Readers who have spent much time peering at cultured cells through a microscope may have noticed the membranous debris that accumulates in the culture medium over time. We now know that this “debris” actually comprises components of an elaborate intercellular communication system mediated by membranous extracellular organelles collectively called microvesicles. Given their capacity to transmit information between cells, the types, contents, and functions of these microvesicles are being studied for various applications in many fields. Although a number of reports have shown that proteins overexpressed in cells are incorporated into microvesicles derived from them, their potential for directed informational protein delivery is just now being explored. In this issue of Molecular Therapy, Mangeot et al.1 document microvesicle-mediated transfer of two different proteins that are able to temporarily manipulate the phenotype of the recipient cells. The microvesicles were generated by expression of the spike glycoprotein of vesicular stomatitis virus (VSV-G), which stimulates their production. The authors coined the term “gesicles” to describe the modified microvesicles, which represent an important new functional twist in the expanding and diverse armamentarium of molecular information transfer for therapeutic and experimental applications.
“Microvesicle” is a collective term for different types of membranous elements, ranging from 20 to 1,000 nm in diameter, that are released from and taken up by most types of cells (Figure 1). The associated terminology is rapidly expanding and can be confounding because many preparations reported in the literature represent heterogeneous mixtures of extracellular membranous organelles. Indeed, at present, there are few firm criteria that distinguish one type of microvesicle from another.2,3,4 Nanoparticles, exosomes, microparticles, shedding microvesicles, apoptotic blebs, and human endogenous retroviral particles are all different types of microvesicles. Indeed, one of the difficulties in the microvesicle field is that these disparate terms are often used interchangeably, and into this Gemisch we now must add gesicles.
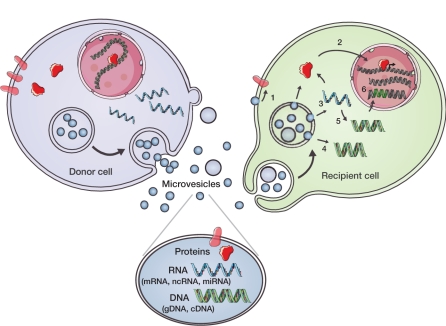
Figure 1.
Microvesicle-mediated intercellular communication. Components of donor cells are incorporated into microvesicles (e.g., exosomes, shedding microvesicles, and apoptotic blebs) that contain proteins, e.g., signaling proteins, transcriptional regulators, reverse transcriptase, and transmembrane proteins; RNA (messenger RNA (mRNA), noncoding RNA (ncRNA), and microRNA (miRNA)); and DNA (genomic DNA (gDNA) and complementary DNA (cDNA)). Microvesicles may initiate signals through interaction between ligands on their surface and receptors on the recipient cell and/or have their contents taken up by recipient cells through endocytosis or fusion at the plasma membrane. (1) Transmembrane proteins can be transferred to the plasma membrane and trigger signaling. (2) Transcriptional regulators can be transferred into the nucleus and regulate promoter activity. (3) mRNAs/miRNAs can be transferred and influence the translational profile. (4) Donor cell–derived cDNAs (e.g., for c-Myc) can be delivered into the recipient cytoplasm (5) or generated from reverse-transcribed mRNAs. (6) Retrotransposon and other DNA elements from microvesicles may integrate into the recipient cell genome. These microvesicle delivery events have the potential to change the phenotype of recipient cells on a short- or long-term basis. (Reprinted with permission from ref. 22.)
Native microvesicles released from different cells have been implicated in a host of normal cell functions. These include immune enhancement and repression,3 tissue repair,5 reproduction,6 and cancer progression (invasion, metastases, and angiogenesis7). The typical microvesicle carries a range of proteins, which can include Rabs, annexins, tetraspanins, heat shock proteins, metabolic enzymes, antigen-presenting proteins, signal transduction molecules, and cell adhesion elements.4 Other proteins shown to be delivered by microvesicles include oncoproteins, such as the mutant activated epidermal growth factor receptor (EGFRvIII), which promotes cell proliferation8; the chemokine receptor CCR5, which expands the cell type range of HIV infection9; ephrins and ephrin receptors involved in cell adhesion and signaling;4 and reverse transcriptase, which can promote pseudogene integration into the recipient genome.10 One area in which this form of protein transport plays an important biological role is during formation of blood clots, where microvesicles (here called microparticles) serve as natural circulating carriers of tissue factor, an essential membrane receptor for activation of the coagulation cascade.11
Workers have also begun to exploit these agents for therapeutic application. For example, microvesicles from dendritic cells (called exosomes, see below) have been loaded with viral or tumor antigens or with chemokines for use in vaccination strategies, and have been shown to greatly enhance the immune response. In addition to protein delivery, microvesicles also transport mRNA, microRNA, noncoding RNA, retrotransposon elements, and DNA fragments (both genomic and cDNAs) with the potential to alter the fate of the recipient cells.10,12,13,14,,15,16 In vivo, microvesicles are released into body fluids, and their contents have been shown to serve as biomarkers for disease states, such as cancer. The content and mode of release and uptake of microvesicles vary between different types of cells, and, in addition to serving as a means of communication, they have been shown to play a role in the elimination of unwanted cellular components or drugs.
In their new study, Mangeot and colleagues exploited the capacity of microvesicles for information transfer so as to deliver functional proteins that can alter the phenotype of the recipient cells in a transient nature, in a fashion somewhat analogous to that of the “cookies” that transmit information back and forth between computers. They first report increased production of microvesicles following transfection of cells with the spike VSV-G. As noted above, they use the term “gesicles” to refer to these modified microvesicles, which represent only one of a variety of membrane-bound particles released from the transfected cells. When cells were cotransfected with expression cassettes for a viral receptor protein or a transactivating protein, these directive proteins were incorporated into the gesicles, which efficiently delivered the functional proteins to recipient cells. The authors validated the method by generating induced pluripotent stem cells from human fibroblasts following gesicle-mediated delivery of the entry receptor for a murine retrovirus. This renders the recipient cells temporarily permissive to infection with murine leukemia virus–pseudotyped ecotropic (which thus normally only infect mouse cells) lentivirus vectors encoding reprogramming transcription factors. Importantly, this strategy allows the whole process to be undertaken at a lower biosafety level (BL2) than that (BL3) usually required for transduction of human cells with amphotropic lentivirus vectors bearing transformative genes.
The authors also show that they can deliver drug-inducible transactivation factors, including modified versions of the TET-off nuclear transactivator (tTA) and the tTRKrab regulator, so as to achieve temporary, dose-dependent, and tightly regulated reporter transgene expression in recipient cells. They used an interesting strategy to increase the content of tTA in the microvesicles by fusing the former to a farnesylated peptide motif from the C terminus of HRAS, which directs the fusion protein to the plasma membrane. Intriguingly, the tTA fusion protein retains the ability to function in the nucleus of the recipient cell. Another method used to incorporate proteins into microvesicles is to incorporate a plasma membrane anchor onto an oligomeric cytoplasmic protein, as a trigger for plasma membrane extrusion.17
Interestingly, the protein-loaded gesicles differ with respect to both biophysical properties and proteome profile from the best-characterized type of microvesicle released from cells, namely, exosomes. Exosomes are of endosomal origin and derive from inward budding of the endosomal membrane so as to generate multivesicular bodies that release exosomes from the cells following fusion with the plasma membrane. Although exosomes and gesicles are both spherical nanomembranous vesicles, gesicles are more heterogeneous in size (average diameter 100 nm) and appear slightly less dense (flotation density ~1.09 g/ml) compared with exosomes (40–80 nm diameter and 1.11 g/ml flotation density). Furthermore, the exosomal marker proteins Tsg101 and Alix, which belong to the family of proteins known as the endosomal sorting complex required for transport, and trans–Golgi network proteins found in exosomes (R.J.S., unpublished data), are absent from gesicles. These observations suggest that gesicles exocytose by a hitherto-unknown trafficking mechanism, and that the means of their uptake by recipient cells remains to be elucidated. Thus, gesicles appear to represent a fundamentally new type of microvesicle that will require much additional characterization before application as a therapeutic delivery vehicle can be contemplated.
Nevertheless, beyond their technical usefulness, gesicles and other microvesicles may eventually provide a new type of vector for gene/protein delivery that should be very efficient with low toxicity and immunogenicity because they can be derived from host cells and should thus be compatible with clinical applications, as in cancer immunotherapy vaccinations.18 Microvesicles can be loaded ex vivo, for example, by first transfecting producer cells with expression cassettes encoding proteins, mRNA, or microRNA, and then isolated by centrifugation or other methods. Isolated microvesicles can also be used for macromolecular “drug” delivery. In a recent seminal article, Alvarez-Erviti et al.19 showed that microvesicles electrophoretically filled with small interfering RNA could be targeted to selective tissues in vivo by incorporating a ligand in the plasma membrane of the producer cells. Furthermore, a poster presented at the recent annual meeting of the American Society of Gene and Cell Therapy showed that microvesicles can also carry rAAV vectors.20 In general, microvesicles are avidly taken up by recipient cells, thereby effecting a change in state that can be either temporary or long term. They have the advantages of a relatively stable constitution both in vitro and in vivo and the potential of being immune-compatible with the host when derived from isogenic cells. Their main disadvantage at this stage is that we lack a clear understanding of both their full complexity and their biogenesis, and—returning to the cookie-software analogy—they pose the risk of codelivery of a wide range of other biologically active “malware” that might adversely impact recipient cells. Indeed, the diversity of microvesicle content is retained in gesicles. The overexpressed proteins introduced by Mangeot et al. represent only about 2.5% of the protein content in gesicles, and the broad range of their other cellular components may have unexpected consequences. Indeed, the cultured cells used to generate gesicles usually exhibit some degree of immortality or transformation, processes typically associated with the expression of mutant proteins and nucleic acids—some of which may be oncogenic or capable of other permanent changes.21 This important caveat notwithstanding, Mangeot et al. clearly document new uses for these novel delivery vehicles, and expand their promise to facilitate gene and cell therapy.
REFERENCES
- Mangeot P-E, Dollet S, Girard M, Ciancia C, Joly S, Peschanski M.et al. (2011Protein transfer into human cells by VSV-G-induced nanovesicles Mol Ther 191656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G., and, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Théry C, Ostrowski M., and, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Ji H., and, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Tetta C, Bruno S, Fonsato V, Deregibus MC., and, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7:105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheva-Nilsson L., and, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S.et al. (2008Tumour-released exosomes and their implications in cancer immunity Cell Death Diff 1580–88. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A.et al. (2008Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells Nat Cell Biol 10619–624. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J.et al. (2000Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection Nat Med 6769–775. [DOI] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakefield XO.et al. (2011Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences Nat Commun 2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JI, Trenor CC, Furie BC., and, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31:728–733. doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P.et al. (2006Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery Leukemia 20847–856. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ., and, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L.et al. (2008Detection of microRNA expression in human peripheral blood microvesicles PLoS One 3e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD., and, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer D, Gainche L, Curry WTJ.et al. (2008Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers Nat Cell Biol 101470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Wu N, Yang JM., and, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286:14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O.et al. (2010Dendritic cell-derived exosomes for cancer immunotherapy: what's next Cancer Res 701281–1285. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S., and, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Balaj L, Sivaraman S, Skog J, Sena-Esteves M, Breakefield XO.et al. (2011AAV vectors copurify with microvesicles in producer cell media: implications for gene transfer and vector purification [abstr 130] Mol Ther 19suppl. 1S51 [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL.et al. (2011Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells Proc Natl Acad Sci USA 1084852–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vos KE, Balaj L, Skog J., and, Breakefield XO.2011Brain tumor microvesicles: insights into intercellular communication in the nervous system Cell Mol Neurobiole-pub ahead of print 8 May 2011. [DOI] [PMC free article] [PubMed]