Abstract
Identification of new techniques to express proteins into mammal cells is of particular interest for both research and medical purposes. The present study describes the use of engineered vesicles to deliver exogenous proteins into human cells. We show that overexpression of the spike glycoprotein of the vesicular stomatitis virus (VSV-G) in human cells induces the release of fusogenic vesicles named gesicles. Biochemical and functional studies revealed that gesicles incorporated proteins from producer cells and could deliver them to recipient cells. This protein-transduction method allows the direct transport of cytoplasmic, nuclear or surface proteins in target cells. This was demonstrated by showing that the TetR transactivator and the receptor for the murine leukemia virus (MLV) envelope [murine cationic amino acid transporter-1 (mCAT-1)] were efficiently delivered by gesicles in various cell types. We further shows that gesicle-mediated transfer of mCAT-1 confers to human fibroblasts a robust permissiveness to ecotropic vectors, allowing the generation of human-induced pluripotent stem cells in level 2 biosafety facilities. This highlights the great potential of mCAT-1 gesicles to increase the safety of experiments using retro/lentivectors. Besides this, gesicles is a versatile tool highly valuable for the nongenetic delivery of functions such as transcription factors or genome engineering agents.
Introduction
Improving the methods for expressing proteins into human cells is of major interest for research and medical purposes. In spite of constant evolution of transfection methods and performances of viral vectors, efficiencies of these approaches can drop dramatically under specific conditions especially in primary cells. Furthermore, production and handling of virus-derived particles for gene delivery may require an access to biosafety level 3 laboratory facilities (BSL-3). Although gene transfer procedures are robust in many cases, they hardly allow a time- and dose-controlled expression of the exogenous protein, which remains an issue in numerous studies. These limitations motivated the exploration of alternative approaches to modify cell functions.
Among these, direct transfer of exogenous proteins into mammalian cells has been previously assessed by using penetrating peptides1,2 and proteoliposomes.3 However, these techniques require the production and tedious purification of recombinant proteins. Vesicular material derived from cells expressing viral proteins (virus-like particles) are also used as protein vehicles to elicit immune response4,5,6 or to deliver proteins embedded with a gammaretrovirus structural polyprotein.7
We report here the characterization and the use of another type of protein-carrying vesicles prepared from conditioned medium of cells expressing the envelope glycoprotein of vesicular stomatitis virus (VSV-G). We show that VSV-G overexpression promotes the release of vesicles that incorporate proteins from producer cells. Due to the binding and fusion properties of this envelope, these vesicles can efficiently transfer their cargo into recipient cells, including primary fibroblasts and peripheral blood mononuclear cells (PBMCs). This protein delivery technique based on fusogenic vesicles has been used for the transfer of membrane, cytoplasmic, and nuclear proteins.
Results
VSV-G overexpression in human cells promotes the release of a protein transfer agent
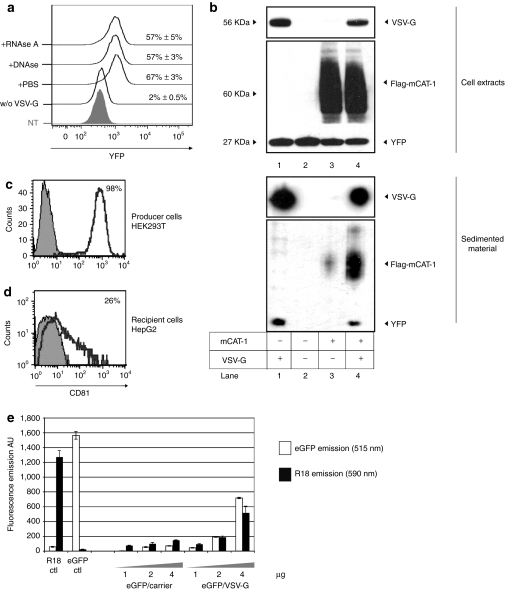
Besides protein delivery by natural exosomes,8,9,10 previous works described the unexpected transfer of proteins by agents coprepared with retroviral vectors.11,12 This phenomenon named pseudotransduction was associated with the use of concentrated VSV-G coated retroviral particles. This prompted us to assess whether expression of this particular viral protein could induce the release of a material responsible for protein delivery. To test this hypothesis, HEK-293T stably expressing yellow fluorescence protein (YFP) (293T-Y) were transfected with VSV-G and conditioned supernatant was harvested 2 days later, filtered, and concentrated by ultracentrifugation. As a control, supernatant from mock-transfected 293T-Y cells was similarly processed. Normalized amounts of both preparations were next incubated with target-cells and YFP transfer was monitored 24 hours later in recipient cells. Figure 1a shows that YFP was transferred only with the sedimented material prepared from VSV-G-expressing cells. Treatment of the YFP/VSV-G agent with RNAseA or DNAse did not significantly affect this result, suggesting a direct protein transfer. Biochemical analysis of both preparations revealed that YFP was highly sedimented in the VSV-G condition (Figure 1b, lanes 1 and 2) as compared with the mock-transfected condition. A similar result was obtained when YFP was co-transfected with VSV-G in producer cells and extended to other fluorescent proteins such as enhanced green fluorescent protein (eGFP) (Figure 1e).
Figure 1.
Vesicular stomatitis virus (VSV-G) promotes the cell-release of a sedimentable agent capable of pseudotransduction. (a) FACS-analyzed pseudotransduction mediated by the VSVG/YFP agent. Two micrograms of sedimented material prepared from VSV-G/YFP cells were treated with RNAse A (10 µg/ml), DNAse I (50 U/ml) or with phosphate-buffered saline (PBS) before incubation with recipient HEK-293T cells. Material shedding from mock-transfected yellow fluorescence protein (YFP)-cells was similarly prepared and incubated with target cells, but failed at transferring YFP (w/o VSV-G). Histogram for nontreated cells is shown (NT). YFP histograms in gated populations and percentages of YFP+ cells are given for each condition. (b) Immunoblot analysis of the sedimented materials shedding from YFP-HEK-293T cells transfected with VSV-G (lane 1), mock-transfected (lane 2), flag-murine cationic amino acid transporter-1 (mCAT-1) (lane 3), flag-mCAT-1 and VSV-G (lane 4). Immunostaining of VSV-G, flag-mCAT-1, and YFP were performed. Each lane was loaded with 5 µg of protein as dosed by Bradford. Extracts from producer cells were similarly analyzed (upper panel). (c) CD81 expression on HEK-293T producer cells (open line). An isotypic control was used as a control staining (gray-filled histogram). (d) CD81 expression on HepG2 cells after incubation with a concentrated material derived from VSV-G-expressing producer cells (open line). Staining of nontreated HepG2 cell is shown (gray-filled histogram). (e) Fluorometric analysis of the VSV-G induced material produced from cells expressing enhanced green fluorescent protein (eGFP) and probed with a membrane tracer (R18). Producer cells were treated with octadecyl-Rhodamine B (R18) prior VSV-G transfection and sedimentation of the shedding material. Increasing amounts of the resulting material (eGFP/VSV-G) were analyzed by fluorometry for eGFP- and R18-emission. A preparation produced without VSV-G was also analyzed (eGFP/carrrier). eGFP-free gesicles loaded with R18 (R18 ctl) and R18-free gesicles-containing eGFP (eGFP ctl) were used as controls, showing the absence of overlapping between the two spectra. The experiments were performed in triplicate and the data represent means ± s.d.
To further study the protein transfer achieved by the VSV-G-induced material, we monitored the delivery of the human CD81 antigen, a tetraspanin protein highly expressed in HEK-293T (Figure 1c). Conditioned supernatant from VSV-G-expressing HEK-293T was added to the culture medium of HepG2 cells, a human hepatic cell line lacking CD81. Cells were next washed and cultivated for 24 hours before CD81 staining. Figure 1d shows that this treatment resulted in an efficient transfer of CD81 to recipient cells.
Altogether these data indicate that expression of VSV-G in human HEK-293T cells induces the release of a sedimentable agent containing cytoplasmic and surface proteins derived from producer cells and able to transfer its cargo into recipient cells.
As previously reported in another cell type,13 we observed that the shedding of cell membranes is enhanced by VSV-G expression (Figure 1e), supporting the notion that the VSV-G-induced material produced from HEK-293T is vesicular. This vehicle will be hereafter named as “X-gesicle,” where X is the protein of interest coexpressed with VSV-G in producer cells.
Gesicle-mediated transfer of mCAT-1 into human cells
As very different proteins like YFP and CD81 could be transferred into target cells by gesicles, we reasoned that a large variety of proteins overexpressed in producer cells could be similarly incorporated in fusogenic vesicles and delivered. This hypothesis was tested using the murine cationic amino acid transporter (mCAT-1, Slc7a1) as a first model. This protein is also known as the receptor for the murine leukemia virus (MLV) ecotropic envelope.14,15 A plasmid encoding a flagged version of mCAT-1 was co-transfected with VSV-G into 293T-Y cells. mCAT-1 gesicles were next prepared as described (Supplementary Figure S1) and analyzed by western blot. As shown in Figure 1b (lanes 3 and 4), the sedimented sample was enriched in mCAT-1 when prepared from VSV-G-expressing cells. We further noted that mCAT-1 gesicles also contained YFP (Figure 1b, lane 4), showing that VSV-G vesicles can incorporate multiple proteins from different cell compartments.
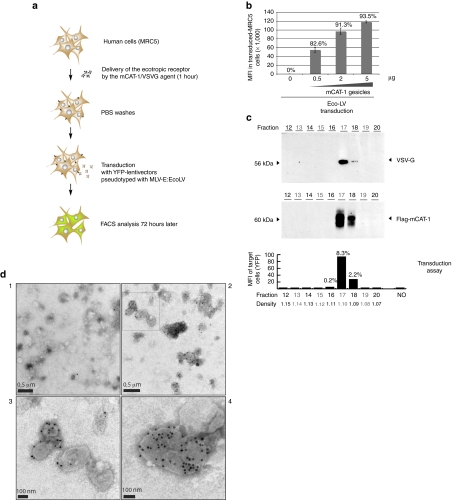
To evaluate the capacity of mCAT-1 gesicles to transfer the receptor function, we developed a transduction assay based on YFP-encoding lentivectors pseudotyped with the MLV ecotropic envelope (Figure 2a). Due to the particular tropism of this glycoprotein, these lentivectors can exclusively transduce mouse/rat cells or modified human cells expressing mCAT-1. If mCAT-1-gesicles deliver the receptor on the surface of human recipient-cells, these cells should become permissive to an ecotropic transduction.
Figure 2.
Delivery of murine cationic amino acid transporter-1 (mCAT-1) in human cells by characterized gesicles. (a) Principle of the ecotropic transduction assay using simian immunodeficiency virus (SIV)-derived lentivectors pseudotyped with the murine leukemia virus (MLV)-E envelope (EcoLV). (b) Ecotropic transduction assay in human MRC-5 fibroblasts treated with increasing amounts of mCAT-1 gesicles. Seventy hours after transduction [multiplicity of infection (MOI) 40, 43 transducing units (TU)/pg P24], mean fluorescence intensity (MFIs) of gated populations and percentage of transduced cells were measured by FACS. The error bars represent the s.d. of triplicate MFI values. (c) Fractionation of the mCAT-1/vesicular stomatitis virus (VSV-G) pellet on a iodixanol gradient. After ultracentrifugation, fractions were collected and analyzed by immunoblotting. VSV-G and mCAT-1 proteins were immunodetected mainly in fractions 17 and 18. mCAT-1 transfer activity of each fraction was measured by an ecotropic transduction using HEK-293T target cells. Percentages of transduced cells and MFI are given. Nonrepresented fractions 1–11 revealed no staining neither receptor-transfer activity. Density of each fraction is indicated. (d) Electron microscopy observation of mCAT-1 gesicles after immunostaining with a control antibody (panel 1) or an anti-flag antibody recognizing flag-mCAT-1 (panels 2–4). A higher magnification of the upper-left region of panel 2 (open square) is shown in panel 3.
A large batch of mCAT-1 gesicles was prepared from HEK-293T cells (Supplementary Figure S1a) and increasing amounts of this preparation were incubated with MRC-5 cells prior the ecotropic transduction assay. Transduction efficiencies obtained with this method are shown in Figure 2b. While nontreated cells were restrictive to transduction with ecotropic lentivectors, cells treated with 0.5–5 µg of mCAT-1 gesicles became permissive to ecotropic transduction in a dose-dependent manner. Trypsination and phosphate-buffered saline (PBS) washes of cells after incubation with gesicles did not affect this result, suggesting that the receptor was delivered in the target cell and not adsorbed on the cell surface. This observation also excludes the formation of complexes between gesicles and ecotropic vectors in the transduction medium.
This gesicle-mediated delivery of mCAT-1 into human cells was further confirmed in various cell types by a binding assay. This well-characterized test is based on the recognition of mCAT-1 by the receptor-binding domain of MLV-E, a soluble protein released in the medium of expressing cells.16 A conditioned medium containing the his-tagged receptor-binding domain of MLV-E was prepared and used to stain human cells treated by mCAT-1 gesicles. Results shown Supplementary Figure S1b indicates that gesicles can deliver mCAT-1 in all tested cell lines as well as in the adherent fraction of human PBMCs (Supplementary Figure S1c). Cell viability was monitored in naive and gesicle-treated PBMCs by propidium iodide staining and reached 95 and 93%, respectively, indicating that gesicles are not toxic for PBMCs under described conditions. We next measured the permissiveness of HEK-293T cells treated by mCAT-1 gesicles as compared with HEK-293T cells stably expressing mCAT-1. This ecotropic transduction assay was performed with two low multiplicities of infection (MOIs) (Supplementary Figure S1d). Under such undersaturated conditions, expected copy number per transduced cell is close to 1.17 Results show that the mCAT-1 stable cell line is threefold more permissive than gesicle-treated cells at low MOIs. However, this difference is no longer observed at high MOIs (data not shown). We can hypothesize that continuous expression of mCAT-1 results in an homogenous expression at the cell surface and a higher number of molecules/cells, as compared to gesicles which deliver a fixed quantity of receptor in a short period of time.
Characterization of mCAT-1 gesicles
To further characterize this original vehicle, the mCAT-1 gesicle preparation was fractionated in an iodixanol-density gradient. All fractions obtained after ultracentrifugation were analyzed by immunoblotting and assessed for their capacity to transfer mCAT-1. To this aim, 1/10 of the iodixanol-fraction was introduced in the medium of target cells prior the ecotropic transduction test. This revealed that the mCAT-1 transferring agent mainly sedimented in fractions 17 and 18, corresponding to densities around 1.10 (Figure 2c). Western blot analysis of collected samples showed that mCAT-1 and VSV-G cosedimented in the same two fractions, supporting the idea that both proteins could be present on the same particles.
To precise the structure of mCAT-1 gesicles, highly purified 6,000× concentrated mCAT-1 gesicles were prepared and stained with a gold-labeled antibody directed against the flag mCAT-1 protein as described. Electron microscopy revealed the presence of mCAT-1 on spherical heterogeneous vesicles with an average diameter of 100 nm (Figure 2d). Of note, gesicles appear larger and less dense that related exosomes.18 Clusters of stained vesicles were also observed in the preparation (Figure 2d, panel 4), possibly resulting from the fusion of smaller units or aggregation due to sample processing. VSV-G typical spikes protruding from vesicle membranes were also observed, as it is described for VSV-G pseudotyped vectors. Thus, we envisaged that mCAT-1 vesicles could benefit from VSV-G binding and fusion properties.
To verify this hypothesis, target cells were treated with chloroquine (CQ) before gesicle treatment. By raising endosomal-pH, CQ prevents membrane-fusion induced by VSV-G but not the fusion triggered by the MLV-E envelope.19,20 The pH-dependence and pH-independence of the two envelopes are recapitulated (Supplementary Figure S2). As shown in Supplementary Figure S2 (lane 3), the CQ-treatment abrogated the transfer of mCAT-1 by gesicles. This suggests that a VSV-G dependent fusion step must take place into the endosome of recipient cells prior the functional release of mCAT-1. We hypothesize that the CQ treatment, by impairing the endosomal fusion of gesicles with cell membranes, prevents the consecutive recycling of mCAT-1 to the cell surface where it acts as a viral receptor. Interestingly, we noted that CQ did not affect the gesicle-mediated transfer of YFP in recipient cells (data not shown) confirming that the drug does not affect the entry of gesicles.
To gain a better insight into gesicle composition, mass spectrometry analysis (MS) of highly purified mCAT-1 gesicles was performed. An overview of abundant proteins identified by MS are listed in Supplementary Figure S3a and a table indicating the cellular components significantly enriched in this list is given in Supplementary Figure S3b. Among the enriched gene ontology terms pointed out by this analysis, terms related to plasma membrane, cytoskeleton, and vesicles were highlighted which is consistent with the vesicular nature of gesicles. It is worthy of note that 10% of the listed proteins were found to be highly represented in exosomes as defined by the ExoCarta database.21 We estimated that mCAT-1 represent around 2.5% of the total protein content in this gesicle preparation. The human CD81 antigen mentioned Figure 1 was also identified by the proteomic analysis of mCAT-1 gesicles but was not found within the most abundant proteins listed in Supplementary Figure S3a. We can speculate that the proteic composition of other types of gesicles derived from HEK-293T might be subject to slight variations depending on the nature of overexpressed proteins in producer cells.
The ecotropic receptor function transferred by mCAT-1 gesicles is not encoded by nucleic acids
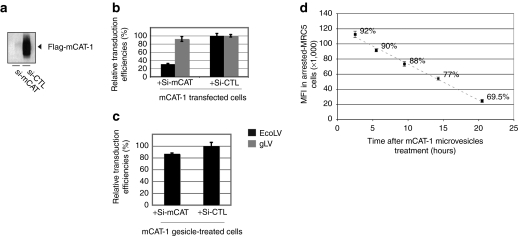
Exosome-like vesicles released from human cells can vehicle proteins as well as mRNA or miRNA, affecting the biology of recipient cells.9,22 Moreover, expression of VSV-G by cells expressing a Semliki Forest Replicon was sufficient to induce the budding of pseudovirions transmitting the replicons to neighbor cells.13,23 These data prompted us to verify that the transfer of the ecotropic receptor function described above was not imputable to coding DNA or mRNA embarked into gesicles in association with the mCAT-1 protein. For this purpose, we expressed a small interfering RNA (siRNA) directed against mCAT-1 in recipient cells prior gesicle treatment. Should gesicles deliver traces of mRNA allowing translation of mCAT-1, it would be degraded by this specific siRNA with a resulting decrease in mCAT-1 expression. On the other hand, if gesicles strictly transfer the mCAT-1 protein, the mCAT-1 siRNA should not affect the ecotropic receptor function.
When coexpressed with a plasmid encoding mCAT-1, the mCAT-1 siRNA strongly suppressed mCAT-1 expression and decreased the permissiveness of cells to an ecotropic transduction (>70% inhibition) (Figure 3a,b). We further show that the siRNA treatment does not decrease the transduction efficiency of pantropic vectors that do not use mCAT-1 as a receptor for their entry. This shows that the mCAT-1 siRNA does not affect the reverse transcription steps and the proviral integration process following viral entry.
Figure 3.
Gesicles delivered murine cationic amino acid transporter-1 (mCAT-1) as a functional protein. (a) Validation of the mCAT-1 small interfering RNA (siRNA). Flag mCAT-1 immunodetection was performed in cell extracts derived from HEK-293T transfected with mCAT-1 in addition with the mCAT-1 siRNA (Si-mCAT lane) or a control siRNA (Si-CTL lane). Ten microgram of each protein extract were loaded per lane. (b) Ecotropic transduction assay in HEK-293T cells after delivery of the receptor by mCAT-1 transfection. EcoLV (black bars) and gLV (gray bars) stand for ecotropic lentivector transduction and pantropic lentivector transduction [vesicular stomatitis virus (VSV-G) pseudotyped], respectively. EcoLV transduction was impaired by the mCAT-1 siRNA. (c) Ecotropic transduction assay in HEK-293T cells after delivery of the receptor by mCAT-1 gesicles. The mCAT-1 siRNA poorly affected the receptor activity when mCAT-1 was delivered by gesicles. (d) Residence time of mCAT-1 in cells treated by gesicles. Mitomycin arrested MRC-5 cells (105 cells in a 12-well plate) were treated with 2 µg of mCAT-1 gesicles and transduced with an ecotropic yellow fluorescence protein (YFP) lentivector [multiplicity of infection (MOI) 20] 3 hours, 5 hours, 9 hours, 14 hours, and 20 hours post-treatment. Transduction efficiencies were measured 72 hours later by FACS. Mean fluorescence intensity (MFIs) of total living populations and percentages of transduced MRC-5 cells are given for each condition. Error bars indicates s.d. (n = 3).
The mCAT-1 siRNA was next transfected in cells prior treatment by mCAT-1 gesicles. Transfer of mCAT-1 function was next monitored by an ecotropic transduction assay. Under these conditions, the mCAT-1 siRNA did not significantly affect the permissiveness of gesicle-treated cells to ecotropic vectors (Figure 3c). These data demonstrate that gesicles essentially transferred the mCAT-1 protein and not mRNA nor DNA molecules encoding it.
As a consequence, one can expect that the function delivered by gesicles will be transient in recipient cells. This was verified by an assay monitoring the presence of functional mCAT-1 receptors at the cell surface several hours after gesicle treatment. Growth-arrested human fibroblasts were treated by mCAT-1 gesicles, washed and transduced with YFP ecotropic lentivectors after a delay ranging from 1 to 20 hours. Results in Figure 3d show the progressive reduction of receptor function after gesicle delivery. In various cell types, half of the mCAT-1 function transmitted by gesicles was lost 12 hours after treatment. This supports the notion that mCAT-1 gesicles deliver a fixed quantity of proteins and not a coding DNA.
iPS reprogrammation of human fibroblasts assisted with mCAT-1 gesicles
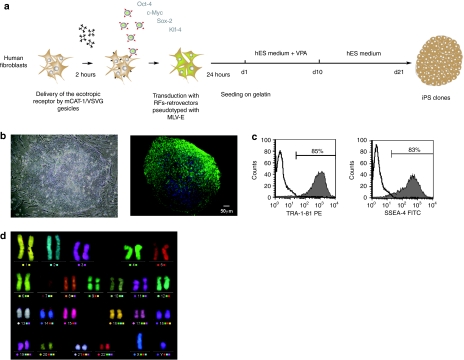
In spite of emerging techniques to generate induced-pluripotent stem cells, the most common methodology still relies on the transduction of fibroblasts in biosafety level 3 laboratories (BSL-3) using retro- or lentiviral vectors encoding the reprogramming factors. We examined whether mCAT-1 gesicles could facilitate this challenging quadri-transduction step by rendering human fibroblasts permissive to ecotropic retrovectors, therefore allowing transduction of reprogramming factors in BSL-2.
Human fibroblasts were first incubated with mCAT-1 gesicles for 2 hours before transduction with ecotropic retrovectors encoding the four factors Oct-4, Sox-2, Klf-4, and c-Myc (Figure 4a). GFP-encoding vectors pseudotyped with MLV-E were used in parallel to follow transduction efficiency that reached 85%. This efficiency was similar to that obtained with amphotropic vectors (not shown). iPS clones were picked between day 20 and day 35 after transduction and amplified on feeder cells in hES medium (Figure 4b). Immunocytochemistry and flow cytometry analysis of pluripotency markers showed staining for SSEA-4 and TRA-1-81 typical of pluripotent stem cells (Figure 4b,c). These iPS clones were expanded in hESC culture conditions for over 40 passages without either any change in morphology or karyotype anomalies (Figure 4e). This transduction process assisted with mCAT-1 gesicles was further validated in a larger reprogrammation session with primary fibroblasts from a panel of 15 individual donors. Reprogrammation was achieved in 13 cases, similarly to the reprogrammation yield obtained with amphotropic vectors. Therefore, gesicles treatment was well tolerated by freshly isolated primary cells and efficiently transferred permissiveness for ecotropic transduction in BSL-2.
Figure 4.
iPS generation assisted with murine cationic amino acid transporter-1 (mCAT-1) gesicles. (a) Reprogramming protocol. (b) Left: Phase contrast representation of an iPSC clone obtained from ecotropic retroviral reprogramming. Right: Immunostaining of the established iPSC clone. Green SSEA-4 staining. Nuclei appear in blue (DAPI staining). (c) FACS analysis of iPSC clone after immunostaining with pluripotency markers. (d) mFISH analysis showing normal karyotype at passage 41.
Gesicle-mediated delivery of TetR transcriptional regulators
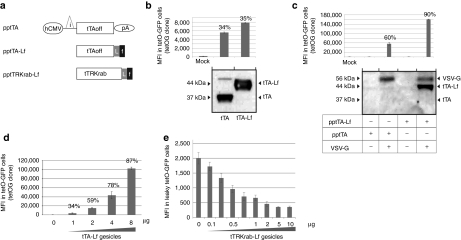
Besides delivery of cytoplasmic and membrane proteins, we explored whether gesicles could deliver nuclear proteins like the TEToff transactivator (tTA), a transcriptional activator resulting from the fusion of the herpes VP16 transactivator protein to the TetR domain which binds to the tetO operator (tetO).24 Due to the capacity of VP16 to translocate into the cell nucleus,25 we anticipated that the tTA would be poorly packaged into gesicles and envisaged to modify its cellular localization to facilitate its incorporation into shedding vesicles. For this purpose, the tTA was fused to the H-RAS C-terminus motif that is farnelysed in vivo and redirects proteins embedded with this motif toward cell membranes.26 Constructs encoding tTA (pptTA) and farnesylated tTA (pptTA-Lf) are depicted in Figure 5a.
Figure 5.
Gesicle-mediated delivery of TetR-regulators in human-cells. (a) Schematic representation of constructs encoding for TetR-regulators. Expression is driven by the human cytomegalovirus early promoter (hCMV) upstream the rabbit b-globin intron (i), and the rabbit b-globin polyadenylation signal (pA). Engineered tTA sequences incorporating a linker (L) and the C-terminal farnesyl motif of H-RAS are depicted (f). (b) Functional validation of the pptTA and pptTA-Lf constructs. (Up): Low amounts of both plasmids (0.1 µg/105 cells) were transfected in a reporter cell line-expressing GFP under the control of the tetO promoter (tetOG cells). Percentages of transfected cells and fluorescence intensities were measured 24 hours later. (Bottom): Immunodetection of the two proteins in cell-extracts. Ten microgram of total protein were loaded per lane. Mock stands for control mock-transfection. (c) Transfer of TetR-activators by gesicles. (Up): Activation of green fluorescent protein (GFP)-transcription in tetOG cells 48 hours after treatment with vesicles derived from producer cells transfected with pptTA or pptTA-Lf with or without vesicular stomatitis virus (VSV-G). (10 µg of gesicles/105 cells). Mock stands for non treated reporter cells. Percentages of GFP+ cells targeted by gesicles are given. (Bottom): Western blot analysis of resulting sedimented vesicles (d) Dose-dependent activation of GFP-transcription in tetOG cells after treatment with tTA-Lf gesicles. Mean fluorescence intensity (MFIs) of recipient cells and percentages of GFP+ cells were measured by FACS 48 hours after treatment. Doses of gesicles are given in µg. (e) Downregulation of transgene expression in human cells by tTRKrab gesicles. A leaky tetO-GFP cell line was generated which emits a fluorescence signal without induction (left bar). 95% of this reporter cell line was GFP+ as compared with parental HEK-293T. GFP downregulation was analyzed by FACS 60 hours after gesicle treatment. The data represent means ± s.d. (n = 3).
To check the functionality of tTA constructs and their expression into human cells, a tTA-responding cell line was established upon stable insertion of a tetO-eGFP expression cassette delivered by lentiviral transduction. A single-cell derived clone whose eGFP expression was tightly controlled by tTA induction was next selected and used as a reporter cell line (tetOG clone). When transfected in the reporter cell line, both pptTA and pptTA-Lf were expressed and induced comparable levels of eGFP activation (Figure 5b), indicating that both constructs were functional. tTA- and tTA-Lf-gesicles were next prepared from HEK-293T cells and analyzed by immunoblotting and for their capacity to deliver the transcription regulators in recipients cells. As revealed by the biochemical analysis of both preparations, farnesylation of the tTA protein resulted in a marked enhancement of its incorporation into gesicles (Figure 5c). Due to the low sensitivity of this immunoblotting assay, tTA was not or poorly detected in the gesicle preparation whereas detection of the farnesylated tTA-Lf protein was highly reproducible in this test. We further show that both gesicle types were efficient in activating transcription in recipient cells, with increased performances for the farnesylated protein (Figure 5c). Interestingly, gesicle-mediated transactivation was shown to be highly dose-dependent and allowed a tunable control of GFP expression in reporter cells (Figure 5d).
We next investigated whether gesicles could transfer another TetR chimeric protein like the tTRKrab regulator which binds the tetO and represses transcription.27 A farnesylated version of tTRKrab was contructed (Figure 5a) and tTRKrab gesicles were produced. To test the transfer of the tTRKrab repressor, we used another reporter cell-line emitting a low GFP-fluorescence under the control of a leaky tetO-GFP cassette. When these reporter cells were exposed to increasing doses of tTRKrab gesicles, the GFP-fluorescence gradually decreased as shown in Figure 5e.
Altogether these results indicate that precise amounts of nuclear factors can be transferred in human cells with gesicles. We noted that tTA-Lf gesicles were two- to threefold more efficient than tTA-gesicles which illustrates that proteins of interest can be optimized to favor their incorporation into gesicles and subsequent transfer. As for other related proteins,28 tTA-Lf remains strikingly active in the nucleus in spite of its farnesyl motif. Further investigations would be necessary to clarify the cellular localization of tTA-Lf in recipient cells.
Discussion
We report here an original method to deliver proteins in human cells by VSV-G induced vesicles named gesicles. Upon overexpression of VSV-G in HEK-293T cells, these shedding vesicles are released and can be concentrated, following a procedure similar to concentration of lenti/retrovectors. Therefore, it is likely that gesicles coexist with bona fide VSV-G pseudotyped viral vectors in vector preparations. Combined with GAG-induced virus-like particles, these by-products may be responsible for the transmission of small quantities of proteins in recipient cells during transduction as previously shown.11,12 By characterizing VSV-G-induced vesicles, this study provides a new insight into the pseudotransduction phenomenon.
Additionally, we show that gesicles are versatile vehicles that can be engineered for the transient delivery of membrane cytoplasmic and nuclear proteins. Among the various protein-transfer assays, we focused on two proofs of concept. First, we developed a method for the nongenetic transfer of the mCAT-1 protein, the receptor for the MLV-E envelope. We show that treatment of human cells with mCAT-1 gesicles permits their immediate transduction with ecotropic retro/lentivectors. This result opens the gate to a general use of ecotropic vectors. As reported by others, high titers of ecotropic lentivectors (>108 transducing units/ml) can be prepared in BSL-2 regardless of the transgene type and safely used for challenging cell transgenesis.29 By providing the little help which allows the entry of ecotropic vectors into human cells, mCAT-1 gesicles offer the possibility to increase the safety of experiments based on retro/lentiviral transductions, including ex vivo gene therapy trials or iPSc generation. Although this work demonstrates that gesicles can efficiently assist the generation of iPSc, further studies remain necessary to assess the full pluripotency of cells described in this report.
Evidence is also provided that gesicles can package and deliver a transcription factor such as the TET-transactivator (tTA). This method of delivery allows a tight and rapid control of transgene expression in human cells. Conditional gene expression systems have been widely used to precisely control the time of transgene expression in vitro and in vivo, rendering possible the integration of deleterious genes in mammalian cells or in transgenic animals. In contrast with other inducible systems which roughly oscillate between the ON or the OFF expression status,24,30 the gesicle-induction system allows a precise tuning of transgene expression in cultured cells by addition of increasing quantities of tTA-Lf or tTRKrab gesicles. This new gene induction system might be valuable for numerous studies exploring biological thresholds of proteins such as transcription factors, apoptotic or oncogenic genes. Furthermore, transient delivery of tTA by gesicles allows a substantial simplification of constructs required to establish a TET-inducible cell system. Since gene induction can be controlled by gesicles, the transactivator coding gene is dispensable either in the construct driving transgene expression or in the studied cell.
Whether gesicles can be used as a universal protein-transferring agent remains to be evaluated. Due to the low amount of material packaged in gesicles, it is conceivable that protein vectorization by fusogenic vesicles will be restricted to proteins with a high biological activity. Although gesicle content can be partly modulated, we assume that packaging of biomaterial in shedding vesicles is a nonspecific process. This implies that gesicles may contain and deliver traces of mRNA, small noncoding RNA or undesirable proteins as it is reported for other vesicles.9,22,31,32 Under the light of the proteomic analysis shown in Supplementary Figure S3, it appears that mCAT-1 represent a small fraction of the total protein load which confirms that gesicles deliver many other proteins derived from producer cells. Interestingly, neither the SV40 Large T antigen nor the adenoviral E1A protein expressed in HEK-293T cells were detected by two MS analysis performed on gesicle preparations. Although their presence in gesicles cannot be totally ruled out, these oncogenic proteins may only be present as traces (<10 pg/µl). Nevertheless, we cannot exclude that some proteins transported by gesicles such as integrins might transiently alter the biology of recipient cells by modifying their adhesion properties or their response to external signals. Various production systems exploiting other cell types, like insect cells or GMP-approved cell lines could be used for a better control of gesicle composition. Beyond this, various optimizations of this method can be envisaged such as the replacement of VSV-G by another fusogenic protein enabling transfer of proteins in restricted cell-types.
As opposed to gene delivery by viral vectors, protein delivery by gesicles allows a rapid transfer of function in primary or immortalized human cells without the involvement of the transcription machinery or any viral integration process that could limit viral transduction in specific cell types. This method is of particular interest for the delivery of surface receptors in human cells and more generally for the transient transfer of proteins with technological and biomedical potentials.
Materials and Methods
Cell culture. HEK-293T and MRC-5 cells (P22-ECACC) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, -glutamine penicillin and streptomycin. The mCAT-1/HEK-293T and the YFP/HEK-293Tcell lines were generated upon saturating transduction with lentivectors-encoding mCAT-1 and YFP, respectively. Complete transduction was assessed upon FACS analysis of YFP target cells. mCAT-1 cells remain permissive to ecotropic retrovectors after at least ten passages.
Human normal adult fibroblasts (Coriell cell repository) were maintained in DMEM high glucose supplemented with 10% fetal calf serum, 1 mmol/l sodium pyruvate and 2 mmol/l GlutaMAX.
Human iPS cells were maintained on mytomycin-C growth-arrested MEF feeder cells in human ES-cell medium, i.e., knockout DMEM supplemented with 20% knockout serum replacement, 10 ng/ml bFGF, 0.1 mmol/l nonessential amino acids, 50 mmol/l β-mercaptoethanol, 2 mmol/l GlutaMAX and 0.1% penicillin/streptomycin.
MRC-5-derived iPSC were cultivated in mTESR-1 (StemCell Technologies, Vancouver, British Columbia, Canada) supplemented with valproic acid (0.5 mmol/l) during the first ten days of culture following transduction.
Constructs and retro/lentiviral production. The mCAT-1 expression cassette, and the TetR-regulators were inserted after PCR amplification in an eucaryotic expression vector upstream the human cytomegalovirus early promoter (CMV) and the rabbit β-globin intron.
The flagged mCAT-1 expression cassette was constructed by fusing the triple flag motif (DYKDHDGDYKDHDIDYKDDDDKG) upstream the mCAT-1 cDNA. The cassette was next inserted in a simian immunodeficiency virus-derived lentivector under the control of the CMV promoter to generate fling-mCAT-1 that was used either as a single expression plasmid or as a vector plasmid for lentiviral preparation and generation of stable EcoR cell lines. The pptTA-Lf was generated by PCR on the basis of the pptTA construct in which the stop codon was deleted and replaced by a linker sequence (RAKRGKPIPNPLLGLDSTSGSGAPVKQTLNFDLLKLAGDVESNP-GP) followed by the H-RAS farnesyl motif (KLNPPDESGPGCMSCKCVLS). The pptTRKrab was constructed by replacing the tTA-coding sequence in pptTA-Lf by the tTRKrab sequence amplified from the pLV-tTRKrab plasmid (gift from D. Trono through Addgene plasmid 12249). All sequences and detailed maps are available on request.
For iPSC generation Moloney-based retroviral vectors containing cDNAs of c-Myc, Oct-4, Sox-2, and Klf-4 were obtained from Addgene (Cambridge, MA) (Addgene plasmids 17220, 17225, 17226, 17227). These plasmids were individually transfected using FuGene into PLAT-A (for amphotropic viral production) or PLAT-E (for ecotropic viral production) packaging cells. PLAT cells medium was replaced 24 hours post-transfection. Viral supernatants were collected 48 hours post-transfection, filtered through a 0.45 µm filter, then mixed at a 1:1:1:1 ratio.
For MRC-5 transductions and genetic reprogramming, retroviral vectors were also prepared with the tritransfection method where the RF-encoding vector, a GAG POL-expressing plasmid and the ecotropic envelope were co-transfected in HEK-293T cells (ratio 40, 40, and 20%, respectively).
Procedures to prepare lentivectors and retrovectors were previously described.33 YFP or GFP-vector preparations were titered on HEK-293T cells prior calculation of MOI. The YFP-lentivector preparation used in most of experiments of this manuscript was titered at 8.6 × 107 transducing units/ml and dosed at 2 × 106 pg of P24/ml (43 transducing units/pg of P24).
Production of gesicles for the delivery of mCAT-1 or TetR-derived regulators. TetR-factors or mCAT-1 gesicles were produced after calcium phosphate transfection of semiconfluent HEK-293T cells seeded at 4 × 106 cells in a 10-cm dish the day before transfection. Fifteen micro gram of plasmid encoding the relevant TetR-factor or mCAT-1 were co-transfected with a VSV-G plasmid (15 µg). Twenty hours after transfection, medium was carefully replaced by fresh medium supplemented with ATP (100 µm final; Sigma-Aldrich, St Louis, MO). Supernatants were harvested 48 hours after transfection. For large scale productions, media were also collected 72 hours after transfection and pooled with the 48-hour supernatants. After clarification and filtration through a 0.45-µm pore filter, gesicle-containing media were ultracentrifuged at 60 minutes at 30,000 r.p.m. in a SW41 rotor. Pelleted material was next resuspended overnight in cold PBS (100× concentration), aliquoted, stored at −80 °C. Gesicles were stable at least 3 months. For each preparation, protein concentration was systematically measured by Bio-Rad protein assay.
General procedure for ecotropic transduction of human cells assisted by mCAT-1 gesicles. Human cells of various origins are exposed with mCAT-1 gesicles in a minimal volume (800 µl for a 6-well plate). Two microgram of dosed mCAT-1 gesicles incubated for 3 hours is optimal to confer ecotropic permissiveness to 105 HEK-293T cells. Gesicles can be added in suspension or on adherent cells without modification of efficacy. Cell washes or trypsination do not affect mCAT-1 availability on treated cells. Transduction with ecotropic lentivectors is maximal if vectors are added between 0 and 3 hours after incubation with gesicles and decreases slightly with time (loss of 50% activity in 12 hours).
Rate zonal centrifugation through continuous iodixanol gradients and immunoblotting analysis. Crude concentrated gesicles were overlaid on a continuous optiprep gradient (6% iodixanol in 215 mmol/l sucrose, 2 mmol/l EDTA, 10 mmol/l Tris-HCl pH 8/56.4% iodixanol in 5 mmol/l sucrose, 2 mmol/l EDTA, 10 mmol/l Tris-HCl pH 8) and centrifuged 15 hours at 30,000 r.p.m. in a SW41 rotor. Fractions (0.5 ml) were collected from the bottom of the gradient and kept at 4 °C before western blot analysis. Densities were measured by weighing 100 µl of each fraction.
Proteins of the different fractions were separated via SDS/PAGE using 4–12% Bis–Tris Gel Nupage gels (Invitrogen) runned in MOPS buffer. 1/100 of each fraction (5 µl) was analyzed. Upon electroblot onto a nitrocellulose membrane, proteins were revealed by a peroxidase-conjugated antibody directed against VSV-G (P5D4-horseradish peroxidase; Sigma) dilution 1/1,000 or a anti-flag-horseradish peroxidase antibody (M2 Sigma) dilution 1/1,000 recognizing the flag-mCAT-1 protein.
Detections of TetR-regulators were performed using the rabbit polyclonal TetR antibody (Abcam 14075, 1/1,000; Abcam, Cambridge, UK) and revealed by an anti-rabbit-horseradish peroxidase antibody (Jackson ImmunoResearch 1/10,000; Jackson ImmunoResearch, Newmarket, UK).
Production of soluble receptor-binding domain fragment and detection of mCAT-1 by binding assay. Conditioned supernatant containing the his-tagged receptor-binding domain of MLV-E (His-receptor-binding domain) was obtained from transfected human cells as previously described.16 Collected medium was filtered through a 0.45-µm filter and stored at 4 °C. For binding assay on cell lines, 2 × 105 target cells were incubated with 10 µg of mCAT-1 gesicles for 3 hours at 37 °C, washed and cultivated in the his-receptor-binding domain-containing medium for one additional hour at 37 °C. Cells were detached by versen, washed and stained for 45 minutes at 4 °C with an anti-His-antibody conjugated with Alexa-fluor 488 (1/100) (Qiagen). After a last wash, cells were analyzed by FACS.
For blood-derived cells, human PBMCs from one healthy donor were prepared using the lymphocyte separation medium (Eurobio, Les Ulis, France) as described by the manufacturer. One million PBMCs were plated in a 12-well dish and cultivated 1 hour at 37 °C in DMEM. The adherent fraction (1.4 × 105 cells) was washed twice with PBS and treated with 10 µg of mCAT-1 gesicles. The binding assay was performed 20 hours later as described elsewhere.
Fluorometric analysis of eGFP-gesicles probed with the membrane tracer R18. eGFP-Gesicles were prepared from HEK-293T cells pretreated with octadecyl-Rhodamine B (R18), a lipophilic cation which probes membranes. Besides VSV-G/eGFP gesicles, control vesicles were produced from HEK-239T cells transfected with eGFP and a carrier plasmid. Increasing amounts of both preparations were next diluted in PBS and analyzed by a fluorometer detecting eGFP-emission upon excitation at 485 nm and R18-emission upon excitation at 560 nm. Emission signals were collected at 515 and 590 nm, respectively and represented values were obtained after background substraction (emission of excited PBS). GFP-free gesicles loaded with R18 and R18-free gesicles-containing GFP were used as controls, showing the absence of overlapping between the two spectra.
CQ treatment. HEK-293T cells were cultivated (105 cells/well in a 12-well plate) with or without CQ (0.5 µmol/l), a drug raising endosomal pH and inhibiting pH-dependent viral entry. After 1 hour of treatment, cells were incubated with mCAT-1-microvesicles (2 µg) for 1 hour before transduction with YFP-coding lentivector pseudotyped with the VSV-G envelope or the ecotropic envelope as indicated. The transduction assay with both vector preparations was performed in HEK-293T cells (naive), in HEK-293T cells stably expressing mCAT-1 (stable mCAT-1) or in cells where mCAT-1 was transferred by gesicles (mCAT-1 gesicles).
siRNA-mediated downregulation of mCAT-1. A synthetic siRNA raised against Slc7a1 and Stealth RNA interference control duplexes (Invitrogen, Paisley, UK) were transfected in 3 × 105 HEK-293T by Lipofectamine RNA interference max (Invitrogen) combined with a plasmid encoding a flagged mCAT-1. Treated cells were split 48 hours later in two equal populations each of them being dedicated to (i) WB analysis and (ii) an ecotropic transduction assay. For the immunostaining of mCAT-1, the anti-Flag M2 antibody conjugated with horseradish peroxidase (dilution 1/1,000; Sigma) was used to detect the flagged version of mCAT-1. For the transduction assay, populations transfected by mCAT-1 and siRNAs were plated 48 hours after transfection in a 6-well plate. Transductions were performed 2 hours later by YFP-lentivectors pseudotyped with either MLV-E or VSV-G (MOI 10) and transduction efficiencies were analyzed 72 hours later.
To test the impact of the mCAT-1 siRNA on gesicle-mediated receptor delivery, target HEK-293T cells were first transfected by siRNAs in a 12-plate dish. Forty-eight hours later, each population was split in three 12-well plate dishes and mCAT-1 gesicles were added directly in the medium (5 µg per well). After 2 hours of incubation, cells were carefully washed with DMEM and transduced with YFP-encoding lentivectors pseudotyped with MLV-E or with the VSV-G pantropic envelope (MOI 10). Transduced cells were analyzed by FACS 72 hours after transduction.
Induction of pluripotent stem cells. For the generation of iPS cells with amphotropic vectors, human fibroblasts were transduced by the amphotropic retrovector mix for 24 hours, then replated in fibroblast medium at 3 × 104 cells per well in gelatin-coated 6-well plates, which is defined as day 0. Generation of iPSc with ecotropic vectors followed the same protocol except that mCAT-1 gesicles were added on cells 90 minutes prior to transduction with ecotropic retrovectors. Starting from day 1 postinfection, cells were cultured for 10 days in human ES-cell medium supplemented with 0.5 mmol/l valproic acid, then in nonsupplemented hES cell medium until emergence of iPSC colonies. To establish iPS cell lines, iPSC colonies were picked about 3–5 weeks postinfection based on ES cell–like colony morphology.
For Coriell-fibroblast-derived iPSC, the picked colonies were subsequently expanded and maintained on mytomycin-C growth-arrested MEF feeder layers in human ES-cell medium without valproic acid. The Y-27632 ROCK inhibitor was used at 10 µmol/l to increase the seeding efficiency of iPS cells for the initial colony expansion after picking and for the first day after each passaging.
MRC-5-derived iPSC were obtained following a similar protocol but were expanded and maintained in mTESR1 (Stemcell Technologies) on Matrigel-coated plates (BD Biosciences, Franklin Lakes, NJ) after apparition of clones in the hES medium.
Preparation of iPS cells for mFISH karyotyping. Actively growing iPSc colonies were treated with colchicine at 20 mg/ml (Eurobio) for 90 minutes at 37 °C. After washing, cells were incubated in trypsin–EDTA 0.05% (Eurobio) for 2–3 minutes and then harvested. Cells were incubated in 0.075 mol/l KCl (Sigma) for 10–14 minutes at 37 °C, followed by fixation with 3:1 methyl alcohol/glacial acetic acid. Fixed cells were dropped on wet slides and dried at 37 °C for 24 hours.
For mFISH, fixed cells were hybridized overnight at 37 °C with a denatured “cocktail painting mFISH” probe (MetaSystems, Altlussheim, Germany). Slides were washed in successive baths of 1× SSC and 0.4× SSC, and nuclei were stained with DAPI. Biotinylated probe was revealed using Cy5 MetaSystems B-tect detection kit. Ten to twenty metaphases were captured using a Zeiss Z1 fluorescence microscope equipped with a UV HBO 100-W lamp coupled to an AxioCam camera and ×20 and ×63 objectives. All the analyzed metaphases were karyotyped using the MetaSystems Isis software.
Immunocytochemistry and flow cytometry analysis of iPS clones. iPS cells were washed with PBS, fixed in 4% paraformaldehyde in PBS for 15 minutes, then rinsed with PBS. To allow nuclear permeation, the cells were treated with 50 mmol/l NH4Cl (Sigma) for 10 minutes, rinsed with PBS and treated with 0.2% Triton (Sigma) in PBS for 4 minutes. After PBS washes, cells were blocked in 0.5% bovine serum albumin (Sigma) in PBS for 30 minutes. Cells were stained with following primary antibodies: hOCT4-3/4 (1:200; goat; Santa Cruz Biotechnology, Santa Cruz, CA); SSEA-4 (1:100; goat; BD Biosciences). Appropriate Alexa Fluor 488 conjugated secondary antibodies (Invitrogen) were used at a 1:1,000 dilution. DAPI was added at 0.1 ng/ml. Stained cultures were photographed in Zeiss microscope.
HEK-293T cells and HepG2 cells were stained after versen treatment with the mouse monoclonal TAPA1/CD81 antibody (Abcam ab59477, 1:750; Abcam).
Preparation of purified gesicles for electron microscopy (TEM) analysis and mass spectrometry. mCAT-1 gesicles prepared from a total of 2 × 107 producers cells transfected with flag-mCAT-1 and VSV-G were first filtered and concentrated 100-fold by overnight centrifugation at 3,800g. This preparation was next laid overlaid on a continuous optiprep gradient and ultracentrifuged as described upper to obtain density fractions. Three 500 µl fractions containing the mCAT-1 transfer activity were next pooled and centrifuged overnight at 3,800g before PBS resuspension to obtain a 6,000×-concentrated sample.
Specimen processing and TEM observation. Immunostaining of purified gesicles was performed by the CeCIL-facility using the control mouse anti-AcV5 antibody (Sigma) and the mouse anti-Flag M2 antibody (Sigma) subsequently revealed by a goat-antimouse antibody coupled with 10-nm colloidal gold beads. After a flash-fixation in glutaraldehyde, staining was amplified using the R-Gent Kit (Biovalley, Marne-la-Vallee, France) before the negative coloration (phosphotungstic acid 2%). Specimen were observed under a JEM-1400 microscope (Jeol, Tokyo, Japan) coupled with the Orius-600 camera (Gatan, Pleasanton, CA).
Proteomic analysis of gesicles and bioinformatic treatment of MS dataset. Protein and peptides were prepared as described.34 Nanoliquid chromatography and LTQ-Orbitrap analyses were performed and peptides/proteins were identified using Mascot and a concatenated Swissprot and TrEMBL database. Results were filtered using IRMa.35 False discovery rate was set inferior to 1% for peptide identification and only proteins identified with a minimum of two peptides were automatically validated.
Gene Ontology cellular component term enrichment was performed using the DAVID web-based tool (#12734009, #19131956). Enrichment analyses were performed using UNIPROT accession IDs as input for the top 100 most abundant proteins as sorted on the basis of the emPAi index.36 Nonhuman proteins like bovine serum-related proteins were excluded from the list. Enriched GO cellular component terms associated with the input list were determined using Fisher's exact tests. Benjamini and Hochberg correction was used to adjust P values associated to multiple testing. GO terms with a corrected P value <0.001 were collected.
SUPPLEMENTARY MATERIAL Figure S1. mCAT-1 transfer in human cells by mCAT-1 gesicles. (a) Production process of mCAT-1 vesicles (b) Surface staining of mCAT-1 on gesicle-treated cells. Human cell lines treated by mCAT-1 gesicles (10μg of gesicles per 2x105 cells) were cultivated in a conditioned medium containing a soluble 6xhis-tagged-receptor binding domain of MLV-E (his-RBD) which binds mCAT-1. After staining with an anti his-Alexa488 antibody, non treated cells (grey-filled) and gesicle-treated cells (open line) were analysed by FACS. Overlay histograms and percentage of cells having bound the his-RBD are given. HEK-293T cells modified to stably express mCAT-1 were also analysed (HEK-293T mCAT-1). In this specific condition, control cells were not exposed to the his-RBD medium. All cell lines were permissive to ecotropic transduction with YFP-lentivectors after gesicle treatment (not shown). (b) Binding assay on human peripheral blood mononuclear cells (adherent fraction of PBMCs) (c). Comparison of transduction efficiencies mediated by ecotropic lentivectors in HEK-293T treated with mCAT-1 gesicles (2μg of gesicles per 105 cells), HEK-293T stably expressing mCAT-1 or non-modified HEK-293T. Under these low MOIs (0,2 and 1), expected copy number per transduced cell is close to 1 [17]. Results are given as the percentage of succesfully transduced cells as monitored by FACS 48 hours after transduction. Error bars represent s.d. (n=3). Figure S2. Gesicle-mediated transfer of mCAT-1 depends on endosomal acidification. The mCAT-1 receptor function delivered by gesicles was monitored by an ecotropic transduction assay in target cells pretreated by chloroquine (grey bars), a drug raising endosomal pH. Non treated cells are shown (black bars). YFP encoding lentiviral vectors (MOI 5, 43TU/pgP24) pseudotyped with the ecotropic envelope (Eco) were used to transduce HEK-293T cells in which mCAT-1 was stably expressed (lane 2), or delivered by gesicles (lane 3). A control transduction assay using lentivectors pseudotyped with VSV-G is shown (lane 1), illustrating the pH-dependence of this envelope and the efficacy of the drug. Transduced cells were analysed by FACS 72 hours post transduction. Results are given as the relative YFP transduction efficiencies in each condition. Error bars represent s. d. (n=3). Figure S3. Proteomic analysis of purified mCAT-1 gesicles. (a) Overview of abundant human proteins identified by mass spectrometry in mCAT-1 gesicles. The list represents the Top-100 of one analysis after a sort based on the Exponentially Modified Protein Abundance Index (emPAI) which gives a relative quantitation of proteins in the mixture [36]. Gene names and descriptions are provided. Both mCAT-1 and VSVG are shown in black. Proteins frequently identified in exosomes are shadowed in grey after comparision with the top 25 list of the ExoCarta database [21]. High confidence proteins indicated by an asterisk (*) were identifed in the Top-100 of a second independent proteomic analysis performed on another batch of mCAT-1 gesicles. (b) Gene Ontology cellular component term enrichment analysis. The analysis was performed using UNIPROT accession IDs of the 100 most abundant proteins in the gesicle preparation. For each Gene Ontology cellular component term (GO term), the number of genes associated with this term and the corrected p-value are indicated.
Acknowledgments
We acknowledge Toshio Kitamura and George Q Daley for the gift of the pMXs-c-Myc construct and the pMIG series. We thank Didier Trono for having provided the pLV-tTRKrab. All these plasmids were obtained through the addgene plasmid depository. We are grateful to Gaël Cristofari for helpful discussions and to Christine Varela (karyotype), Pascal Fragner, and Elise Simonazzi for their technical assistance. The authors thank Elisabeth Errazuriz-Cerda and the CeCIL-facility (Lyon-France) for the preparation and the observation of samples by TEM, yohann Couté and the edyp-service (Grenoble-France) for the proteomic analysis. Thanks go to Laurène Meyniel-Schicklin for her bioinformatic analysis and savoir-faire. This work was funded by INSERM and INSERM transfert.
Supplementary Material
mCAT-1 transfer in human cells by mCAT-1 gesicles. (a) Production process of mCAT-1 vesicles (b) Surface staining of mCAT-1 on gesicle-treated cells. Human cell lines treated by mCAT-1 gesicles (10μg of gesicles per 2x105 cells) were cultivated in a conditioned medium containing a soluble 6xhis-tagged-receptor binding domain of MLV-E (his-RBD) which binds mCAT-1. After staining with an anti his-Alexa488 antibody, non treated cells (grey-filled) and gesicle-treated cells (open line) were analysed by FACS. Overlay histograms and percentage of cells having bound the his-RBD are given. HEK-293T cells modified to stably express mCAT-1 were also analysed (HEK-293T mCAT-1). In this specific condition, control cells were not exposed to the his-RBD medium. All cell lines were permissive to ecotropic transduction with YFP-lentivectors after gesicle treatment (not shown). (b) Binding assay on human peripheral blood mononuclear cells (adherent fraction of PBMCs) (c). Comparison of transduction efficiencies mediated by ecotropic lentivectors in HEK-293T treated with mCAT-1 gesicles (2μg of gesicles per 105 cells), HEK-293T stably expressing mCAT-1 or non-modified HEK-293T. Under these low MOIs (0,2 and 1), expected copy number per transduced cell is close to 1 [17]. Results are given as the percentage of succesfully transduced cells as monitored by FACS 48 hours after transduction. Error bars represent s.d. (n=3).
Gesicle-mediated transfer of mCAT-1 depends on endosomal acidification. The mCAT-1 receptor function delivered by gesicles was monitored by an ecotropic transduction assay in target cells pretreated by chloroquine (grey bars), a drug raising endosomal pH. Non treated cells are shown (black bars). YFP encoding lentiviral vectors (MOI 5, 43TU/pgP24) pseudotyped with the ecotropic envelope (Eco) were used to transduce HEK-293T cells in which mCAT-1 was stably expressed (lane 2), or delivered by gesicles (lane 3). A control transduction assay using lentivectors pseudotyped with VSV-G is shown (lane 1), illustrating the pH-dependence of this envelope and the efficacy of the drug. Transduced cells were analysed by FACS 72 hours post transduction. Results are given as the relative YFP transduction efficiencies in each condition. Error bars represent s. d. (n=3).
Proteomic analysis of purified mCAT-1 gesicles. (a) Overview of abundant human proteins identified by mass spectrometry in mCAT-1 gesicles. The list represents the Top-100 of one analysis after a sort based on the Exponentially Modified Protein Abundance Index (emPAI) which gives a relative quantitation of proteins in the mixture [36]. Gene names and descriptions are provided. Both mCAT-1 and VSVG are shown in black. Proteins frequently identified in exosomes are shadowed in grey after comparision with the top 25 list of the ExoCarta database [21]. High confidence proteins indicated by an asterisk (*) were identifed in the Top-100 of a second independent proteomic analysis performed on another batch of mCAT-1 gesicles. (b) Gene Ontology cellular component term enrichment analysis. The analysis was performed using UNIPROT accession IDs of the 100 most abundant proteins in the gesicle preparation. For each Gene Ontology cellular component term (GO term), the number of genes associated with this term and the corrected p-value are indicated.
REFERENCES
- Wagstaff KM., and, Jans DA. Protein transduction: cell penetrating peptides and their therapeutic applications. Curr Med Chem. 2006;13:1371–1387. doi: 10.2174/092986706776872871. [DOI] [PubMed] [Google Scholar]
- Mäe M., and, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6:509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Liguori L., and, Lenormand JL. Production of recombinant proteoliposomes for therapeutic uses. Meth Enzymol. 2009;465:209–223. doi: 10.1016/S0076-6879(09)65011-4. [DOI] [PubMed] [Google Scholar]
- Xu YF, Zhang YQ, Xu XM., and, Song GX. Papillomavirus virus-like particles as vehicles for the delivery of epitopes or genes. Arch Virol. 2006;151:2133–2148. doi: 10.1007/s00705-006-0798-8. [DOI] [PubMed] [Google Scholar]
- Temchura VV, Tenbusch M, Nchinda G, Nabi G, Tippler B, Zelenyuk M.et al. (2008Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus Vaccine 263662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noad R., and, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D.et al. (2010Protein transduction from retroviral Gag precursors Proc Natl Acad Sci USA 1077805–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Jensen SS., and, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P.et al. (2006Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery Leukemia 20847–856. [DOI] [PubMed] [Google Scholar]
- Liu ML, Winther BL., and, Kay MA. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo HF, Tan C, Ory D., and, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- Rolls MM, Webster P, Balba NH., and, Rose JK. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Albritton LM, Tseng L, Scadden D., and, Cunningham JM. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Kavanaugh MP, North RA., and, Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Lavillette D, Ruggieri A, Russell SJ., and, Cosset FL. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J Virol. 2000;74:295–304. doi: 10.1128/jvi.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustikova OS, Wahlers A, Kuhlcke K, Stahle B, Zander AR, Baum C.et al. (2003Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population Blood 1023934–3937. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G., and, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Matlin KS, Reggio H, Helenius A., and, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Ragheb JA., and, Anderson WF. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S., and, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ., and, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Dorange F, Piver E, Bru T, Collin C, Roingeard P., and, Pagès JC. Vesicular stomatitis virus glycoprotein: a transducing coat for SFV-based RNA vectors. J Gene Med. 2004;6:1014–1022. doi: 10.1002/jgm.582. [DOI] [PubMed] [Google Scholar]
- Gossen M., and, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Boissière S, Hughes T., and, O'Hare P. HCF-dependent nuclear import of VP16. EMBO J. 1999;18:480–489. doi: 10.1093/emboj/18.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J., and, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu J, Pierson T, O'Malley BW., and, Tsai SY. Positive and negative regulation of gene expression in eukaryotic cells with an inducible transcriptional regulator. Gene Ther. 1997;4:432–441. doi: 10.1038/sj.gt.3300402. [DOI] [PubMed] [Google Scholar]
- Elam C, Hesson L, Vos MD, Eckfeld K, Ellis CA, Bell A.et al. (2005RRP22 is a farnesylated, nucleolar, Ras-related protein with tumor suppressor potential Cancer Res 653117–3125. [DOI] [PubMed] [Google Scholar]
- Schambach A, Galla M, Modlich U, Will E, Chandra S, Reeves L.et al. (2006Lentiviral vectors pseudotyped with murine ecotropic envelope: increased biosafety and convenience in preclinical research Exp Hematol 34588–592. [DOI] [PubMed] [Google Scholar]
- Sauer B., and, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Février B, Vilette D, Laude H., and, Raposo G. Exosomes: a bubble ride for prions. Traffic. 2005;6:10–17. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J.et al. (2000Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection Nat Med 6769–775. [DOI] [PubMed] [Google Scholar]
- Mangeot PE, Cosset FL, Colas P., and, Mikaelian I. A universal transgene silencing method based on RNA interference. Nucleic Acids Res. 2004;32:e102. doi: 10.1093/nar/gnh105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut A, Marcellin M, Adrait A, Kuhn L, Louwagie M, Kieffer-Jaquinod S.et al. (2009Peptide storage: are you getting the best return on your investment? Defining optimal storage conditions for proteomics samples J Proteome Res 83778–3785. [DOI] [PubMed] [Google Scholar]
- Dupierris V, Masselon C, Court M, Kieffer-Jaquinod S., and, Bruley C. A toolbox for validation of mass spectrometry peptides identification and generation of database: IRMa. Bioinformatics. 2009;25:1980–1981. doi: 10.1093/bioinformatics/btp301. [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J.et al. (2005Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein Mol Cell Proteomics 41265–1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mCAT-1 transfer in human cells by mCAT-1 gesicles. (a) Production process of mCAT-1 vesicles (b) Surface staining of mCAT-1 on gesicle-treated cells. Human cell lines treated by mCAT-1 gesicles (10μg of gesicles per 2x105 cells) were cultivated in a conditioned medium containing a soluble 6xhis-tagged-receptor binding domain of MLV-E (his-RBD) which binds mCAT-1. After staining with an anti his-Alexa488 antibody, non treated cells (grey-filled) and gesicle-treated cells (open line) were analysed by FACS. Overlay histograms and percentage of cells having bound the his-RBD are given. HEK-293T cells modified to stably express mCAT-1 were also analysed (HEK-293T mCAT-1). In this specific condition, control cells were not exposed to the his-RBD medium. All cell lines were permissive to ecotropic transduction with YFP-lentivectors after gesicle treatment (not shown). (b) Binding assay on human peripheral blood mononuclear cells (adherent fraction of PBMCs) (c). Comparison of transduction efficiencies mediated by ecotropic lentivectors in HEK-293T treated with mCAT-1 gesicles (2μg of gesicles per 105 cells), HEK-293T stably expressing mCAT-1 or non-modified HEK-293T. Under these low MOIs (0,2 and 1), expected copy number per transduced cell is close to 1 [17]. Results are given as the percentage of succesfully transduced cells as monitored by FACS 48 hours after transduction. Error bars represent s.d. (n=3).
Gesicle-mediated transfer of mCAT-1 depends on endosomal acidification. The mCAT-1 receptor function delivered by gesicles was monitored by an ecotropic transduction assay in target cells pretreated by chloroquine (grey bars), a drug raising endosomal pH. Non treated cells are shown (black bars). YFP encoding lentiviral vectors (MOI 5, 43TU/pgP24) pseudotyped with the ecotropic envelope (Eco) were used to transduce HEK-293T cells in which mCAT-1 was stably expressed (lane 2), or delivered by gesicles (lane 3). A control transduction assay using lentivectors pseudotyped with VSV-G is shown (lane 1), illustrating the pH-dependence of this envelope and the efficacy of the drug. Transduced cells were analysed by FACS 72 hours post transduction. Results are given as the relative YFP transduction efficiencies in each condition. Error bars represent s. d. (n=3).
Proteomic analysis of purified mCAT-1 gesicles. (a) Overview of abundant human proteins identified by mass spectrometry in mCAT-1 gesicles. The list represents the Top-100 of one analysis after a sort based on the Exponentially Modified Protein Abundance Index (emPAI) which gives a relative quantitation of proteins in the mixture [36]. Gene names and descriptions are provided. Both mCAT-1 and VSVG are shown in black. Proteins frequently identified in exosomes are shadowed in grey after comparision with the top 25 list of the ExoCarta database [21]. High confidence proteins indicated by an asterisk (*) were identifed in the Top-100 of a second independent proteomic analysis performed on another batch of mCAT-1 gesicles. (b) Gene Ontology cellular component term enrichment analysis. The analysis was performed using UNIPROT accession IDs of the 100 most abundant proteins in the gesicle preparation. For each Gene Ontology cellular component term (GO term), the number of genes associated with this term and the corrected p-value are indicated.