Abstract
Vaccines that aim to expand tumor-specific CD8+ T cells have yielded disappointing results in cancer patients although they showed efficacy in transplantable tumor mouse models. Using a system that more faithfully mimics a progressing cancer and its immunoinhibitory microenvironment, we here show that in transgenic mice, which gradually develop adenocarcinomas due to expression of HPV-16 E7 within their thyroid, a highly immunogenic vaccine expressing E7 only induces low E7-specific CD8+ T-cell responses, which fail to affect the size of the tumors. In contrast, the same type of vaccine expressing E7 fused to herpes simplex virus (HSV)-1 glycoprotein D (gD), an antagonist of the coinhibitory B- and T-lymphocyte attenuator (BTLA)/CD160-herpes virus entry mediator (HVEM) pathways, stimulates potent E7-specific CD8+ T-cell responses, which can be augmented by repeated vaccination, resulting in initial regression of even large tumor masses in all mice with sustained regression in more than half of them. These results indicate that active immunization concomitantly with blockade of the immunoinhibitory HVEM-BTLA/CD160 pathways through HSV-1 gD may result in sustained tumor regression.
Introduction
In vitro expanded tumor antigen (TA)-specific T cells either isolated from patients' tumor material1 or genetically modified to express a chimeric antigen receptor composed of an antigen-specific single-chain immunoglobulin variable fragment linked to a T-cell-receptor-signaling domain with intracellular cosignaling motifs from CD28, 4-1BB, or others2 can cause regression of even large tumor masses when transferred back into partially myeloablated human subjects. This supports the concept that T cells, especially CD8+ T cells, can have substantial clinical benefit to end-stage cancer patients. Nevertheless, even ex vivo manipulated autologous TA-specific T cells appear to be susceptible to the immunosuppressive tumor microenvironment (TME) as upon transfer they commonly fail to survive long enough to affect tumor regression.3 Cancer vaccines aiming to induce or expand TA-specific CD8+ T cells in vivo have in general yielded disappointing results in clinical trials,4 presumably also reflecting that a tumor during its progression causes a gradual impairment of the patients' TA-specific CD8+ T cells, which cannot be reversed by traditional vaccines. In addition, cancer vaccines have been shown to increase frequencies of TA-specific regulatory T (Treg) cells, thus further weakening vaccine-induced CD8+ T-cell responses.5,6
Here, we tested the hypothesis that blockade of a coinhibitory pathway concomitantly with active immunization would overcome defects of TA-specific CD8+ T-cell responses and thus improve the efficacy of a therapeutic vaccine in cancer-prone transgenic (tg) mice. Specifically, we tested blockade of the coinhibitory herpes virus entry mediator (HVEM)–B- and T-lymphocyte attenuator (BTLA)/CD160 pathways. HVEM upon binding to BTLA and CD160 sends inhibitory signals to CD8+ T cells,7,8 and this is blocked by herpes simplex virus (HSV)-1 glycoprotein D (gD)9 resulting in more potent CD8+ T-cell responses to an antigen fused into the C-terminus of gD.10,11,12 The HVEM pathway is further used by FoxP3+CD25+CD4+ Treg cells, which through expression of HVEM can bind and signal through BTLA to effector T cells.13
We had shown previously that the augmented CD8+ T-cell response to E7 oncoprotein of human papilloma virus (HPV)-16 expressed within gD allows for complete rejection of rapidly growing transplanted tumor cells even if animals were vaccinated shortly after they developed small tumors.12,14 Transplantable tumor models have the limitation that they fail to accurately mimic the immunoinhibitory effects of the microenvironment of slowly progressing tumors and thus commonly paint an unduly optimistic picture on the effectiveness of immunotherapeutic treatments of cancer. We, therefore, tested vaccines based on adenovirus (Ad) vectors expressing only E7 or E7 fused into the C-terminus of HSV-1 gD in mice that had been genetically engineered to express the E7 under a thyroid-specific promoter.15 The E7-tg mice, which gradually develop thyroid hyperplasia and by 6–8 months of age thyroid adenocarcinomas with a 100% penetrance, clearly fail to mimic cervical cancer, the most common clinical sequela of persisting HPV-16 infections, but provide a suitable model to assess therapeutic vaccines that target a TA to which the immune system is partially tolerant due to its presence during early development.16 The model also allows an evaluation of the effects of a slowly progressing cancer on functions of vaccine-induced immune responses. We show here that in this model vaccination with E7 expressed as a fusion protein within gD induces a potent E7-specific CD8+ T-cell response without increasing frequencies or numbers of FoxP3+CD25+CD4+ Treg cells and most importantly affects tumor regression in all mice with large tumor masses, which in more than half of the vaccinated mice is sustained for at least 6 months.
Results
Magnitude of vaccine-induced E7-specific CD8+ T-cell responses in wild-type, HVEM-KO, and E7-tg mice
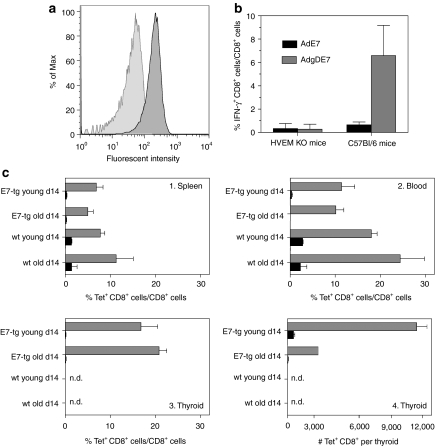
The initial experiments were designed first to test whether expressing an antigen within gD augmented CD8+ T-cell responses through blockade of the immunoinhibitory HVEM pathway and second to assess whether E7 expressed on the thyroid affected stimulation of E7-specific CD8+ T cells in young E7-tg mice with thyroid gland hyperplasia or in older E7-tg mice with adenocarcinomas by a vaccine. To address the first question, groups of HVEM-knockout (HVEM-KO; ref. 17) mice, which lack HVEM expression (Figure 1a), were immunized once with 5 × 1010 virus particles of replication-defective Ad vectors of human serotype 5 expressing E7 (AdE7) or E7 fused into gD (AdgDE7). Mice were bled 2 and 4 weeks later and frequencies of E7-specific CD8+ T cells were determined upon culture of peripheral blood mononuclear cells with the E7 peptide or an unrelated peptide by intracellular cytokine staining (ICS) of CD8+ T cells for interferon-γ (IFN-γ). Wild-type (wt) C57Bl/6 mice immunized with the same set of vaccines were tested by ICS for comparison at 3 weeks after vaccination. As shown in Figure 1b, wt mice immunized with the AdgDE7 vaccine developed significantly higher frequencies of IFN-γ producing E7-specific CD8+ T cells compared to those vaccines with the AdE7 vector. In contrast, in HVEM-KO mice both vaccines resulted at either time point (Figure 1b shows result for the analyses conducted at 4 weeks after immunization) in CD8+ T cells of comparable magnitude confirming that the gD-mediated enhancement of CD8+ T-cell responses depended on HVEM.
Figure 1.
Vaccine-induced E7-specific CD8+ T-cell responses. (a) Peripheral blood mononuclear cells (PBMCs) of herpes virus entry mediator knockout (HVEM-KO) mice or wild-type (wt) C57Bl/6 mice were stained with antibodies to CD3 and HVEM. Graphs shows intensity for the HVEM stain on CD3+ cells from wt C57Bl/6 mice (dark gray) and HVEM-KO mice (light gray) over % of maximal events. (b) HVEM-KO mice or wt C57Bl/6 mice were vaccinated with AdE7 (black squares) or AdgDE7 (open squares). HVEM-KO mice were bled 4 weeks later and frequencies of E7-specific CD8+ T cells were determined by intracellular cytokine staining for interferon-γ. PBMCs from C57Bl/6 mice were tested 3 weeks after immunization. Significance of differences between the groups was determined by one-tailed Student t-tests. For HVEM-KO mice comparing responses to AdE7 to those of AdgDE7 P value was 0.43; for wt C57Bl/6 mice the difference between these two vaccine groups was statistically significant (P = 0.0004). (c) Young (2-month-old) and older (8- to 10-month-old) wt C57Bl/6 and E7-tg mice were intramuscularly (i.m.) immunized with 5 × 1010 virus particles of E1-deleted Ad vectors carrying either E7 (black bars) or gDE7 (gray bars). Fourteen days later (1) spleens, (2) PBMCs isolated from wt and E7-tg mice and lymphocytes isolated from (3, 4) thyroid glands of E7-tg mice were analyzed by staining with the E7-tetramer. Graphs c1–3 show frequencies of E7-tet+CD44highCD8+ cells over total CD8+ cells, and c4 shows numbers of E7-tet+CD44highCD8+ cells per thyroid gland. Experiments were conducted with 3–10 mice per group. Graphs show means ± SD. Significance of differences between groups was calculated by one-tailed Student t-test. Comparing mice matched for strain (E7-tg versus wt mice), tissue (spleen, blood, or thyroid), and age (young versus older) groups immunized with AdE7 versus AdgDE7 showed significant differences in all comparisons with P values <0.01. Comparing young versus older wt mice immunized with either of AdE7 or AdgDE7 vaccines showed no significant age-related differences (P > 0.1). Comparing young wt to young E7-tg mice or older wt to older E7-tg mice vaccinated with AdE7 showed significant differences (P values <0.002). Conducting the same comparison for AdgDE7-vaccinated mice showed for young wt and E7-tg mice significant differences in blood (P < 0.006) but not spleen (P = 0.2), while older wt versus older E7-tg mice showed significant differences in blood and spleens (for both, P < 6E-5). n.d. – not done.
To assess whether growing E7-expressing tumors affected the vaccines' ability to induce CD8+ T-cell responses, groups of E7-tg C57Bl/6 mice and age-matched wt C57Bl/6 mice were immunized at 8–10 weeks (young) or at 8–10 months (older) of age once with 5 × 1010 virus particles of AdE7, AdgDE7, or AdgD. Frequencies of E7-specific CD8+ T cells were tested with an E7-specific major histocompatibility complex class I tetramer (E7-tet) 14 days later (Figure 1c). In young and older mice, the AdE7 vaccine induced approximately tenfold lower frequencies in E7-tg mice than in wt mice and this difference was highly significant. AdgDE7 induced significantly higher E7-specific CD8+ T-cell responses than AdE7 in all cohorts tested and responses in E7-tg mice were only approximately twofold lower than those in age-matched wt mice. Responses in the thyroid were tested in E7-tg mice but not in wt mice as their small thyroids failed to yield sufficient numbers of lymphocytes. E7-specific CD8+ T-cell responses were high only in AdgDE7-vaccinated E7-tg mice shown by both frequencies (Figure 1c) and absolute numbers of E7-specific CD8+ T cells per thyroid (Figure 1d). The AdgD control vaccine failed to induce E7-specific CD8+ T-cell responses in any of the groups (data not shown). As reported previously E7-tg mice are tolerant to E7 and fail to mount strong CD8+ T-cell responses to traditional vaccines expressing this antigen.16,17 As shown here a vaccine that concomitant to the TA expresses an antagonist of the coinhibitory HVEM pathways can readily overcome the CD8+ T cells' tolerance and induce responses that are only marginally lower than those achieved in wt mice.
Kinetics of responses in E7-tg mice
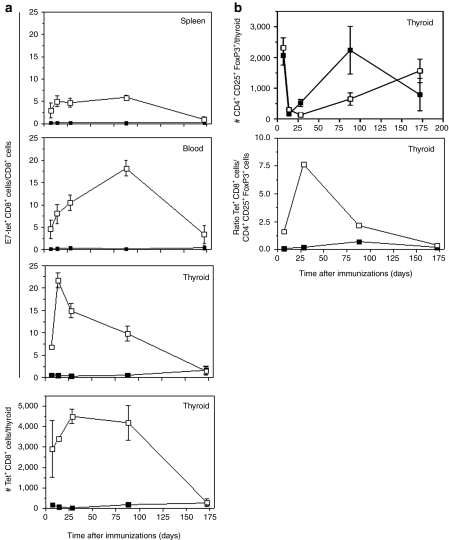
Upon intramuscular injection into a mouse E1-deleted Ad vectors can persist at low levels in T cells for at least a year. Their genome continues to be transcribed so that frequencies of CD8+ T cells to an Ad vector's transgene product are maintained at exceptionally high levels for long periods of time.18,19 To assess whether the Ad vectors achieved sustained E7-specific CD8+ T-cell responses in tumor-bearing mice, 8-month-old E7-tg mice were vaccinated either with AdE7, AdgD, or AdgDE7 and the magnitude of the E7-specific CD8+ T-cell response was tested over a period of 172 days from spleens, blood, and thyroid glands (Figure 2a). Again AdE7 induced only a low CD8+ T-cell response to E7. Mice immunized with AdgD failed to show detectable frequencies of E7-specific CD8+ T cells indicating that the tumor per se was poorly immunogenic (data not shown). The time course of the response to AdgDE7 detected by E7-tet staining was unexpected and differed markedly from previous reports, which had shown that CD8+ T cell responses to an Ad vector's transgene product including those fused to gD typically peak within the first 2 weeks, slightly decline thereafter, and then remain stable.12,19 In spleens of AdgDE7-immunized mice, frequencies of E7-specific CD8+ T cells detected by E7-tet staining were stable between days 7 and 88, but then by day 172 became markedly lower. In blood, frequencies continued to rise between days 7 and 88 following vaccination and then also sharply declined by day 172. In tumor-bearing thyroids, frequencies and numbers of E7-tet+CD8+ T cells peaked on day 14 and then gradually declined. Aliquots of cells from the same mice were tested for frequencies of E7-specific CD8+ T cells by ICS for IFN-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α. Using this functional assay, frequencies of E7-specific CD8+ T cells were slightly lower in spleens than those detected by E7-tet staining but the overall pattern was similar. In blood, responses peaked slightly later, i.e., by day 28 and then showed a marked drop by day 88 and declined further by day 172. An enrichment of E7-specific CD8+ T cells within the tumor-bearing thyroid glands was only seen early after immunization; by day 88 frequencies in thyroids had dropped below those in blood. Staining for cytokines showed markedly lower frequencies in blood and thyroid glands than E7-tet-staining, suggesting that a substantial fraction of circulating and tumor-infiltrating E7-specific CD8+ T cells either produced factors other than those we tested for or had lost functions.
Figure 2.
Kinetics of vaccine-induced CD8+ T and regulatory T cells (Treg) cells. Analyses were carried out using cells isolated from spleen, blood and thyroid glands of older E7-tg mice intramuscularly immunized with Ad vectors carrying either E7 (black squares) or gDE7 (open squares). (a) Frequencies of E7-tet+CD44highCD8+ were determined from lymphocytes isolated from spleen, blood, and thyroid glands. Bottom graph shows numbers of E7-tet+CD44highCD8+ cells isolated from thyroid glands. (b) FoxP3+CD25+CD4+ cells were isolated from thyroid glands of AdE7 (black squares) or AdgDE7 (open squares) mice. Top graph shows numbers of FoxP3+CD25+CD4+ cells isolated from individual thyroid glands; middle graph shows ratios of E7-Tet+CD8+ T cells over CD4+CD25+FoxP3+ cells. Day 0 reflects data gained from unvaccinated age-matched E7-tg mice.
To test whether vaccination affected tumor-infiltrating Treg cells, numbers of CD4+CD25+FoxP3+ cells within the thyroid glands were determined (Figure 2b). In unvaccinated tumor-bearing E7-tg mice, substantial numbers of infiltrating CD4+ cells stained positive for FoxP3 and most of those (~90%) were also positive for CD25. Early after immunization with either of the two vaccines numbers of CD4+CD25+FoxP3+ cells within the thyroid decreased (Figure 2b). Such a decrease was not seen in blood or spleen (not shown) suggesting that the decrease in CD4+CD25+FoxP3+ cells may have depended on the presence of antigen. Frequencies of CD4+CD25+FoxP3+ cells eventually increased again in thyroids of AdgDE7- or AdE7-immunized mice albeit with a delay in the latter. Overall differences in numbers of Treg cells comparing lymphocyte samples from spleens, blood (not shown), and thyroids of naive to vaccinated E7-tg mice were subtle and results did not indicate that vaccination supported their expansion. It has previously been shown that ratios of TA-specific CD8+ T cells to Treg cells rather than absolute Treg cell numbers are predictive for the speed of cancer progression.20,21 Ratios for E7-Tet+CD8+ T cells over CD4+CD25+FoxP3+ T cells were significantly higher in thyroids of AdgDE7 as compared to AdE7-vaccinated mice with peaks around day 28 and then gradually decreased till day 172 when ratios became comparable between the two cohorts.
Expansion of E7-specific CD8+ T cells upon repeated immunization
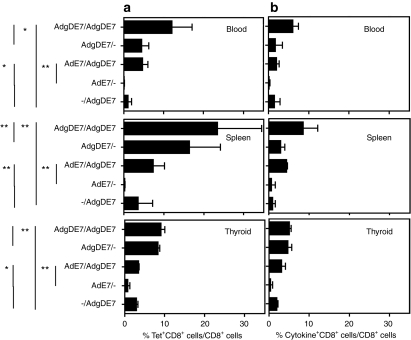
The marked and early contraction of E7-specific CD8+ T cells in thyroids and the delayed contraction in blood and spleen observed in AdgDE7-vaccinated mice by E7-tet staining could reflect removal of antigen upon destruction of E7+ tumor cells or alternatively could indicate loss of CD8+ T cells due to the immunosuppressive microenvironment of the tumor that affected their ability to expand and instead induced their death. To assess whether E7-specific CD8+ T cells were able to expand, tumor-bearing E7-tg mice were primed with AdE7 or AdgDE7. Some of the mice were boosted 2 months later with an AdgDE7 vector. To avoid reduction of recall responses by neutralizing antibodies to the Ad vaccine, mice were primed with vectors based on human serotype 5 Ad, they were boosted with a serologically distinct vector based on chimpanzee serotype 68 Ad; additional E7-tg mice only received the 2nd vaccine. Frequencies of E7-specific CD8+ T cells were tested 14 days later from spleen, blood, and thyroid glands by tetramer (Figure 3a) or ICS (Figure 3b). In spleen, the staining booster immunization resulted in a significant increase in frequencies of E7-specific CD8+ T cells compared to those achieved in mice that received only the 1st or 2nd vaccine, suggesting that E7-specific CD8+ T cells were able to expand. In blood and thyroids, AdgDE7 booster immunization failed to increase frequencies of E7-specific CD8+ T cells of mice that had been primed with the serologically distinct Ad vector expressing the same antigen. The same trends were seen upon measuring frequencies by ICS for IFN-γ, IL-2 and TNF-α (Figure 3b).
Figure 3.
T cell responses upon prime boost immunization. E7-specific (a) CD8+ and (b) cytokine+CD8+ T-cell response were analyzed ~80 days after the first immunization and 14 days after the booster immunization. To calculate % cytokine producing CD8+ T cells over CD8+ T cells as shown in b, the percentages of cells producing either of seven possible combinations of cytokines (i.e., IFN-γ, IL-2, or TNF-α only, IFN-γ together with TNF-α or IL-2, TNF-α together with IL-2 or all three) in presence of the E7 peptide were calculated as follows: Background data were subtracted from frequencies of each of the E7 peptide triggered seven population and then the sum of all seven frequencies was used to determine total % cytokine producing CD8+ T cells. Older E7-tg mice immunized with either AdE7 or AdgDE7 were boosted with a chimpanzee-derived adenovirus carrying gDE7. Some animals were not boosted. Graphs show means ± SD. *P < 0.05; **P < 0.01; no asterisk, not significant.
Functionality of E7-specific CD8+ T cells
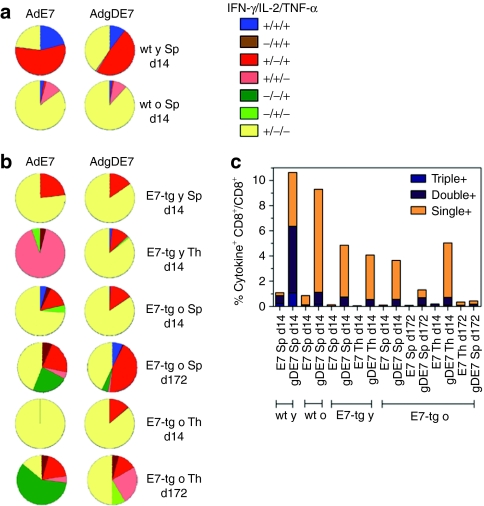
To further compare the functions of E7-specific CD8+ T lymphocytes, those from young and older wt and E7-tg mice were analyzed 14 days after vaccination for cytokine production profiles in response to the E7 peptide (Figure 4a,b). In young wt mice, AdE7 and AdgDE7 immunization induced E7-specific CD8+ T cells that produced IFN-γ and TNF-α or IFN-γ only; a small fraction produced all three cytokines. In spleens of older wt mice and young or older E7-tg mice, CD8+ T cells producing IFN-γ only dominated the response. The same profile was seen in thyroid-infiltrating specific CD8+ T cell from mice immunized with the AdgDE7 vaccine; upon AdE7 immunization those from young E7-tg mice produced only IFN-γ while those from older mice produced IFN-γ in combination with IL-2.
Figure 4.
Cytokine expression profiles. Young and older (a) wt and (b) E7-tg mice immunized with AdE7 or AdgDE7 were analyzed for production of IFN-γ, IL-2, and TNF-α by CD8+ T cells upon stimulation with E7 peptide. Samples were analyzed 14 and 172 days after a single immunization. IFN-γ+/IL-2+/TNF-α+: blue; IFN-γ−/IL-2+/TNF-α+: brown; IFN-γ+/IL-2−/TNF-α+: red; IFN-γ+/IL-2+/TNF-α−: pink; IFN-γ−/IL-2−/TNF-α+: dark green; IFN-γ−/IL-2+/TNF-α−: green; and IFN-γ+/IL-2−/TNF-α−, yellow. (c) Graph shows frequencies of responding CD8+ T cells producing three, two, or one of any of the cytokines. IFN-γ, interferon-γ IL-2, interleukin-2; TNF-α, tumor necrosis factor-α y, young; o, old.
To assess whether the cytokine profile changed over time in tumor-bearing E7-tg mice, IFN-γ, IL-2, and TNF-α production was analyzed on day 172 following immunization. At the later time point, the response in spleens was less dominated by CD8+ T cells producing only IFN-γ and in mice that had received the AdgDE7 vaccine nearly half of the splenic CD8+ T cells produced IFN-γ together with TNF-α in response to the E7 peptide. In mice immunized with AdE7, a sizable fraction produced only TNF-α. Differences between the cohorts were more pronounced for E7-specific CD8+ T cells isolated from thyroid glands. Those from mice immunized with AdE7 produced mainly TNF-α alone or in combination with IFN-γ or IL-2; CD8+ T cells that produced IFN-γ only presented less than a quarter of the overall populations. In mice that had received the AdgDE7 vaccine, IFN-γ-producing CD8+ T cells were more common. As in spleens, a sizable fraction of E7-specific CD8+ T cells from thyroids produced both IFN-γ and TNF-α. Unlike in spleens, in thyroid glands a fraction of CD8+ T cells produced only IL-2 and about a quarter of the CD8+ T cells produced only TNF-α. Overall, these results show dynamic changes in cytokine profiles over time and indicate that although numbers of CD8+ T cell markedly declined over time in tumor-bearing E7-tg mice, this was not linked to a loss of cytokine production by those that remained. Furthermore, of interest was the observation that while early after AdgDE7 vaccination cytokine production profiles of tumor-infiltrating E7-specific CD8+ T cells largely mirrored those of cells isolated from spleen, patterns became markedly different at the later time point.
Phenotypes of E7-specific CD8+ T cells
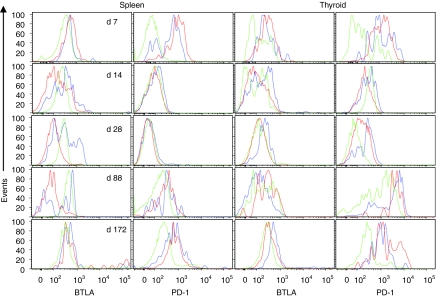
To determine whether blockade of the HVEM pathways affects differentiation of vaccine-induced CD8+ T cells, their phenotypes were analyzed from naive CD44lowCD8+ T cells and E7-tet+CD8+ T cells from spleens and thyroid glands of E7-tg mice immunized 14 days previously with the Ad vectors. There were no marked differences in expression levels of activation markers (CD44, CD62L, and CD127) or coinhibitory markers (PD-1, CD160, CTLA-4, BTLA) on E7-tet+CD8+ T cells from the spleens of young or older wt or E7-tg mice (data not shown). Differences in expression levels of BTLA and PD-1 were mainly seen in the thyroids of tumor-bearing older E7-tg mice. To test whether phenotypes changed over time in these mice, E7-tet+CD8+ T cells were analyzed from spleens and thyroids of tumor-bearing E7-tg mice on days 7, 14, 28, 88, and 172 following vaccination with AdE7 or AdgDE7 for expression levels of BTLA and PD-1 (Figure 5). On day 7, E7-tet+CD8+ T cells from spleens and thyroid expressed BTLA at slightly and PD-1 at markedly higher levels as compared to naive CD8+ T cells as would be expected early after their activation. By day 14, expression levels of BTLA declined on E7-tet+CD8+ T cells from spleen and thyroids of AdgDE7-vaccinated mice, while those of AdE7-induced CD8+ T cells stayed high. PD-1 levels decreased to those on naive T cells in both tissues and in both vaccine groups. On day 28 after immunization, BTLA levels remained low on E7-tet+CD8+ T cells from spleens and thyroids of AdgDE7-vaccinated mice but increased in those from mice that had received the AdE7 vaccine. PD-1 remained low on cells from spleens but was markedly increased on E7-tet+CD8+ T cells from thyroids of AdE7-immune mice and to a lesser degree on those from AdgDE7-vaccinated mice. By day 88 after vaccination, BTLA remained low on AdgDE7-induced splenic E7-tet+CD8+ T cells and levels were slightly higher on those from AdE7-immunized mice. Expression of BTLA was markedly higher on E7-tet+CD8+ T cells from thyroids than on naive CD8+ T cells. PD-1 was markedly increased on E7-tet+CD8+ T cells in spleens and thyroids of mice receiving either of the vaccines. By day 172 after vaccination, the pattern of BTLA expression remained constant compared to day 88. PD-1 expression appeared to decrease on thyroid-infiltrating E7-specific CD8+ T cells although they continued to express higher levels of PD-1 than naive T cells isolated from the same tissue. Overall, increases of coinhibitors, especially PD-1, suggest that vaccine-induced E7-tet+CD8+ T cells may become rapidly susceptible to suppressive signals in tumor-bearing mice and this is not prevented but rather delayed upon vaccine-mediated blockade of the HVEM-BTLA/CD160 pathways.
Figure 5.
Phenotypes of vaccine-induced E7-specific CD8+ T cells. Older E7-tg mice were immunized with Ad vectors carrying either E7 (blue lines) or gDE7 (red lines). Lymphocytes from spleens and thyroids were isolated 7, 14, 28, 88, and 172 days later, stained with antibodies to CD8, CD44, BTLA and PD-1, and the E7-tetramer. Tet+CD44hiCD8+ T cells were analyzed for expression of PD-1 or BTLA in comparison to tet−CD44lowCD8+ cells from naive mice (green). For each time point, spleens from five individual animals and 1 or 2 pools of lymphocytes from thyroids were analyzed. Data were then concatenated by FlowJo to generate the histograms. BTLA, B- and T-lymphocyte attenuator.
Efficacy of vaccination
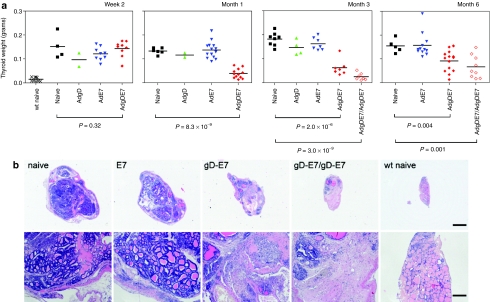
To determine whether vaccination affected tumor progression, older E7-tg mice were vaccinated once with AdE7, AdgD, AdgDE7, or nothing and the weights of their thyroid glands were measured 2 weeks, 1, 3, and 6 months later (Figure 6a). Other groups were primed with AdgDE7 and then boosted with a heterologous Ad vector 2 months later. Weights of their thyroid glands were measured 3 or 6 months after the 1st immunization (i.e., 1 or 4 months after the boost). Weights of thyroid glands isolated from the different groups of E7-tg mice were comparable by 2 weeks after vaccination and were significantly higher than those of age-matched wt mice. By 1 month after immunization, the weights of thyroid glands from AdgDE7-vaccinated E7-tg mice were significantly lower than those that received AdE7, AdgD, or nothing. Although the average weights of the thyroid glands increased by 3 months after AdgDE7 vaccination, thyroid glands from this group continued to show a significant reduction in weight compared to those from the other groups and 6 out of 7 mice still had thyroid weights below 0.1 g while average weight of thyroids from control mice were 0.18 g. By 6 months, thyroid gland weights of 7 out of 17 of the AdgDE7-vaccinated mice remained low while those of the other 10 mice increased. A prime boost regimen further decreased the weight of the thyroids by 1 month after the boost (i.e., 3 months after priming), but again at the later time point 6 out of 9 mice showed evidence of slowed tumor progression. Reduction in thyroid gland weight in AdgDE7-vaccinated E7-tg mice was linked to destruction of tumor cells; histological analyses of thyroid glands showed pronounce lymphocytic infiltrates, extensive fibrosis and signs of tumor cell necrosis (Figure 6b), which was not seen in thyroids of AdE7-vaccinated or naive E7-tg mice. An effect on metastases could not be evaluated in this model, as the thyroid tumors only very rarely spread to other sites; from over 300 mice tested only one of the aged E7-tg mice had a single metastatic tumor in the lung.
Figure 6.
Effect of vaccination on tumor progression. Older E7-tg mice were immunized with Ad vectors carrying either E7 or gDE7. Naive age-matched E7-tg animals and AdgD-immunized mice were used as controls. The weight of thyroids of age-matched C57Bl/6 mice was determined to provide a reference. (a) Weights of the thyroid gland from each individual E7-tg mouse 2 weeks, 1, 3, and 6 months days after immunization with AdE7 or AdgDE7 are shown. Two months after priming some AdE7- and AdgDE7-immunized mice were boosted with an AdgDE7 vaccine based on chimpanzee serotype 68. (b) Sections of thyroid glands from naive or vaccinated E7-tg mice or wt mice were analyzed by microscopy (3 months after immunization). Thyroid gland sections from one representative mouse of the most pertinent groups are shown at 2× (top panels) and 10× (bottom panels) magnification. Bar = 1 mm (top panels) and 200 µm (bottom panels).
Discussion
Therapeutic cancer vaccines are commonly ineffective in achieving the robust expansion of functional TA-specific CD8+ T cells needed to achieve tumor regression.4,22 This may in part relate to immunological tolerance to those TAs that as differentiation antigens are expressed during development. Alternatively the immunosuppressive TME may affect CD8+ T-cell functionality and survival23 or effector T-cell responses may become impaired due to Tregs.24 Additionally, cancer vaccines have been shown to not only expand TA-specific effector cells but also Tregs, which appear better equipped to withstand the oxidative stress of the TME. The net results of vaccination can be immunosuppression rather than activation of beneficial immune responses.24 Immunotherapy can be achieved in some types of cancers, such as melanoma, by adoptive transfer of ex vivo expanded tumor-infiltrating lymphocytes or in other types of cancer by transfer of the patients' T cells genetic engineered to carry an chimeric T-cell receptor that binds with high affinity to a cell-surface-expressed TA.1,2 Either type of in vitro–manipulated T cell has shown clinical benefit and in some cases even achieved complete remission especially upon transfer into myeloablated patients. Nevertheless, in many patients, including those that receive lymphodepleting regimens, the transferred T cells fail to engraft and hence are unable to achieve reduction of tumor masses again stressing the detrimental effects of tumors on adaptive TA-specific immune responses. Immunosuppression within the TME is a multifaceted and still poorly defined process; antigen presentation in the absence of costimulators25 but rather in the presence of coinhibitors,26 presence of Treg cells24 and metabolic stress27 within the oxygen-deprived TME have been identified as common denominators in a variety of cancers. Attempts to circumvent some of the immunosuppressive effects of the TME by depletion of Tregs cells,28 blockade of the PDL1/PD-129 or CD28/CTLA-430 pathways, or adjuvanting cancer vaccines with immunostimulatory moieties, such as Toll-like receptor agonists,31 costimulators of the B7 family32 or cytokines,33 have shown promise in supplementing cancer therapies in animal models but less so in early clinical trials.
Our studies have focused on improving CD8+ T-cell responses to a therapeutic cancer vaccine through gD-mediated blockade of HVEM binding to BTLA or CD160. HVEM serves as a bifunctional ligand that submits costimulatory signals upon binding to LIGHT and coinhibitor signals upon binding to BTLA or CD160.7 HSV-1 gD binds close to the BTLA/CD160 sites and thus blocks their ligation to HVEM without affecting binding of LIGHT.7,34,35 HSV-1 gD-mediated blockade of the coinhibitory HVEM interactions changes HVEM into an exclusively costimulatory molecule, which results in enhanced adaptive immune responses. As we had shown previously with the transplantable TC-1 tumor model, Ad vectors12 or DNA14 vaccines expressing E7 fused into gD induce potent CD8+ T-cell responses that are able to prevent tumor formation and even affect tumor regression if immunizations are given shortly after the injection of tumor cells. As shown here, this augmentation requires expression of HVEM as it was not observed in HVEM-KO mice. Of equal importance to cancer is our subsequent finding that a viral antigen expressed within gD significantly enhances CD8+ T-cell responses not only in young but also in very old mice10 suggesting that blockade of this pathway could at least in part overcome immunosenescence, which is crucial for active immunotherapy of cancer, a disease that most commonly affects the elderly.
To assess whether blockade of the HVEM pathway could induce regression of slowly progressing tumors in an animal model, we here tested Ad vectors expressing only E7 or E7 within gD in mice that due to expression of E7 under a thyroid-specific promoter develop papillary thyroid adenocarcinomas. Similarly, mice expressing E7 together with E6 have been used previously to study the therapeutic benefits of Listeria-based vaccines to E7. In this model, Listeria-induced E7-specific CD8+ T-cell responses were markedly lower in E7/E6-tg mice than in wt mice, the T-cell receptor affinity to E7 appeared to be reduced, and decreases in tumor progression were only seen upon multiple immunizations given before tumor development.16,17 Similarly an Ad vector expressing only E7 that we had shown previously to protect against tumor formation in the TC-1 model36 neither induced robust E7-specific CD8+ T-cell responses nor provided clinical benefit to tumor-bearing E7-tg mice. In contrast, the vaccine expressing E7 within gD induced high frequencies of E7-specific CD8+ T cells and achieved initial reduction in tumor size in all of the vaccinated mice and in ~60% of mice lack of tumor progression was observed for up to 6 months. The AdgDE7 vaccine not only induced higher frequencies of E7-specific CD8+ T cells than the AdE7 vaccine, but also T cells that within the tumor environment had a distinct cytokine production profile and phenotypes compared to those induced by AdE7 with the former showing a delay in upregulation of BTLA, which can provide a target for Tregs13 and PD-1, a marker for T-cell exhaustion.23 Whether these differences were caused by the initial activation of T cell in the presence of the HVEM antagonist or were secondary due to differences in tumor burden due to increased numbers of E7-specific CD8+ T cells in AdgDE7-vaccinated mice remains to be investigated in more depth in adoptive transfer studies.
Although the AdgDE7 vaccine induced high-frequency E7-specific CD8+ T-cell responses and initial reduction in tumor burden in all mice tested, the caveat should be pointed out that the response was not sustained and 1 out of 7 and 8 out of 13 of the vaccinated mice showed evidence of renewed tumor progression after 3 and 6 months, respectively. Although a 2nd immunization with a serological distinct Ad vector initially further reduced tumor burden, the effect of the boost was also transient and 3 out of 9 mice that received two immunizations also showed tumor progression at 4 months after the 2nd immunization. Additional studies will be needed to further elucidate the mechanism that in the end in spite of an impressive initial reduction in tumor size in AdgDE7 vaccinated E7-tg mice allowed for a gradual decline of vaccine-induced E7-specific CD8+ T cells, which was especially pronounced within the tumor-bearing thyroids. We saw no evidence for increases in FoxP3+CD25+CD4+ T-cell frequencies or numbers upon Ad vector immunization, which may in part relate to peculiarities of this particular vaccine platform as Ad vectors induce very strong innate cytokine responses such as IL-6,37 which has been shown previously to disfavor Treg cell induction.38 Alternatively or in addition, HVEM is also one of the effector molecules on Treg cells and its blockade may have prevented vaccine-induced expansion of Treg cells. We did observe in thyroids of AdgDE7 vaccinated mice over time an increase of an atypical CD4+ population that expressed high levels of FoxP3 but lacked expression of CD25 (data not shown), a molecule that is thought essential for Treg cells with their high demands for IL-2. Unlike thyroid-infiltrating FoxP3+CD25+CD4+ Treg cells, FoxP3+CD25−CD4+ cells, described previously in chronic infectious human diseases39 were also observed albeit at lower frequencies in the thyroids of naive E7-tg mice but their phenotypes were modulated in mice that were vaccinated with reduced expression levels of PD-1, CD160, and HVEM. Whether these cells are indeed suppressive and contributed to the decline of vaccine-induced E7-specific CD8+ T cells remains to be investigated in more depth.
Overall, data presented here conclusively show that a vaccine that expresses a TA within HSV-1gD unlike a traditional vaccine that only expresses the TA induces a robust TA-specific CD8+ T-cell response that achieves tumor regression in a tg mouse model with slowly progressing thyroid gland tumors. This suggests that vaccines that not only aim to provide antigen in the context of an immunostimulatory carrier, such as an Ad vector, but also concomitantly target coinhibitory pathways may be one avenue to render active cancer immunotherapy more efficacious. Incorporation of antagonists into the vaccine rather than their systemic application has the added advantage that effects are expected to be limited thus lessening the risk of unwanted global side effects caused by disruption of key immunoregulatory pathways.
Materials and Methods
Construction of recombinant Ad vectors. The gDE7 gene was constructed by fusion of the HPV-16 E7, E6, and E5 genes into a complementary DNA encoding HSV-1 gD as described.12 To construct Ad vectors, genes were inserted into a pShuttle vector behind a cytomegalovirus promoter. The expression cassette was then cloned into the E1 domain of a molecular clone of E1-deleted Ad viruses from human serotype 5 or chimpanzee serotype 68.36 Recombinant Ad vectors were rescued and propagated on HEK 293 cells, and purified by CsCl-gradient centrifugation and titrated as described.40
Animals and immunization. Young C57Bl/6 mice were purchased from Charles River Laboratories (Boston, MA). E7-tg mice have been described previously.15 The plasmid used to create E7-tg mice (kindly provided by Dr C. Ledent, Université Libre de Bruxelles) contains the bovine thyroglobulin promoter for selective high expression in thyrocytes,41,42 a rabbit β-intron, the E7 gene, and a polyadenylation signal. C57Bl/6 founder mice were produced by the University of Pennsylvania School of Medicine Transgenic Facility and mated to wt C57BL/6 mice. The progeny was back-crossed and screened for homozygosity. HVEM-KO mice were described previously.43 Mice were vaccinated intramuscularly with a total of 5 × 1010 virus particles of Ad vector given into the tibialis anterior muscle of each hindlimb. Mice were housed at the Animal Facility of the Wistar Institute and all procedures used approved institutional protocols.
Isolation of lymphocytes. Peripheral blood mononuclear cells and spleens were harvested as described.11 Thyroid-infiltrating lymphocytes were harvested upon treatment of tissue fragments with 2 mg/ml collagenase P (Roche, Indianapolis, IN) and 1 mg/ml DNase I (Invitrogen, Carlsbad, CA). After 15 min, the thyroid glands were homogenized, filtrated through a 70-mm cell strainer and purified by Percoll-gradient centrifugation.
ICS. Intracellular IFN-γ, IL-2, and TNF-α staining was carried out upon stimulation with the E7-specific RAHYNIVTF peptide as described;12 the SIINFEKL peptide (Alpha Diagnostic International) was used as a control peptide. Cells were stained with AmCyan fluorescent reactive dye (Invitrogen) and anti-mouse CD8-PerCP-Cy5.5 and upon being permeabilized with anti-mouse IFN-γ-FITC, IL-2-APC, and TNF-α-PECy7 (all from BD Biosciences, San Jose, CA). Cells were washed twice, fixed with BD Stabilizing Fixative (BD Biosciences), and then analyzed by FACS using LSRII (BD Biosciences) and DiVa software. Flow cytometric acquisition and analysis of samples was performed on at least 300,000 events. Postacquisition analyses were performed with FlowJo (TreeStar, Ashland, OR). Data shown on graphs represent values of E7 peptide-stimulated cells from which control peptide values have been subtracted. Polyfunctionality pie-chart graphs were generated using SPICE software (NIH, Bethesda, MD). Single color controls used CompBeads Anti-Mouse Igκ (BD Biosciences) were used for compensation.
Tetramer and lymphocyte marker staining. Lymphocytes were stained with APC-labeled major histocompatibility complex class I E7 peptide (RAHYNIVTF) tetramers (E7-tet, MHC Tetramer Core Facility, Emory University, Atlanta, GA), AmCyan fluorescent reactive dye (Invitrogen), anti-CD8a-PerCPCy5.5, or CD8-PE-Texas Red (Invitrogen), CD44-Alexa700, CD62L-FITC, BTLA-PE (eBioscience, San Diego, CA), PD-1-PECy7, CD4-PerCPCy5.5, CD25-APC, FoxP3-PacificBlue, and CTLA-4-FITC. For FoxP3 and CTLA-4 intracellular staining, cells were permeabilized and then stained. Unless otherwise noted, antibodies were purchased from BD Biosciences. Cells were fixed and analyzed as described for ICS. To ensure that HVEM-KO mice lacked HVEM expression, peripheral blood mononuclear cells were incubated with a live cell AmCyan fluorescent reactive dye (Invitrogen) and fluorochrome-labeled antibodies to CD3 (labeled with APC) and HVEM (labeled with PE; both BD, Biosciences) and analyzed as described above.
Thyroid gland weight and histopathology analysis. Thyroid glands from each mouse were excised and weighted. They were fixed in 10% neutral-buffered formalin for 24 hours, embedded in paraffin wax, cut at 5 µm, and stained with hematoxylin and eosin. All sections were screened by a board-certified veterinary pathologist for lymphocyte accumulation in the stroma within neoplastic lobules, lymphocytes surrounding neoplastic lobules, necrosis of neoplastic cells and lymphocytes in neoplastic acini including colloid, fibrous connective tissue proliferation (scarring) at sites where neoplastic lobules were considered to have been effaced by an acquired immune response, and lymphocytes within the fibrous connective tissue.
Statistical analysis. Experiments were conducted repeatedly using 3–14 mice per group. Results show the means ± SD. Significances between two groups or more than two groups were analyzed, respectively, by one-tailed Student's t-test or one-way analysis of variance. P values that showed significant differences are shown in the graphs. The other groups were not significantly different from the controls.
Acknowledgments
This work was sponsored by institutional grants to the Wistar Institute including an NCI Cancer Core Grant (CA10815), and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health. Financial support for the E7-tg mouse colony was provided by RO1 CA 69632 (Y.P.). We thank Wistar Institute flow cytometry and microscopy facilities, and the MHC Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for providing the E7-tetramer. We are grateful to Bin Wang, Zhen-Kun Pan, and Alex Rodriguez for assistance in developing and maintaining the E7-tg mouse colony.
M.O.L. and H.C.J.E. have filed for US patent on use of gD to enhance immune responses.
REFERENCES
- Rosenberg SA., and, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G, Savoldo B., and, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: “are we nearly there yet?”. Hum Gene Ther. 2009;20:1229–1239. doi: 10.1089/hum.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM.et al. (2006Cancer regression in patients after transfer of genetically engineered lymphocytes Science 314126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizée G, Cantu MA., and, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N.et al. (2009Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma Clin Cancer Res 152166–2173. [DOI] [PubMed] [Google Scholar]
- Zhou G, Drake CG., and, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B., and, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK.et al. (2003BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1 Nat Immunol 4670–679. [DOI] [PubMed] [Google Scholar]
- Carfí A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ.et al. (2001Herpes simplex virus glycoprotein D bound to the human receptor HveA Mol Cell 8169–179. [DOI] [PubMed] [Google Scholar]
- DiMenna L, Latimer B, Parzych E, Haut LH, Töpfer K, Abdulla S.et al. (2010Augmentation of primary influenza A virus-specific CD8+ T cell responses in aged mice through blockade of an immunoinhibitory pathway J Immunol 1845475–5484. [DOI] [PubMed] [Google Scholar]
- Lasaro MO, Diniz MO, Reyes-Sandoval A, Ertl HC., and, Ferreira LC. Anti-tumor DNA vaccines based on the expression of human papillomavirus-16 E6/E7 oncoproteins genetically fused with the glycoprotein D from herpes simplex virus-1. Microbes Infect. 2005;7:1541–1550. doi: 10.1016/j.micinf.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Lasaro MO, Tatsis N, Hensley SE, Whitbeck JC, Lin SW, Rux JJ.et al. (2008Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses Nat Med 14205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Wang L, Murphy KM, Fraser CC., and, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol. 2008;180:6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- Diniz MO, Lasaro MO, Ertl HC., and, Ferreira LC. Immune responses and therapeutic antitumor effects of an experimental DNA vaccine encoding human papillomavirus type 16 oncoproteins genetically fused to herpesvirus glycoprotein D. Clin Vaccine Immunol. 2010;17:1576–1583. doi: 10.1128/CVI.00264-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Marcotte A, Dumont JE, Vassart G., and, Parmentier M. Differentiated carcinomas develop as a consequence of the thyroid specific expression of a thyroglobulin-human papillomavirus type 16 E7 transgene. Oncogene. 1995;10:1789–1797. [PubMed] [Google Scholar]
- Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A.et al. (2007Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer Cancer Immun 72. [PMC free article] [PubMed] [Google Scholar]
- Sewell DA, Pan ZK., and, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26:5315–5320. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro MO., and, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N., and, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y.et al. (2010Intratumoral CD8+ T/FOXP3+ cell ratio is a predictive marker for survival in patients with colorectal cancer Cancer Immunol Immunother 59653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O., and, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8+ T cells and CCR4+ regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:744–752. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Lasaro MO., and, Ertl HC. Targeting inhibitory pathways in cancer immunotherapy. Curr Opin Immunol. 2010;22:385–390. doi: 10.1016/j.coi.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A.et al. (2009Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection Nat Immunol 1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougiakakos D, Choudhury A, Lladser A, Kiessling R., and, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z.et al. (1999Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40 Nat Med 5780–787. [DOI] [PubMed] [Google Scholar]
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N.et al. (2003Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase Nat Med 91269–1274. [DOI] [PubMed] [Google Scholar]
- Rech AJ., and, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH.et al. (2010Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates J Clin Oncol 283167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA.et al. (2006Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma Cancer 1062437–2444. [DOI] [PubMed] [Google Scholar]
- Kowalski ML, Wolska A, Grzegorczyk J, Hilt J, Jarzebska M, Drobniewski M.et al. (2008Increased responsiveness to toll-like receptor 4 stimulation in peripheral blood mononuclear cells from patients with recent onset rheumatoid arthritis Mediators Inflamm 2008132732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X., and, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- Hege KM, Jooss K., and, Pardoll D. GM-CSF gene-modified cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25:321–352. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D., and, Hymowitz SG. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J Biol Chem. 2005;280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Eisenberg RJ., and, Cohen GH. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM) J Virol. 2002;76:10894–10904. doi: 10.1128/JVI.76.21.10894-10904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wlazlo AP, Kowalczyk DW, Cheng J, Xiang ZQ, Giles-Davis W.et al. (2000Viral recombinant vaccines to the E6 and E7 antigens of HPV-16 Virology 270146–161. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B.et al. (2001Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages Mol Ther 35 Pt 1697–707. [DOI] [PubMed] [Google Scholar]
- Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M.et al. (2009Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease Blood 114891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Floess S, Hamann A, Gaudieri S, Lucas A, Hellard M.et al. (2009Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection PLoS Pathog 5e1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP.et al. (2003A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag J Immunol 1701416–1422. [DOI] [PubMed] [Google Scholar]
- Ledent C, Parmentier M., and, Vassart G. Tissue-specific expression and methylation of a thyroglobulin-chloramphenicol acetyltransferase fusion gene in transgenic mice. Proc Natl Acad Sci USA. 1990;87:6176–6180. doi: 10.1073/pnas.87.16.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Avarbock MR, Allen DD., and, Brinster RL. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci USA. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S.et al. (2005The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses J Clin Invest 115711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]