Abstract
Random integration of conventional gene delivery vectors such as viruses, plasmids, P1 phage-derived artificial chromosomes, bacterial artificial chromosomes and yeast artificial chromosomes can be associated with transgene silencing. Furthermore, integrated viral sequences can activate oncogenes adjacent to the insertion site resulting in cancer. Various human artificial chromosomes (HACs) exhibit several potential characteristics desired for an ideal gene delivery vector, including stable episomal maintenance and the capacity to carry large genomic loci with their regulatory elements, thus allowing the physiological regulation of the introduced gene in a manner similar to that of native chromosomes. HACs have been generated mainly using either a “top-down approach” (engineered chromosomes), or a “bottom-up approach” (de novo artificial chromosomes). The recent emergence of stem cell–based tissue engineering has opened up new avenues for gene and cell therapies. This review describes the lessons learned and prospects identified mainly from studies in the construction of HACs and HAC-mediated gene expression systems in cultured cells, as well as in animals.
Introduction
Gene therapy has been envisioned to provide a direct and permanent correction of genetic defects. To achieve the desired effects, therapeutic genes need to be carried by safe and effective vectors that can deliver foreign genes to specific cells and thereafter sustain their expression in a physiologically regulated fashion. Gene delivery vectors with the following properties may further add to the applications for gene and cell therapies: (i) high transfection efficiency; (ii) long-term stable maintenance in host cells without integration into the host genome; (iii) appropriate levels of spatial and temporal expression of therapeutic genes in specifically desired cells; and (iv) no risk of cellular transformation or stimulation of the host's immune system. Although a number of different approaches have been attempted to achieve efficient gene transfer and long-term gene expression, this challenging task remains unfulfilled as all current methods have some limitations. For example, adenovirus-derived vectors have been widely employed in many current gene therapies because of their high infectivity in a wide variety of cell types and tissues, independent of the proliferative state.1,2 However, adenovirus-mediated transgene expression is transient and needs high-titer administration. The consequent toxicity and undesired immunological response make adenovirus-based gene therapies risky.3 Other popular choices include well-characterized retroviral vectors, which enable sustained transgene expression in dividing cells by integration of target genes into the host cell genome. However, retroviruses have been reported to favor integration into sites near transcription start regions of the host genome, thus causing insertional mutagenesis with retrovirus-based vectors.4,5,6 In addition, transcriptional silencing caused by retroviral vector integration is often observed in mouse stem cells and transfected hematopoietic stem cells.7,8 In comparison to the other approaches, lentiviral vectors offer an attractive means of gene delivery because such viruses can transduce dividing and quiescent cells.9,10,11,12 HIV-based vectors have recently been utilized successfully for human gene correction, although integrations of the vectors into the host genome were observed.13,14 Recently, integration-deficient lentiviral vectors were developed through the use of integrase mutations.12,15 Integration-deficient lentiviral vectors have a much lower risk of insertional mutagenesis and replication-component recombinant (RCR) generation than integrating lentiviral vectors. Although integration-deficient lentiviral vectors can mediate stable expression in non-dividing cells, integration-deficient lentiviral vectors show transient expression in proliferating cells. An alternative solution to these problems could be the use of human artificial chromosome (HAC) vectors. Although the efficiency of transferring the HACs into the recipient cells is lower than that of conventional viral vectors, HACs replicate and segregate as natural chromosomes, independently from the host genome, thus overcoming the problems.16,17,18,19,20,21,22 This review describes various types of HACs and their potential characteristics. Methods of chromosome transfer, examples of gene delivery including genetic correction, pharmaceutical protein production and animal transgenesis, as well as possible usage for other purposes are also introduced.
Advantages of HAC for Gene Delivery
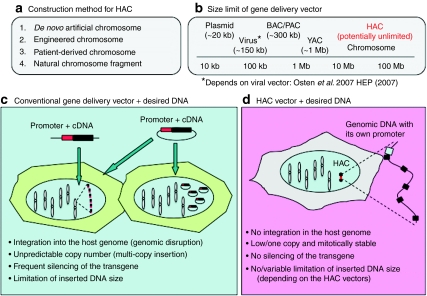
Method for constructing HAC vectors and potential characteristics of HACs are described in Figure 1. HACs are exogenous mini-chromosomes artificially created mainly by either a “top-down approach” (engineered chromosomes), or a “bottom-up approach” (de novo artificial chromosomes). The chromosomes can be transferred into host cells, mainly by microcell-mediated chromosome transfer (MMCT), as described in a following section. HACs can faithfully mimic the normal pattern of gene expression because they can hold complete genomic loci, including upstream and downstream regulatory elements.22,23 In addition, because of their episomal nature, many complications, such as silencing of the therapeutic gene or oncogenesis resulting from integration at unfavorable sites, should be minimized. Although there are potential gene silencing problems in several HAC vectors, due to the insertion of the transgenes close to a functional kinetochore consisting a large blocks of heterochromatin that could inactivate the gene, surrounding transgenes with insulators can protect them from the positional effects of the neighboring sequences at the insertional site of a transferred gene.24,25 It might also be possible to maintain long-term correction of defective genes because these vectors are mitotically stable throughout many cell divisions, at least in human cells. Such a capability would be advantageous over current gene delivery methods.
Figure 1.
Potential characteristics of human artificial chromosomes (HACs). (a) Method for constructing HACs. (b) Size limits for gene delivery vectors. Maximum deliverable DNA size in each vector is described. HAC vectors as well as chromosomes, can carry DNA larger than 1 Mb. The size limits depend on each vector. (c,d) Limitations and consequences of gene delivery with conventional vectors such as a virus or plasmid, and with HACs, respectively.
Various Types of HACs and Episomal Vectors
The de novo assembly of HACs using the bottom-up approach was developed in human fibrosarcoma HT1080 cells.16,17,26,27,28,29,30,31,32,33,34 In most cases, de novo-generated HACs range from 1 to 10 Mb in size, and have been shown to be mitotically stable. Recently, other systems for the construction of HACs have been developed to rapidly create bacterial artificial chromosome (BAC)-based HACs using the red-recombination system from bacteriophage λ,35 or using a modified bacterial Tn5 transposon.31 Utilization of invasive Escherichia coli systems may facilitate de novo HAC formation.36 Recently, a technique based on the HSV1 amplicon greatly improved de novo HAC formation protocols (i.e., much higher efficiency and applicability to many different cell lines other than HT1080).37
Conversely, HACs engineered via the top-down approach can also be constructed by telomere-associated chromosome fragmentation techniques in the homologous recombination-proficient chicken cell line, DT40.38 Such an approach can generate mitotically stable, linear mini-chromosomes. Initially, mini-chromosomes ranging in size from 0.5 to 10 Mb have been produced from both the human X39,40,41 and Y chromosomes.42,43,44,45,46 These mini-chromosomes retain a normal centromere and are mitotically stable in human cells with only minor rearrangements.
There are other types of gene delivery systems that aim to achieve episomal maintenance and introduction of large sized genomic DNA (>100 kb).47,48 They include utilization of (i) naturally occurring or patient-derived mini-chromosomes,18,49,50,51 (ii) neocentromere-based mini-chromosomes,52 (iii) murine satellite-based artificial chromosomes,53,54,55 and (iv) self-replicating viral vector amplicons.56,57,58,59 The following sections provide an overview and in depth discussions of the work on HACs with specified acceptor sites for delivery of desired gene(s).
HACs with acceptor sites
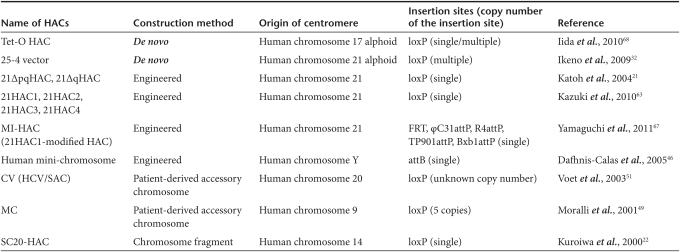
The HACs described above require a cloning site for inserting exogenic genes. Recently, HACs with an acceptor site(s) for gene delivery have been developed and characterized as follows. For example, the 21ΔqHAC and 21ΔpqHAC have been constructed by telomere-directed breakage of a human chromosome 21 (hChr. 21) in DT40 cells via the top-down approach.21 To construct this HAC vector, substantial segments of both the p- and q-arm of an hChr. 21 were deleted through two rounds of telomere-directed chromosome truncation. Next, a single loxP sequence (a target sequence for homologous recombination by Cre recombinase) with a neo gene lacking a promoter was introduced into a euchromatic region of the remaining q-arm. Therefore, at least in theory, any circular DNA (plasmid, BAC, P1 phage-derived artificial chromosome, or circular yeast artificial chromosome) with a loxP site and a promoter can restore the neo gene expression by Cre-mediated insertion at the loxP site of the 21ΔqHAC or 21ΔpqHAC. A summary of HACs with acceptor site(s) and their characteristics are described in Table 1. Single or multiple cloning site(s), including loxP, FRT, attP and attB,60,61,62 have been inserted into HAC vectors, including de novo-created HACs, engineered HACs, patient-derived chromosomes and natural chromosomal fragments. Several characteristics are needed for HAC vectors to be ideal for human gene and cell therapy, including physiological and functional expression of desired gene(s), independent maintenance of the HACs in the host cells, high mitotic stability of the HACs, at least in human cells, and harmlessness to the host cells or bodies. Since detailed characteristics of the HACs in Table 1 have not been compared among those in published papers, the most feasible HAC is currently uncertain. The recently developed 21HACs (including 21HAC1, 21HAC2, 21HAC3 and 21HAC4) may be very useful tools for human gene and cell therapy, since they exhibit many of the characteristics described above, and gene delivery has been accomplished using these vectors.63,64,65,66,67 The next sections mainly describe and discuss two of newly developed HAC vector systems, the 21HACs and tet-O-HAC.68
Table 1. List of human artificial chromosomes (HACs) with acceptor site (s) for gene delivery.
Characterization of two novel HAC systems
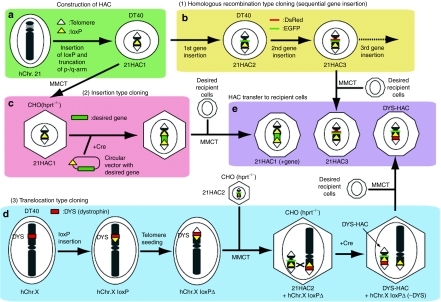
HAC vectors were developed from a normal hChr.14 or hChr.21 using an engineering approach (named SC20-HAC and 21ΔqHAC/21ΔpqHAC) that incorporates a loxP gene acceptor site.21,22 The SC20-HAC was transmittable through the germline and was rather mitotically stable in mice and cattle.22,69,70,71 Both 21ΔqHAC and 21ΔpqHAC were very stable, especially when introduced in human cell lines.21,24,72 However, SC20-HAC and 21ΔqHAC/21ΔpqHAC contain several structurally undefined regions carrying many endogenous genes, which causes a genetic imbalance in cells propagating these HACs. This may affect physiological gene expression patterns, resulting in abnormal development. Although these HACs showed significant potential for gene therapy and animal transgenesis, the ideal gene delivery vector should be structurally defined and should not contain endogenous genes from the original chromosome. Thus, a novel 21HAC vector was developed without known endogenous genes using the top-down approach63 (Figure 2a). This 21HAC was physically characterized using a transformation-associated recombination-cloning strategy followed by sequencing of transformation-associated recombination-BAC clones,73 confirming that no known endogenous genes remained in the 21HAC. Although the newer 21HAC could be producing unknown noncoding RNAs of physiological consequence, since non-α satellite-based 21 pericentromeric sequences remain on the 21HAC, any remaining pericentromeric non-α satellite-based sequences from the 21HAC can be removed prior to future clinical application. Since the suicide gene herpes simplex virus thymidine kinase, as a safeguard system against tumor formation, was also inserted into the HAC vector together with a loxP site, tumor cells containing the HAC carrying the suicide gene can be selectively killed by ganciclovir in vitro and in vivo.63,65 Thus, the resulting 21HAC vector contains four useful features: (i) it has a well-defined genetic architecture; (ii) it is episomally present, independent of the host chromosomes; (iii) it is mitotically stable in human somatic cells in vitro and mouse cells both in vitro and in vivo; and (iv) it has a system for safeguarding against tumor formation. Furthermore, any desired gene could be cloned into the 21HAC using the Cre-loxP system in Chinese hamster ovary cells, or by a homologous recombination system in DT40 cells.63 Using the homologous recombination system, two different vectors, each containing a desired gene, were inserted sequentially into 21HAC1 by homologous recombination in DT40 cells (Figure 2b). Two or more vectors containing desired genes can be inserted sequentially into the HAC. Using the Cre-loxP system, insertion-type cloning and translocation-type cloning were applied for gene cloning. Circular vectors containing desired genes, such as plasmids, P1 phage-derived artificial chromosomes and BACs, can be inserted into the HAC vector using insertion-type cloning (Figure 2c). Mb-sized genes, which cannot be cloned into the circular vectors, can be cloned into the HAC vector using translocation-type cloning64 (Figure 2d). More recently, a HAC vector containing five recombination platforms (πC31 attP, R4 attP, TP901-1 attP, Bxb1 attP and FRT; multi-integrase-HAC vector) was developed.67 The insertion frequency of target genes into each platform on multi-integrase-HAC using the de novo mammalian codon-optimized integrases, including πC31, R4, TP901-1 and Bxb1, was much higher than that using FLPe. Thus, five different circular vectors, such as plasmids and BACs containing genes of interest, can be cloned into the multi-integrase-HAC vector. Therefore, any combination of genes, including full-length genomic DNA, can in theory be cloned into the HAC derivatives by a combination of these cloning systems and transferred into a desired recipient cell type using the HAC (Figure 2e). Thus, these novel 21HAC vectors may be useful for gene and cell therapies as well as for animal transgenesis.
Figure 2.
An example for the construction of engineered human artificial chromosomes (HACs) via top-down approach and subsequent gene delivery. (a) HAC construction. 21HAC1 is generated by insertion of a loxP site into a pericentromeric region of the q-arm of an hChr. 21 and subsequent truncation of the p- and q-arms. (b) Homologous recombination type cloning (sequential gene insertion). The desired gene can be sequentially cloned into a specific site on the HAC in DT40 cells by homologous recombination. (c) Insertion-type cloning. A circular vector containing a loxP site and a desired gene can be cloned into a HAC in Chinese hamster ovary (CHO) (hprt−/−) cells by Cre-loxP mediated gene insertion with reconstitution of the HPRT gene. (d) Translocation-type cloning. An example of this method is the cloning of human dystrophin on the p-arm of a human X chromosome. Chromosome manipulation is carried out in homologous recombination-proficient DT40 cells. To clone the human dystrophin gene into the 21HAC2 vector, a loxP site is targeted to the proximal locus of the dystrophin gene on the human X chromosome. Extra genes distal to the dystrophin gene are deleted by the telomere-associated chromosome truncation. The modified human X chromosome fragment is transferred into CHO hybrids containing 21HAC2, including the loxP site by microcell-mediated chromosome transfer (MMCT). The large size of dystrophin gene (2.4 Mb) can be cloned into the 21HAC2 vector in CHO cells using Cre-loxP mediated chromosomal translocation (designated as DYS-HAC). (e) HAC transfer to recipient cells. HACs with gene(s) of interest can be transferred to desired recipient cells via MMCT.
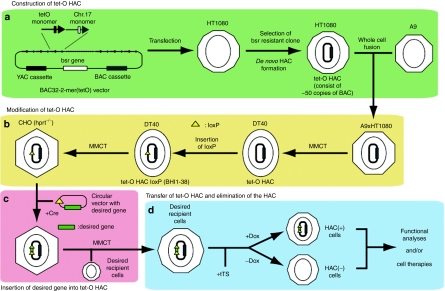
Recently, a HAC with a conditional centromere that includes the tetracycline operator (tet-O) sequence embedded in the alphoid DNA array has been generated74 (Figure 3a). This conditional centromere can be inactivated, loss of the alphoidtet-O HAC (tet-O HAC), by expression of tet-repressor fusion proteins. Since the desired gene cannot be inserted into the tet-O HAC without an acceptor site, such as loxP or FRT, the tet-O HAC vector was adapted for gene delivery and gene expression in human cells68 (Figure 3b). A loxP cassette was inserted into the tet-O HAC by homologous recombination in chicken DT40 cells following MMCT. The tet-O HAC with the loxP cassette was then transferred into Chinese hamster ovary cells. It has been shown that the enhanced green fluorescent protein (EGFP) transgene was efficiently and accurately incorporated into the tet-O HAC vector (Figure 3c). The EGFP transgene was stably expressed in human cells after transfer via MMCT, and the transgenes inserted into the tet-O HAC can be subsequently eliminated from cells by HAC loss due to centromere inactivation (Figure 3d). The tet-O HAC vector has significant advantages over other expression/cloning systems, because it provides a mechanism to compare the phenotype of a mammalian cell with or without a functional copy of any cloned gene of interest. Thus, a rigorous negative control for phenotypic changes attributed to expression of the cloned gene can be conducted easily in any population of dividing cells by simply inactivating the tet-O HAC centromere. Such controls are required for proper interpretation of gene function studies. In addition, the modified tet-O HAC vector can be used for experiments that require transient expression of a cloned gene of interest. For example, the generation of induced pluripotent stem (iPS) cells is possible through the transient expression of specific cellular factors, including OCT4, SOX2, KLF4, cMYC, and LIN28.75,76,77 In this case, HAC elimination and removal of the stem cell–inducing factors could provide a strategy to avoid insertional mutagenesis and cell transformation, complications that are frequently observed during cell reprogramming. Taken together, the natural human centromere sequence with α-satellite is retained on the linear telomere-capped 21HAC (engineered HAC), while the de novo-generated tet-O HAC is formed from transfected α-satellite DNA and is circular. An advantage of 21HAC and tet-O HAC is that they have the ability to control transgene number. Thus, the combination of the 21HACs and tet-O HAC will be useful tools for human gene and cell therapies, since the 21HACs are persistent in human cells and tet-O HAC is removable from the cells.
Figure 3.
An example of construction of de novo-generated human artificial chromosomes (HACs) via bottom-up approach and subsequent gene delivery. (a) Construction of tet-O HAC. The tet-O HAC is generated by transfection of a BAC containing tetO monomer (alphoidtet-O) and hChr. 17 monomer (hChr.17 alphoid) into human HT1080 cells. (b) Modification of tet-O HAC. Since the tet-O HAC cannot be transferred to DT40 from HT1080 directly, HT1080 (tet-O HAC) and A9 cells are fused, then the tetO-HAC are transferred to DT40 from the A9/HT1080 hybrid cells. A loxP site is inserted into the tet-O-HAC by homologous recombination in DT40 cells. The tet-O HAC with the loxP site is transferred to CHO (hprt−/−) cells to insert the desired gene. (c) Insertion of the desired gene into tet-O HAC. A circular vector containing a loxP site and a desired gene can be cloned into the HAC in CHO (hprt−/−) cells by Cre-loxP mediated gene insertion with reconstitution of the HPRT gene. (d) Transfer of tet-O HAC and elimination of the HAC. The tet-O HAC, with gene(s) of interest, can be transferred to desired recipient cells via microcell-mediated chromosome transfer (MMCT). After the expression of a chromatin modifier gene fused with tet-R (tTS), the HAC is maintained in the presence of doxycyclin (Dox) or the HAC is destabilized in the absence of Dox. HAC-positive or HAC-negative cells are utilized for functional analyses and/or cell therapies.
Chromosome Transfer into Desired Host Cells
The efficiency of chromosome transfer into the desired host cells is crucial when using any of the HACs described above. MMCT is a technique by which a chromosome(s) is moved from donor to recipient cells by microcell fusion.78,79 Polyethylene glycol (PEG) has conventionally been used as a fusogen, and has been employed very successfully in various genetic studies.80 However, PEG is not applicable for all types of recipient cell, due to its cell-type-dependent toxicity and low efficiency. Furthermore, PEG produces a low yield of microcell hybrids (10−6–10−5 per recipient cells). Although hemagglutinating virus of JAPAN envelope was utilized for MMCT, the transfer efficiency was approximately three to eight times higher than that of the PEG-mediated MMCT method.81 To harness the full potential of MMCT, a more efficient, less toxic fusion protocol is needed.
The measles virus (MV), which causes an acute contagious disease, has an envelope protein complex that is used for both virus attachment and membrane fusion.82 Thus, the feasibility of using the MV viral fusion machinery was tested as an alternative to PEG for microcell fusion. The introduction of genes encoding MV envelope proteins enabled rodent cells to produce fusogenic microcells that efficiently transmit donor chromosomes to recipient human cells expressing a high level of CD46.83 The maximum efficiency observed was 50 and 100 times greater than that using conventional PEG fusion. This MV-mediated MMCT method has several advantages over the conventional PEG-fusion method for chromosome transfer. First, microcell hybrids can be obtained from a low number of recipients, even from recipients that are too low in number for hybrids to be obtained by PEG-fusion, as long as the recipients express the CD46 receptor over a threshold density. Second, the procedure for microcell fusion is simple. The formation of microcell hybrids requires only the addition of prepared microcells to recipient cells. This ease of application, which avoids the laborious tasks of handling a highly viscous PEG solution and performing repeated washout steps, should improve the reproducibility of microcell fusion. The next issue for microcell fusion via an MV fusogen is whether the tropism for recipient cells can be altered from the default CD46 receptor to arbitrary receptors. Since engineering of viral tropism has been pursued for many gene therapy-based strategies,84,85,86,87,88,89,90 the modification of the viral tropism potentially enables retargeting of fusogenic microcells, not only to recipient cells of interest in vitro, but also to desired cells in vivo.
Using the MMCT technique, HACs cannot be directly transferred to recipient cells from donor cells that cannot form microcells. Recently, Ikeno and colleagues established an alternative transfer technology for the isolation and transfection of HACs into target cell lines.91 They isolated HACs from metaphase cells using a simple 25% sucrose cushion centrifugation under polyamine buffer conditions. Transfer of HACs into target cell lines was achieved using conventional transfection reagents, which enabled direct transfer of the HAC from a variety of donor cells, including those that could not form microcells. The efficiency of chromosome transfer was comparable to PEG fusion in MMCT.
In contrast, flow-sorted chromosome transfer is a modified method based on the isolation of the target chromosome from the host cells by flow sorting.92 During flow-sorted chromosome transfer, donor cells are first treated with iodo-deoxyuridine and subsequently blocked at mitotic metaphase by treatment with colchicine. These chromosomes are then stained with Hoechst 33258 and chromomycin A3, then harvested and purified from the background of host chromosomes. The efficiency of chromosome transfer using chromosome transfection and flow-sorted chromosome transfer are comparable to the normal MMCT method (10–6–10–4).93 Both MMCT and alternative HAC transfer protocols require improvement for higher efficiency.
Genes in HACs
The genes previously loaded on HAC vectors are listed in Table 2. Several studies have demonstrated the feasibility of de novo HAC construction for the delivery and expression of large human transgenes into human cell lines. The successful functional complementation of the hypoxanthine-guaninephosphoribosyltransferase (HPRT) gene has been observed in HPRT-deficient human cells.19,35,37,72,94 HPRT was utilized as a model gene for genetic complementation because it is easy to assay functional complementation of metabolic deficiencies in host cells by enumerating hypoxanthine-aminopterin-thymidine-resistant and 6-thioguanine-susceptible colonies. Although several entire human genomic DNA segments related to recessive hereditary disorders, including CFTR,95,96,97 β-globin34,91,98 and Factor IX,99 were successfully loaded into HACs, most of the studies simply showed the cloning and expression in nonaffected cell lines, such as HT1080 and Chinese hamster ovary. However, HACs containing genetic disorder-related genes will be useful tools for future treatment of their disorders. Ito et al. reported the treatment of nonalbumin rats by transplantation of immortalized hepatocytes using a de novo HAC with the SV40T antigen.100,101 Although several groups have reported functional analyses in vitro using de novo HACs, several factors limit the application of de novo-generated HACs as gene delivery vehicles. The most critical problems are their undefined structure and the unpredictable relationship between the input DNA and resultant HAC, especially in terms of their size and composition.47 This is due to de novo-generated HACs being composed of multimers of the original yeast artificial chromosome or BAC sequences used for transfection. On the other hand, the apparent formation of de novo-generated HACs from introduced DNA alone may be a favorable safety feature, although the structure should be defined before clinical use.
Table 2. List of genes delivered by human artificial chromosomes (HACs).
Several studies using the 21Δq HAC or 21Δpq HAC vectors have been published, including studies that report the following: (i) the persistent expression of the erythropoietin gene in normal human fibroblasts;102 (ii) the tetracycline-inducible expression of the DNA-Pkcs gene;103 (iii) the induction of tissue-specific expression of an EGFP reporter gene accompanying in vitro differentiation in human mesenchymal stem cells;24 (iv) a transformation-associated recombination-cloning mediated gene-cloning system;104 (v) exogenous gene expression and antigen-mediated growth regulation of human hematopoietic cells;105 (vi) heat-regulated production and secretion of insulin;106 (vii) antigen-mediated growth control of hybridoma cells;107 (viii) cell-type–specific gene expression and induction of immunoglobin secretion;108 (ix) telomerase-mediated life-span extension of human primary fibroblasts;109 (x) an evaluation system for bioactive substances;110 and (xi) a ready-made P1 phage-derived artificial chromosome insertion and correction of a genetic deficiency in multipotent germline stem cells.72
Recent studies for the treatment of a genetic disease, Duchenne muscular dystrophy (DMD) using the newly developed 21HAC2 has been reported.66 DMD is caused by dysfunction of the dystrophin gene.111 As some DMD patients show a large deletion in the DMD gene, these defects cannot be restored by exon-skipping approaches.112 Although several vectors have been developed for DMD gene therapy, no episomal vector containing the entire dystrophin genomic region has been reported, due to the extremely large size of this region (2.4 Mb).113 Thus, a 21HAC2 vector containing the entire dystrophin genomic region (DYS-HAC) has been developed for potential application in DMD gene therapy.64 iPS cells have great potential for gene therapy, as such cells can be generated from an individual's own tissues, and when reintroduced can contribute to the specialized function of any tissue.75,76,77 As a proof of concept, the complete correction of a genetic deficiency was shown in iPS cells derived from DMD model (mdx) mice and a human DMD patient using the DYS-HAC.66 Advances in the efficiency of methods used for differentiation and purification of stem cells, including embryonic stem (ES) and iPS cells, are anticipated, and the application of these methods to ES/iPS cells combined with HAC vector systems may enable the development of more sophisticated gene therapies. Thus, stem cells, potentially derived from multiple sources, combined with HAC-mediated gene delivery should permit safe treatment of various genetic defects. The next step in the future of gene therapy is to demonstrate functional restoration in vivo using animal models.
Animal Models
Another important application of HACs or hCFs (human chromosome fragments) is animal transgenesis. The ability of hCFs to act as vectors for introducing large stretches of human DNA into mice was first demonstrated in 1997.23 An hCF containing the human immunoglobulin gene locus was introduced into mouse ES cells, followed by the production of chimeric mice. Transferred hCFs were stably maintained as an extra chromosome in the somatic cells of mice and their human genes were expressed under proper tissue-specific regulation. In some cases they could be transmitted through the germline, resulting in the establishment of novel mouse strains (transchromosomic mice) containing a heritable hCF.23 Therefore, employing chromosome vectors to create transgenic animals is useful for overcoming the size constraints of cloned transgenes used in conventional techniques, and to facilitate functional studies of the human genome. However, it has been reported that the mitotic stability of hCFs in mice varies among chromosomes.69,70 Thus, it is difficult to stably maintain certain types of hCFs in order to perform functional analyses of several chromosomes in mice. Furthermore, it has proven to be difficult to introduce defined regions of hCFs into mice because fragmentation of hCFs can occur randomly. Thus, Kuroiwa and colleagues developed a chromosome-cloning system in which defined regions of human chromosomes can be cloned into a mitotically stable human mini-chromosome vector (hChr.14-derived SC20-HAC) in homologous recombination-proficient chicken DT40 cells.22 The stable SC20-HAC vector allowed a 10 Mb-sized region of the mitotically unstable human chromosome 22 to be stably maintained in mouse ES cells, as well as in mice. Furthermore, they demonstrated functional expression of human genes in mice with this construct. This study clearly demonstrated the possibility of expressing human antibodies in mice. This technology was also applied to cows with nuclear transfer of the fibroblasts containing the HAC.71,114 Furthermore, to generate humanized CYP3A mice for prediction of human xenobiotic metabolism, the region around the CYP3A cluster (~700 kb) in hChr. 7 was cloned into the SC20-HAC vector. Germline-transmittable CYP3A-HAC mice were generated that exhibited CYP3A gene expression in a tissue- and developmentally specific manner (Y. Kazuki, K. Kobayashi, T. Oshima, S. Aueviriyavit, S. Abe, M. Takiguchi et al. unpublished results). Furthermore, this strategy, using a chromosome-cloning system, was also utilized in constructing a HAC containing the entire 2.4 Mb human dystrophin gene described above.64 The availability of HAC vectors would be of great value in the construction of animals carrying human genetic elements to model specific diseases, or in the commercial production of various therapeutic/other products.
Another application of chromosome engineering technology is to generate animal models of human aneuploidy syndromes, which are caused by extra dosage of wild-type genes on human chromosomes. Trisomy of chromosome 21 is the most common live-born human aneuploidy and results in a constellation of features known as Down syndrome.115 Two groups have successfully generated transchromosomic Down syndrome model mice.116,117 These mice contain an extra hChr. 21 and show cardiac abnormalities and behavioral impairment similar to patients with Down syndrome. The critical difference between the two studies was the region of the hChr. 21 transmitted through the germline. Fisher's group succeeded in generating an aneuploid mouse that stably transmits a freely segregating, almost complete hChr. 21 through the germline.117 On the other hand, Oshimura's group generated germline-transmittable mice containing a partial fragment of hChr. 21.118 Transchromosomic mouse technology can be useful in the dissection of other human aneuploidies as well as to identify and map genes that contribute to aneuploidy diseases.
Possible Usage for Other Purposes
Several properties of HACs enable their potential use in a variety of applications. HACs could increase protein production efficiency since a HAC can be loaded with multiple copies of a particular gene. Most recently, we have compared the expression levels of HAC-derived transgene products with a transfected P1 phage-derived artificial chromosome containing the same gene. The former showed expression levels consistent with those of the original clones, even after 50 population doublings, whereas the latter showed a remarkable decrease in expression despite unvarying DNA content, indicating that the gene on the HAC is resistant to gene silencing (H. Kurosaki, M. Hiratsuka, N. Imaoka, Y. Iida, N. Uno, Y. Kazuki et al., unpublished results). The ability to insert different transgenes at multiple locations in the HAC, using site-specific recombinases, such as πC31, R4, TP901-1 and Bxb1, may also have benefits for screening the interaction of gene products.67 Most recently, several research groups have developed a technology to induce ES-like stem cells, so-called iPS cells, from mouse and human fibroblasts.75,76,77 A HAC vector containing the four initiation factors (Oct4, Sox2, c-Myc, and Klf4) and others that enhance pluripotency might be suitable for the development of iPS cells. As stated earlier, a HAC carrying herpes simplex virus thymidine kinase and other suicide genes with proper promoters may be useful in preventing tumor formation. Thus, HACs may prove to be powerful tools for the generation of iPS cells ideal for gene and cell therapies because of the following advantages: (i) efficient generation of iPS cells; (ii) no integration of the transgene into the host genome; (iii) efficient differentiation from iPS cells; (iv) efficient purification of differentiated cells; and (v) safeguarding against tumor formation.
Conclusions and Perspectives
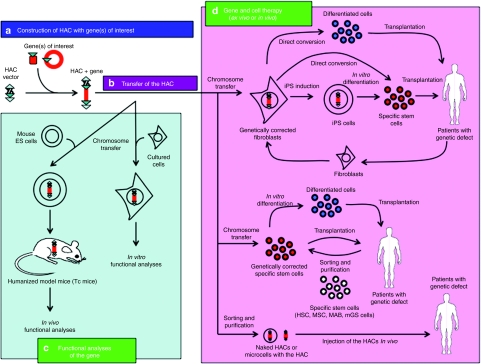
The chromosome vector systems offer complementary and desirable characteristics for use as gene delivery vectors to overcome various problems in existing viral and nonviral vector systems. HACs also have the advantages of being mitotically stable in the absence of selection and they have an indefinite cloning capacity, thus allowing for the insertion of all control elements for the correct expression of the transgene. A schematic diagram of the potential usage of the HAC vector system for functional analyses and for the treatment of genetic disorders is described in Figure 4. A feasible therapeutic application of HAC vectors would be the ex vivo transduction of a gene of interest by the transfer of a HAC into stem cells followed by autologous transplantation. Stem cells possess two characteristic features: the ability for self-renewal, and the ability for multilineage differentiation. In addition, stem cells, including hematopoietic stem cells,119,120 bone marrow-derived mesenchymal stem cells,24 mesoangioblasts,121 multipotent germline stem cells122 and iPS cells,75,76,77 can potentially avoid immune rejection due to their being obtained from the patient's own tissues, although stem cell–based therapies (with the exception of hematopoietic stem cells) are still in their infancy. Most recently, neurons, cardiomyocytes and blood progenitors were induced by defined factors from normal fibroblasts via a direct reprogramming (or direct conversion) approach.123,124,125 Thus, genetically corrected fibroblasts may be useful not only for the iPS production but also for the direct conversion to specific differentiated cells. The potential utility of HACs to correct a genetic defect has been demonstrated in cultured cells and animal models. However, prior to the use of HAC vectors in the clinical treatment of patients, their efficacy and safety as a treatment modality should be evaluated in animal models. Future research will focus on the efficient delivery of such vectors, not only to cells cultured in vitro but also to animal cells in vivo. Taken together, the various HAC vectors developed by several groups will be useful for designing HAC-based gene and cell therapies, as well as for the generation of humanized animal models. Thus, HACs may be designated as “multipotent vectors”.
Figure 4.
Schematic diagram of the human artificial chromosome (HAC) vector system for functional analyses and for the treatment of genetic disorders. (a) HAC construction with gene(s) of interest. (b) Transfer of the HAC to desired recipient cells. (c) Functional analyses of the gene (s) on the HAC. HACs containing desired gene(s) can be utilized for functional analyses in vitro and in vivo, including humanized animal models. (d) Gene and cell therapy (ex vivo or in vivo). HACs containing therapeutic gene(s) can be utilized for the treatment of patients with genetic disorder. Several approaches including ex vivo or in vivo therapies will be potentially utilized in the treatment of genetic diseases. For example, naked HACs or microcells with the HAC containing therapeutic gene(s) are directly injected to patient's tissues. HSC, hematopoietic stem cell; iPS, induced pluripotent stem; MAB, mesoangioblast; mGS, multipotent germline stem; MSC, mesenchymal stem cell.
Acknowledgments
This study was supported in part by JST, CREST (M.O. and Y.K.), and the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.O. and Y.K.), and the New Energy and Industrial Technology Development Organization (NEDO) from the Ministry of Economy, Trade and Industry of Japan (M.O.).
REFERENCES
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81 Pt 11:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- Gugala Z, Olmsted-Davis EA, Gannon FH, Lindsey RW., and, Davis AR. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther. 2003;10:1289–1296. doi: 10.1038/sj.gt.3302006. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP.et al. (2003Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer Mol Genet Metab 80148–158. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B., and, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Li Z, Düllmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J.et al. (2002Murine leukemia induced by retroviral gene marking Science 296497. [DOI] [PubMed] [Google Scholar]
- Challita PM., and, Kohn DB. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug CA, Cheshier S., and, Weissman IL. Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- Kafri T, Blömer U, Peterson DA, Gage FH., and, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S.et al. (2002Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes Mol Ther 6349–358. [DOI] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J.et al. (2003A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference Nat Genet 33401–406. [DOI] [PubMed] [Google Scholar]
- Wanisch K., and, Yáñez-Muñoz RJ. Integration-deficient lentiviral vectors: a slow coming of age. Mol Ther. 2009;17:1316–1332. doi: 10.1038/mt.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F.et al. (2010Transfusion independence and HMGA2 activation after gene therapy of human ß-thalassaemia Nature 467318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasik MB., and, McCray PB., Jr Integrase-defective lentiviral vectors: progress and applications. Gene Ther. 2010;17:150–157. doi: 10.1038/gt.2009.135. [DOI] [PubMed] [Google Scholar]
- Ikeno M, Grimes B, Okazaki T, Nakano M, Saitoh K, Hoshino H.et al. (1998Construction of YAC-based mammalian artificial chromosomes Nat Biotechnol 16431–439. [DOI] [PubMed] [Google Scholar]
- Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K., and, Willard HF. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet. 1997;15:345–355. doi: 10.1038/ng0497-345. [DOI] [PubMed] [Google Scholar]
- Guiducci C, Ascenzioni F, Auriche C, Piccolella E, Guerrini AM., and, Donini P. Use of a human minichromosome as a cloning and expression vector for mammalian cells. Hum Mol Genet. 1999;8:1417–1424. doi: 10.1093/hmg/8.8.1417. [DOI] [PubMed] [Google Scholar]
- Grimes BR, Schindelhauer D, McGill NI, Ross A, Ebersole TA., and, Cooke HJ. Stable gene expression from a mammalian artificial chromosome. EMBO Rep. 2001;2:910–914. doi: 10.1093/embo-reports/kve187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffery R., and, Choo KH. Strategies for engineering human chromosomes with therapeutic potential. J Gene Med. 2002;4:5–13. doi: 10.1002/jgm.236. [DOI] [PubMed] [Google Scholar]
- Katoh M, Ayabe F, Norikane S, Okada T, Masumoto H, Horike S.et al. (2004Construction of a novel human artificial chromosome vector for gene delivery Biochem Biophys Res Commun 321280–290. [DOI] [PubMed] [Google Scholar]
- Kuroiwa Y, Tomizuka K, Shinohara T, Kazuki Y, Yoshida H, Ohguma A.et al. (2000Manipulation of human minichromosomes to carry greater than megabase-sized chromosome inserts Nat Biotechnol 181086–1090. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Yoshida H, Uejima H, Kugoh H, Sato K, Ohguma A.et al. (1997Functional expression and germline transmission of a human chromosome fragment in chimaeric mice Nat Genet 16133–143. [DOI] [PubMed] [Google Scholar]
- Ren X, Katoh M, Hoshiya H, Kurimasa A, Inoue T, Ayabe F.et al. (2005A novel human artificial chromosome vector provides effective cell lineage-specific transgene expression in human mesenchymal stem cells Stem Cells 231608–1616. [DOI] [PubMed] [Google Scholar]
- Bayne RA, Broccoli D, Taggart MH, Thomson EJ, Farr CJ., and, Cooke HJ. Sandwiching of a gene within 12 kb of a functional telomere and alpha satellite does not result in silencing. Hum Mol Genet. 1994;3:539–546. doi: 10.1093/hmg/3.4.539. [DOI] [PubMed] [Google Scholar]
- Masumoto H, Ikeno M, Nakano M, Okazaki T, Grimes B, Cooke H.et al. (1998Assay of centromere function using a human artificial chromosome Chromosoma 107406–416. [DOI] [PubMed] [Google Scholar]
- Henning KA, Novotny EA, Compton ST, Guan XY, Liu PP., and, Ashlock MA. Human artificial chromosomes generated by modification of a yeast artificial chromosome containing both human alpha satellite and single-copy DNA sequences. Proc Natl Acad Sci USA. 1999;96:592–597. doi: 10.1073/pnas.96.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole TA, Ross A, Clark E, McGill N, Schindelhauer D, Cooke H.et al. (2000Mammalian artificial chromosome formation from circular alphoid input DNA does not require telomere repeats Hum Mol Genet 91623–1631. [DOI] [PubMed] [Google Scholar]
- Mejía JE, Alazami A, Willmott A, Marschall P, Levy E, Earnshaw WC.et al. (2002Efficiency of de novo centromere formation in human artificial chromosomes Genomics 79297–304. [DOI] [PubMed] [Google Scholar]
- Kouprina N, Ebersole T, Koriabine M, Pak E, Rogozin IB, Katoh M.et al. (2003Cloning of human centromeres by transformation-associated recombination in yeast and generation of functional human artificial chromosomes Nucleic Acids Res 31922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Stromberg G, Compitello G, Willard HF., and, Van Bokkelen G. Rapid creation of BAC-based human artificial chromosome vectors by transposition with synthetic alpha-satellite arrays. Nucleic Acids Res. 2005;33:587–596. doi: 10.1093/nar/gki207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M, Suzuki N, Hasegawa Y., and, Okazaki T. Manipulating transgenes using a chromosome vector. Nucleic Acids Res. 2009;37:e44. doi: 10.1093/nar/gkp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M, Inagaki H, Nagata K, Morita M, Ichinose H., and, Okazaki T. Generation of human artificial chromosomes expressing naturally controlled guanosine triphosphate cyclohydrolase I gene. Genes Cells. 2002;7:1021–1032. doi: 10.1046/j.1365-2443.2002.00580.x. [DOI] [PubMed] [Google Scholar]
- Basu J, Compitello G, Stromberg G, Willard HF., and, Van Bokkelen G. Efficient assembly of de novo human artificial chromosomes from large genomic loci. BMC Biotechnol. 2005;5:21. doi: 10.1186/1472-6750-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzamanis G, Cheung W, Abdulrazzak H, Perez-Luz S, Howe S, Cooke H.et al. (2005Construction of human artificial chromosome vectors by recombineering Gene 35129–38. [DOI] [PubMed] [Google Scholar]
- Narayanan K., and, Warburton PE. DNA modification and functional delivery into human cells using Escherichia coli DH10B. Nucleic Acids Res. 2003;31:e51. doi: 10.1093/nar/gng051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moralli D, Simpson KM, Wade-Martins R., and, Monaco ZL. A novel human artificial chromosome gene expression system using herpes simplex virus type 1 vectors. EMBO Rep. 2006;7:911–918. doi: 10.1038/sj.embor.7400768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerstedde JM., and, Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- Farr CJ, Stevanovic M, Thomson EJ, Goodfellow PN., and, Cooke HJ. Telomere-associated chromosome fragmentation: applications in genome manipulation and analysis. Nat Genet. 1992;2:275–282. doi: 10.1038/ng1292-275. [DOI] [PubMed] [Google Scholar]
- Mills W, Critcher R, Lee C., and, Farr CJ. Generation of an approximately 2.4 Mb human X centromere-based minichromosome by targeted telomere-associated chromosome fragmentation in DT40. Hum Mol Genet. 1999;8:751–761. doi: 10.1093/hmg/8.5.751. [DOI] [PubMed] [Google Scholar]
- Spence JM, Critcher R, Ebersole TA, Valdivia MM, Earnshaw WC, Fukagawa T.et al. (2002Co-localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha-satellite array EMBO J 215269–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Barnett MA, Burgtorf C, Shaw P, Buckle VJ., and, Brown WR. Dissecting the centromere of the human Y chromosome with cloned telomeric DNA. Hum Mol Genet. 1994;3:1227–1237. doi: 10.1093/hmg/3.8.1227. [DOI] [PubMed] [Google Scholar]
- Heller R, Brown KE, Burgtorf C., and, Brown WR. Mini-chromosomes derived from the human Y chromosome by telomere directed chromosome breakage. Proc Natl Acad Sci USA. 1996;93:7125–7130. doi: 10.1073/pnas.93.14.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MH, Mee PJ, Nichols J, Yang J, Brook F, Gardner RL.et al. (2000A structurally defined mini-chromosome vector for the mouse germ line Curr Biol 1031–34. [DOI] [PubMed] [Google Scholar]
- Yang JW, Pendon C, Yang J, Haywood N, Chand A., and, Brown WR. Human mini-chromosomes with minimal centromeres. Hum Mol Genet. 2000;9:1891–1902. doi: 10.1093/hmg/9.12.1891. [DOI] [PubMed] [Google Scholar]
- Dafhnis-Calas F, Xu Z, Haines S, Malla SK, Smith MC., and, Brown WR. Iterative in vivo assembly of large and complex transgenes by combining the activities of phiC31 integrase and Cre recombinase. Nucleic Acids Res. 2005;33:e189. doi: 10.1093/nar/gni192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J., and, Willard HF. Human artificial chromosomes: potential applications and clinical considerations. Pediatr Clin North Am. 2006;53:843–53, viii. doi: 10.1016/j.pcl.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Monaco ZL., and, Moralli D. Progress in artificial chromosome technology. Biochem Soc Trans. 2006;34 Pt 2:324–327. doi: 10.1042/BST20060324. [DOI] [PubMed] [Google Scholar]
- Moralli D, Vagnarelli P, Bensi M, De Carli L., and, Raimondi E. Insertion of a loxP site in a size-reduced human accessory chromosome. Cytogenet Cell Genet. 2001;94:113–120. doi: 10.1159/000048801. [DOI] [PubMed] [Google Scholar]
- Voet T, Vermeesch J, Carens A, Dürr J, Labaere C, Duhamel H.et al. (2001Efficient male and female germline transmission of a human chromosomal vector in mice Genome Res 11124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet T, Schoenmakers E, Carpentier S, Labaere C., and, Marynen P. Controlled transgene dosage and PAC-mediated transgenesis in mice using a chromosomal vector. Genomics. 2003;82:596–605. doi: 10.1016/s0888-7543(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Saffery R, Wong LH, Irvine DV, Bateman MA, Griffiths B, Cutts SM.et al. (2001Construction of neocentromere-based human minichromosomes by telomere-associated chromosomal truncation Proc Natl Acad Sci USA 985705–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenius H, Szeles A, Keresö J, Csonka E, Praznovszky T, Imreh S.et al. (1999Stability of a functional murine satellite DNA-based artificial chromosome across mammalian species Chromosome Res 73–7. [DOI] [PubMed] [Google Scholar]
- Lindenbaum M, Perkins E, Csonka E, Fleming E, Garcia L, Greene A.et al. (2004A mammalian artificial chromosome engineering system (ACE System) applicable to biopharmaceutical protein production, transgenesis and gene-based cell therapy Nucleic Acids Res 32e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, MacDonald N, Perkins E, DeJong G, Perez C., and, Lindenbaum M. Retrofitting of a satellite repeat DNA-based murine artificial chromosome (ACes) to contain loxP recombination sites. Gene Ther. 2002;9:719–723. doi: 10.1038/sj.gt.3301757. [DOI] [PubMed] [Google Scholar]
- Black J., and, Vos JM. Establishment of an oriP/EBNA1-based episomal vector transcribing human genomic beta-globin in cultured murine fibroblasts. Gene Ther. 2002;9:1447–1454. doi: 10.1038/sj.gt.3301808. [DOI] [PubMed] [Google Scholar]
- Wang J., and, Vos JM. Infectious Epstein-Barr virus vectors for episomal gene therapy. Meth Enzymol. 2002;346:649–660. doi: 10.1016/s0076-6879(02)46083-1. [DOI] [PubMed] [Google Scholar]
- Senior SL., and, Wade-Martins R. Herpes simplex virus type 1 amplicon vectors for the infectious delivery and expression of genomic DNA loci. Curr Opin Mol Ther. 2005;7:337–345. [PubMed] [Google Scholar]
- Hibbitt OC., and, Wade-Martins R. Delivery of large genomic DNA inserts >100 kb using HSV-1 amplicons. Curr Gene Ther. 2006;6:325–336. doi: 10.2174/156652306777592054. [DOI] [PubMed] [Google Scholar]
- Groth AC., and, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- Russell JP, Chang DW, Tretiakova A., and, Padidam M. Phage Bxb1 integrase mediates highly efficient site-specific recombination in mammalian cells. Biotechniques. 2006;40:460, 462, 464. doi: 10.2144/000112150. [DOI] [PubMed] [Google Scholar]
- Wirth D, Gama-Norton L, Riemer P, Sandhu U, Schucht R., and, Hauser H. Road to precision: recombinase-based targeting technologies for genome engineering. Curr Opin Biotechnol. 2007;18:411–419. doi: 10.1016/j.copbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Kazuki Y, Hoshiya H, Takiguchi M, Abe S, Iida Y, Osaki M.et al. (2011Refined human artificial chromosome vectors for gene therapy and animal transgenesis Gene Ther 18384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiya H, Kazuki Y, Abe S, Takiguchi M, Kajitani N, Watanabe Y.et al. (2009A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene Mol Ther 17309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita Y, Kamitani H, Mamun MH, Wasita B, Kazuki Y, Hiratsuka M.et al. (2010A gene delivery system with a human artificial chromosome vector based on migration of mesenchymal stem cells towards human glioblastoma HTB14 cells Neurol Res 32429–437. [DOI] [PubMed] [Google Scholar]
- Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H.et al. (2010Complete genetic correction of ips cells from Duchenne muscular dystrophy Mol Ther 18386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kazuki Y, Nakayama Y, Nanba E, Oshimura M., and, Ohbayashi T. A method for producing transgenic cells using a multi-integrase system on a human artificial chromosome vector. PLoS ONE. 2011;6:e17267. doi: 10.1371/journal.pone.0017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida Y, Kim JH, Kazuki Y, Hoshiya H, Takiguchi M, Hayashi M.et al. (2010Human artificial chromosome with a conditional centromere for gene delivery and gene expression DNA Res 17293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Tomizuka K, Takehara S, Yamauchi K, Katoh M, Ohguma A.et al. (2000Stability of transferred human chromosome fragments in cultured cells and in mice Chromosome Res 8713–725. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Shinohara T, Yoshida H, Uejima H, Ohguma A, Tanaka S.et al. (2000Double trans-chromosomic mice: maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies Proc Natl Acad Sci USA 97722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa Y, Kasinathan P, Choi YJ, Naeem R, Tomizuka K, Sullivan EJ.et al. (2002Cloned transchromosomic calves producing human immunoglobulin Nat Biotechnol 20889–894. [DOI] [PubMed] [Google Scholar]
- Kazuki Y, Hoshiya H, Kai Y, Abe S, Takiguchi M, Osaki M.et al. (2008Correction of a genetic defect in multipotent germline stem cells using a human artificial chromosome Gene Ther 15617–624. [DOI] [PubMed] [Google Scholar]
- Kouprina N., and, Larionov V. TAR cloning: insights into gene function, long-range haplotypes and genome structure and evolution. Nat Rev Genet. 2006;7:805–812. doi: 10.1038/nrg1943. [DOI] [PubMed] [Google Scholar]
- Nakano M, Cardinale S, Noskov VN, Gassmann R, Vagnarelli P, Kandels-Lewis S.et al. (2008Inactivation of a human kinetochore by specific targeting of chromatin modifiers Dev Cell 14507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S.et al. (2007Induced pluripotent stem cell lines derived from human somatic cells Science 3181917–1920. [DOI] [PubMed] [Google Scholar]
- Koi M, Shimizu M, Morita H, Yamada H., and, Oshimura M. Construction of mouse A9 clones containing a single human chromosome tagged with neomycin-resistance gene via microcell fusion. Jpn J Cancer Res. 1989;80:413–418. doi: 10.1111/j.1349-7006.1989.tb02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier RE., and, Ruddle FH. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci USA. 1977;74:319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., and, Shen MH. Polyethylene glycol-mediated cell fusion. Methods Mol Biol. 2006;325:59–66. doi: 10.1385/1-59745-005-7:59. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Ren X, Katoh M, Miyata K, Fukushima H, Inoue T.et al. (2006A new method of microcell-mediated transfer of human artificial chromosomes using a hemagglutinating virus of Japan envelope Chromosome Sci 965–73.. [Google Scholar]
- Navaratnarajah CK, Leonard VH., and, Cattaneo R. Measles virus glycoprotein complex assembly, receptor attachment, and cell entry. Curr Top Microbiol Immunol. 2009;329:59–76. doi: 10.1007/978-3-540-70523-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Kazuki Y, Kazuki K, Kajitani N, Takiguchi M, Nakayama Y.et al. (2010Exploitation of the interaction of measles virus fusogenic envelope proteins with the surface receptor CD46 on human cells for microcell-mediated chromosome transfer BMC Biotechnol 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis JM, Stoff-Khalili MA., and, Curiel DT. Oncolytic adenoviruses - selective retargeting to tumor cells. Oncogene. 2005;24:7775–7791. doi: 10.1038/sj.onc.1209044. [DOI] [PubMed] [Google Scholar]
- Järås M, Brun AC, Karlsson S., and, Fan X. Adenoviral vectors for transient gene expression in human primitive hematopoietic cells: applications and prospects. Exp Hematol. 2007;35:343–349. doi: 10.1016/j.exphem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buchholz CJ, Mühlebach MD., and, Cichutek K. Lentiviral vectors with measles virus glycoproteins - dream team for gene transfer. Trends Biotechnol. 2009;27:259–265. doi: 10.1016/j.tibtech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Schneider U, Bullough F, Vongpunsawad S, Russell SJ., and, Cattaneo R. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol. 2000;74:9928–9936. doi: 10.1128/jvi.74.21.9928-9936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T.et al. (2004Antibody-targeted cell fusion Nat Biotechnol 22331–336. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD.et al. (2005Rescue and propagation of fully retargeted oncolytic measles viruses Nat Biotechnol 23209–214. [DOI] [PubMed] [Google Scholar]
- Jing Y, Tong C, Zhang J, Nakamura T, Iankov I, Russell SJ.et al. (2009Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor Cancer Res 691459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Itou T, Hasegawa Y, Okazaki T., and, Ikeno M. Cell to cell transfer of the chromatin-packaged human beta-globin gene cluster. Nucleic Acids Res. 2010;38:e33. doi: 10.1093/nar/gkp1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deJong G, Telenius AH, Telenius H, Perez CF, Drayer JI., and, Hadlaczky G. Mammalian artificial chromosome pilot production facility: large-scale isolation of functional satellite DNA-based artificial chromosomes. Cytometry. 1999;35:129–133. [PubMed] [Google Scholar]
- de Jong G, Telenius A, Vanderbyl S, Meitz A., and, Drayer J. Efficient in-vitro transfer of a 60-Mb mammalian artificial chromosome into murine and hamster cells using cationic lipids and dendrimers. Chromosome Res. 2001;9:475–485. doi: 10.1023/a:1011680529073. [DOI] [PubMed] [Google Scholar]
- Mejía JE, Willmott A, Levy E, Earnshaw WC., and, Larin Z. Functional complementation of a genetic deficiency with human artificial chromosomes. Am J Hum Genet. 2001;69:315–326. doi: 10.1086/321977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriche C, Carpani D, Conese M, Caci E, Zegarra-Moran O, Donini P.et al. (2002Functional human CFTR produced by a stable minichromosome EMBO Rep 3862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laner A, Goussard S, Ramalho AS, Schwarz T, Amaral MD, Courvalin P.et al. (2005Bacterial transfer of large functional genomic DNA into human cells Gene Ther 121559–1572. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Braz C, Cattani S, Ramalho A, Christan S, Edlinger M.et al. (2010Escherichia coli-cloned CFTR loci relevant for human artificial chromosome therapy Hum Gene Ther 211077–1092. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nishii K, Okazaki T., and, Ikeno M. Human artificial chromosomes constructed using the bottom-up strategy are stably maintained in mitosis and efficiently transmissible to progeny mice. J Biol Chem. 2006;281:26615–26623. doi: 10.1074/jbc.M603053200. [DOI] [PubMed] [Google Scholar]
- Breman AM, Steiner CM, Slee RB., and, Grimes BR. Input DNA ratio determines copy number of the 33 kb Factor IX gene on de novo human artificial chromosomes. Mol Ther. 2008;16:315–323. doi: 10.1038/sj.mt.6300361. [DOI] [PubMed] [Google Scholar]
- Ito M, Ito R, Yoshihara D, Ikeno M, Kamiya M, Suzuki N.et al. (2008Immortalized hepatocytes using human artificial chromosome Cell Transplant 17165–171. [DOI] [PubMed] [Google Scholar]
- Ito M, Ikeno M, Nagata H, Yamamoto T, Hiroguchi A, Fox IJ.et al. (2009Treatment of nonalbumin rats by transplantation of immortalized hepatocytes using artificial human chromosome Transplant Proc 41422–424. [DOI] [PubMed] [Google Scholar]
- Kakeda M, Hiratsuka M, Nagata K, Kuroiwa Y, Kakitani M, Katoh M.et al. (2005Human artificial chromosome (HAC) vector provides long-term therapeutic transgene expression in normal human primary fibroblasts Gene Ther 12852–856. [DOI] [PubMed] [Google Scholar]
- Otsuki A, Tahimic CG, Tomimatsu N, Katoh M, Chen DJ, Kurimasa A.et al. (2005Construction of a novel expression system on a human artificial chromosome Biochem Biophys Res Commun 3291018–1025. [DOI] [PubMed] [Google Scholar]
- Ayabe F, Katoh M, Inoue T, Kouprina N, Larionov V., and, Oshimura M. A novel expression system for genomic DNA loci using a human artificial chromosome vector with transformation-associated recombination cloning. J Hum Genet. 2005;50:592–599. doi: 10.1007/s10038-005-0300-6. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kunisato A, Kawahara M, Tahimic CG, Ren X, Ueda H.et al. (2006Exogenous gene expression and growth regulation of hematopoietic cells via a novel human artificial chromosome J Hum Genet 51147–150. [DOI] [PubMed] [Google Scholar]
- Suda T, Katoh M, Hiratsuka M, Takiguchi M, Kazuki Y, Inoue T.et al. (2006Heat-regulated production and secretion of insulin from a human artificial chromosome vector Biochem Biophys Res Commun 3401053–1061. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Inoue T, Ren X, Sogo T, Yamada H, Katoh M.et al. (2007Antigen-mediated growth control of hybridoma cells via a human artificial chromosome Biochim Biophys Acta 1770206–212. [DOI] [PubMed] [Google Scholar]
- Yamada H, Li YC, Nishikawa M, Oshimura M., and, Inoue T. Introduction of a CD40L genomic fragment via a human artificial chromosome vector permits cell-type-specific gene expression and induces immunoglobulin secretion. J Hum Genet. 2008;53:447–453. doi: 10.1007/s10038-008-0268-0. [DOI] [PubMed] [Google Scholar]
- Shitara S, Kakeda M, Nagata K, Hiratsuka M, Sano A, Osawa K.et al. (2008Telomerase-mediated life-span extension of human primary fibroblasts by human artificial chromosome (HAC) vector Biochem Biophys Res Commun 369807–811. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tsuji S, Kazuki Y, Noguchi M, Arifuku I, Umebayashi Y.et al. (2010Development of evaluation system for bioactive substances using human artificial chromosome-mediated osteocalcin gene expression J Biochem 14829–34. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP., and, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P., and, Chamberlain JS. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim Biophys Acta. 2007;1772:243–262. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C., and, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Kuroiwa Y, Kasinathan P, Sathiyaseelan T, Jiao JA, Matsushita H, Sathiyaseelan J.et al. (2009Antigen-specific human polyclonal antibodies from hyperimmunized cattle Nat Biotechnol 27173–181. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A., and, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5:725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Tomizuka K, Miyabara S, Takehara S, Kazuki Y, Inoue J.et al. (2001Mice containing a human chromosome 21 model behavioral impairment and cardiac anomalies of Down's syndrome Hum Mol Genet 101163–1175. [DOI] [PubMed] [Google Scholar]
- O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S.et al. (2005An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes Science 3092033–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuki Y, Shinohara T, Tomizuka K, Katoh M, Ohguma A, Ishida I.et al. (2001Germline transmission of a transferred human chromosome 21 fragment in transchromosomal mice J Hum Genet 46600–603. [DOI] [PubMed] [Google Scholar]
- Tjønnfjord GE, Steen R, Veiby OP, Friedrich W., and, Egeland T. Evidence for engraftment of donor-type multipotent CD34+ cells in a patient with selective T-lymphocyte reconstitution after bone marrow transplantation for B-SCID. Blood. 1994;84:3584–3589. [PubMed] [Google Scholar]
- Kohn DB, Weinberg KI, Nolta JA, Heiss LN, Lenarsky C, Crooks GM.et al. (1995Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency Nat Med 11017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A.et al. (2006Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs Nature 444574–579. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H.et al. (2004Generation of pluripotent stem cells from neonatal mouse testis Cell 1191001–1012. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC., and, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG.et al. (2010Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors Cell 142375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A.et al. (2010Direct conversion of human fibroblasts to multilineage blood progenitors Nature 468521–526. [DOI] [PubMed] [Google Scholar]