element promoters J Thorac Cardiovasc Surg 11826–4, discussion 34. [DOI] [PubMed] [Google Scholar]
- Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM.et al. (2007Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle Mol Ther 15320–329. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Tang Y, Schmidt-Ott K, Qian K., and, Kagiyama S. Vigilant vector: heart-specific promoter in an adeno-associated virus vector for cardioprotection. Hypertension. 2002;39 2 Pt 2:651–655. doi: 10.1161/hy0202.103472. [DOI] [PubMed] [Google Scholar]
- Aikawa R, Huggins GS., and, Snyder RO. Cardiomyocyte-specific gene expression following recombinant adeno-associated viral vector transduction. J Biol Chem. 2002;277:18979–18985. doi: 10.1074/jbc.M201257200. [DOI] [PubMed] [Google Scholar]
- Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W.et al. (2007Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue Circulation 1152506–2515. [DOI] [PubMed] [Google Scholar]
- Su H, Arakawa-Hoyt J., and, Kan YW. Adeno-associated viral vector-mediated hypoxia response element-regulated gene expression in mouse ischemic heart model. Proc Natl Acad Sci USA. 2002;99:9480–9485. doi: 10.1073/pnas.132275299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Dong H, Zhang Z, Wang W, Zhang Y., and, Xu X. Hypoxic response elements control expression of human vascular endothelial growth factor(165) genes transferred to ischemia myocardium in vivo and in vitro. J Gene Med. 2007;9:788–796. doi: 10.1002/jgm.1070. [DOI] [PubMed] [Google Scholar]
- Su H, Joho S, Huang Y, Barcena A, Arakawa-Hoyt J, Grossman W.et al. (2004Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts Proc Natl Acad Sci USA 10116280–16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., and, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Müller G, Hillen W., and, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- NIH 2011MedlinePlus_Heart FailureRetrieved 4 April 2011. < . < http://www.nlm.nih.gov/medlineplus/heartfailure.html >.
- Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H.et al. (2008Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure J Am Coll Cardiol 511112–1119. [DOI] [PubMed] [Google Scholar]
- Kawase Y, Ladage D., and, Hajjar RJ. Rescuing the failing heart by targeted gene transfer. J Am Coll Cardiol. 2011;57:1169–1180. doi: 10.1016/j.jacc.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y.et al. (2004Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats J Clin Invest 113727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino LM, Takeda M, Kasahara H, Jakymiw A, Byrne BJ., and, Lewin AS. AAV-mediated knockdown of phospholamban leads to improved contractility and calcium handling in cardiomyocytes. J Gene Med. 2008;10:132–142. doi: 10.1002/jgm.1131. [DOI] [PubMed] [Google Scholar]
- Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J.et al. (2009Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy Circulation 1191241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hu SJ, Sun J, Zhu ZH, Zheng X, Wang GZ.et al. (2005Construction of phospholamban antisense RNA recombinant adeno-associated virus vector and its effects in rat cardiomyocytes Acta Pharmacol Sin 2651–55. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ikeda Y, Yano M, Yoshimura K, Nishino S, Aoyama H.et al. (2006Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy FASEB J 201197–1199. [DOI] [PubMed] [Google Scholar]
- Belke DD, Gloss B, Swanson EA., and, Dillmann WH. Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-α1 and -β1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology. 2007;148:2870–2877. doi: 10.1210/en.2007-0009. [DOI] [PubMed] [Google Scholar]
- Opasich C, Pacini F, Ambrosino N, Riccardi PG, Febo O, Ferrari R.et al. (1996Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure Eur Heart J 171860–1866. [DOI] [PubMed] [Google Scholar]
- Brinks H., and, Koch WJ. βARKct: a therapeutic approach for improved adrenergic signaling and function in heart disease. J Cardiovasc Transl Res. 2010;3:499–506. doi: 10.1007/s12265-010-9206-6. [DOI] [PubMed] [Google Scholar]
- Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE.et al. (2009Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure Circulation 11989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHA 2011Silent Ischemia and Ischemic Heart DiseaseRetrieved 4 April 2011. < . < http://www/americanheart.org/presenter.jhtml?identifier=4720 >.
- Dandapat A, Hu CP, Li D, Liu Y, Chen H, Hermonat PL.et al. (2008Overexpression of TGFβ1 by adeno-associated virus type-2 vector protects myocardium from ischemia-reperfusion injury Gene Ther 15415–423. [DOI] [PubMed] [Google Scholar]
- Lin HH, Chen YH, Chang PF, Lee YT, Yet SF., and, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A.et al. (2002Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene Circulation 105602–607. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Deodato B, Squadrito G, Seminara P, Passaniti M, Venuti FS.et al. (2003Gene transfer of IκBα limits infarct size in a mouse model of myocardial ischemia-reperfusion injury Lab Invest 831097–1104. [DOI] [PubMed] [Google Scholar]
- Agrawal RS, Muangman S, Layne MD, Melo L, Perrella MA, Lee RT.et al. (2004Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemic reperfusion injury, improves ventricular function and prolongs survival Gene Ther 11962–969. [DOI] [PubMed] [Google Scholar]
- Pachori AS, Melo LG, Zhang L, Solomon SD., and, Dzau VJ. Chronic recurrent myocardial ischemic injury is significantly attenuated by pre-emptive adeno-associated virus heme oxygenase-1 gene delivery. J Am Coll Cardiol. 2006;47:635–643. doi: 10.1016/j.jacc.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT.et al. (2007Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction Am J Physiol Heart Circ Physiol 293H48–H59. [DOI] [PubMed] [Google Scholar]
- Belke DD, Gloss B, Hollander JM, Swanson EA, Duplain H., and, Dillmann WH. In vivo gene delivery of HSP70i by adenovirus and adeno-associated virus preserves contractile function in mouse heart following ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H2905–H2910. doi: 10.1152/ajpheart.00323.2006. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Ikeda U, Shimpo M, Shibuya M, Monahan J, Urabe M.et al. (2000Adeno-associated virus-mediated vascular endothelial growth factor gene transfer into cardiac myocytes J Cardiovasc Pharmacol 36438–443. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Hu SJ, Li J, Mou Y, Chan CF, Jin J.et al. (2006rAAV-mediated angiogenin gene transfer induces angiogenesis and modifies left ventricular remodeling in rats with myocardial infarction J Mol Med 841033–1046. [DOI] [PubMed] [Google Scholar]
- Kusano K, Tsutsumi Y, Dean J, Gavin M, Ma H, Silver M.et al. (2007Long-term stable expression of human growth hormone by rAAV promotes myocardial protection post-myocardial infarction J Mol Cell Cardiol 42390–399. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R., and, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X.et al. (2006Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs Circ Res 98954–961. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Yajima T, Duplain H, Iwatate M, Kido M, Hoshijima M.et al. (2003The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury J Clin Invest 111469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT.et al. (2003Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer Circulation 1072623–2629. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lu L, Li J, Xiao X, Fung JJ., and, Qian S. Prolonged survival of heart allografts transduced with AAV-CTLA4Ig. Microsurgery. 2003;23:489–493. doi: 10.1002/micr.10181. [DOI] [PubMed] [Google Scholar]
- Genzyme 2011Pompe DiseaseRetrieved 28 March 2011. < . < http://www.pompe.com/en/healthcare-professionals/ genetics-epidemiology/incidence-prevalence.aspx >.
- Fraites TJ, Jr, Schleissing MR, Shanely RA, Walter GA, Cloutier DA, Zolotukhin I.et al. (2002Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors Mol Ther 55 Pt 1571–578. [DOI] [PubMed] [Google Scholar]
- Mah C, Pacak CA, Cresawn KO, Deruisseau LR, Germain S, Lewis MA.et al. (2007Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors Mol Ther 15501–507. [DOI] [PubMed] [Google Scholar]
- Genzyme 2011Fabry DiseaseRetrieved 28 March 2011. < . < http://www.genzyme.ca/thera/fz/ca_en_p_tp_thera-fz.asp >.
- Takahashi H, Hirai Y, Migita M, Seino Y, Fukuda Y, Sakuraba H.et al. (2002Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer Proc Natl Acad Sci USA 9913777–13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Murray GJ, Limaye A, Quirk JM, Gelderman MP, Brady RO.et al. (2003Long-term correction of globotriaosylceramide storage in Fabry mice by recombinant adeno-associated virus-mediated gene transfer Proc Natl Acad Sci USA 1003450–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Han IP, Limaye A, Xu R, Gelderman MP, Zerfas P.et al. (2001Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice Proc Natl Acad Sci USA 982676–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH 2011Mucopolysaccharidosis type VIIRetrieved 28 March 2011. < . < http://ghr.nlm.nih.gov/condition/mucopolysaccharidosis-type-vii >.
- Emedicine 2011Genetics of Mucopolysaccharidosis Type VIIRetrieved 28 March 2011. < . < http://emedicine.medscape.com/article/944298-overview >.
- Sferra TJ, Backstrom K, Wang C, Rennard R, Miller M., and, Hu Y. Widespread correction of lysosomal storage following intrahepatic injection of a recombinant adeno-associated virus in the adult MPS VII mouse. Mol Ther. 2004;10:478–491. doi: 10.1016/j.ymthe.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Leslie 2009Very Long Chain Acyl-CoA Dehydrogenase Deficiency, VLCAD Deficiency Pagon BT.andDolan CR.eds.). University of Washington, Seattle; [PubMed] [Google Scholar]
- Merritt JL., 2nd, , Nguyen T, Daniels J, Matern D., and, Schowalter DB. Biochemical correction of very long-chain acyl-CoA dehydrogenase deficiency following adeno-associated virus gene therapy. Mol Ther. 2009;17:425–429. doi: 10.1038/mt.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C.et al. (2005Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer Circulation 1122650–2659. [DOI] [PubMed] [Google Scholar]
- Kawada T, Nakazawa M, Nakauchi S, Yamazaki K, Shimamoto R, Urabe M.et al. (2002Rescue of hereditary form of dilated cardiomyopathy by rAAV-mediated somatic gene therapy: amelioration of morphological findings, sarcolemmal permeability, cardiac performances, and the prognosis of TO-2 hamsters Proc Natl Acad Sci USA 99901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S.et al. (2007Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer J Am Coll Cardiol 50453–462. [DOI] [PubMed] [Google Scholar]
- Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS., and, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS., and, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Brunner M, Busconi L., and, Koren G. Long-term restitution of 4-aminopyridine-sensitive currents in Kv1DN ventricular myocytes using adeno-associated virus-mediated delivery of Kv1.5. FEBS Lett. 2003;550:74–78. doi: 10.1016/s0014-5793(03)00822-6. [DOI] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Lai Y, Long C, Li D., and, Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008;19:851–856. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykänen AI, Pajusola K, Krebs R, Keränen MA, Raisky O, Koskinen PK.et al. (2006Common protective and diverse smooth muscle cell effects of AAV-mediated angiopoietin-1 and -2 expression in rat cardiac allograft vasculopathy Circ Res 981373–1380. [DOI] [PubMed] [Google Scholar]
- Tuuminen R, Nykänen AI, Krebs R, Soronen J, Pajusola K, Keränen MA.et al. (2009PDGF-A, -C, and -D but not PDGF-B increase TGF-β1 and chronic rejection in rat cardiac allografts Arterioscler Thromb Vasc Biol 29691–698. [DOI] [PubMed] [Google Scholar]
- Collesi C, Zentilin L, Sinagra G., and, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CP, Dandapat A, Liu Y, Hermonat PL., and, Mehta JL. Blockade of hypoxia-reoxygenation-mediated collagen type I expression and MMP activity by overexpression of TGF-β1 delivered by AAV in mouse cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1833–H1838. doi: 10.1152/ajpheart.00488.2007. [DOI] [PubMed] [Google Scholar]
- Masson R, Nicklin SA, Craig MA, McBride M, Gilday K, Gregorevic P.et al. (2009Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2 Hypertension 53694–700. [DOI] [PubMed] [Google Scholar]
- Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC.et al. (2009Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) induces the vascular and hemodynamic changes of pulmonary hypertension Am J Physiol Lung Cell Mol Physiol 296L582–L593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AS, Kizana E, Smith RR, Terrovitis J, Dong P, Leppo MK.et al. (2008Lentiviral vectors bearing the cardiac promoter of the Na+-Ca2+ exchanger report cardiogenic differentiation in stem cells Mol Ther 16957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C, Guo J, Lin G, Hu M., and, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J. 2008;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- Li M, Jayandharan GR, Li B, Ling C, Ma W, Srivastava A.et al. (2010High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy Hum Gene Ther 211527–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss MA, Smith LJ, Zhong L, Srivastava A, Wong KK., Jr, and, Chatterjee S. Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2. Hum Gene Ther. 2010;21:1129–1136. doi: 10.1089/hum.2010.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA.et al. (2008In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses J Virol 825887–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachori AS, Melo LG, Zhang L, Loda M, Pratt RE., and, Dzau VJ. Potential for germ line transmission after intramyocardial gene delivery by adeno-associated virus. Biochem Biophys Res Commun. 2004;313:528–533. doi: 10.1016/j.bbrc.2003.11.140. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Conlon TJ, Poirier A, Campbell-Thompson M., and, Byrne BJ. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum Gene Ther. 2007;18:245–256. doi: 10.1089/hum.2006.113. [DOI] [PubMed] [Google Scholar]
- McTiernan CF, Mathier MA, Zhu X, Xiao X, Klein E, Swan CH.et al. (2007Myocarditis following adeno-associated viral gene expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts Gene Ther 141613–1622. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ., and, Zsebo K. AAV vectors and cardiovascular disease: targeting TNF receptor in the heart: clue to way forward with AAV. Gene Ther. 2007;14:1611–1612. doi: 10.1038/sj.gt.3303047. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey JK, Yerevanian AI., and, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wilson JM., and, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- Clément N, Knop DR., and, Byrne BJ. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum Gene Ther. 2009;20:796–806. doi: 10.1089/hum.2009.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZZ, Cheng KM, Huang W, Dilshat, and, Feng DR. [Study on industrialized extraction technology and function of hyperlipidemic regulating of Laminaria japonica polysaccharides] Zhong Yao Cai. 2010;33:1795–1798. [PubMed] [Google Scholar]
- Wright JF. Transient transfection methods for clinical adeno-associated viral vector production. Hum Gene Ther. 2009;20:698–706. doi: 10.1089/hum.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]