Abstract
Receptor interacting protein 140 (RIP140) is a coregulator for numerous nuclear receptors and transcription factors and primarily exerts gene-repressive activities on various target genes. We previously identified a spectrum of posttranslational modifications on RIP140 that augment its property and biological activity. In T3-triggered biphasic regulation of cellular retinoic acid binding protein 1 (Crabp1) gene along the course of fibroblast-adipocyte differentiation, we found TRAP220(MED1) critical for T3-activated chromatin remodeling whereas RIP140 essential for T3-repressive chromatin remodeling of this gene promoter. In this current study, we aim to examine whether and how RIP140 replaces TRAP220(MED1) on the CrabpI promoter in differentiating adipocyte cultures. We find increasing recruitment of RIP140 to this promoter, with corresponding reduction in TRAP220(MED1) recruitment during the T3-repressive phase. We also uncover direct interaction of RIP140 with cyclin-dependent kinase (CDK)8 through the amino terminus of RIP140, which is stimulated by lysine acetylation on RIP140. We further validate the biological activity of lysine acetylation-mimetic RIP140, which elicits a stronger repressive effect and more efficiently recruits CDK8 and confirm CDK8's function in recruiting repressive components, such as G9a, to the RIP140 complex on this promoter. This underlies the T3-triggered repression of CrabpI gene. This study illustrates a new gene-repressive mechanism of RIP140 that can affect the transcription machinery by directly interacting with CDK8.
Nuclear receptor (NR)-mediated gene regulation involves a complex interplay between transcription factors and various coregulatory complexes (1, 2). In the case of hormone-triggered gene regulation, studies have revealed that hormone-bound NR can activate or repress target genes (3). Further, for certain genes, cellular contexts can differentially affect the outcome of hormonal regulation of the same gene, i.e. the same hormone can activate or repress the same gene, depending on the context. This partially underlies certain types of epigenetic regulation observed in different experimental conditions (4). We previously reported such an example in T3-triggered biphasic regulation of cellular retinoic acid binding protein 1 (Crabp1) gene along the course of fibroblast-adipocyte differentiation in cell culture models such as 3T3-L1 and mouse embryonic fibroblast cells (4, 5).
In these adipocyte differentiation models, the Crabp1 gene can be regulated by a variety of signals but is primarily controlled by T3 (5). Specifically, this gene is activated by T3 in the predifferentiating cells but is repressed by T3 in postdifferentiating cells (4, 6, 7). One important T3-triggered chromatin remodeling event common to both of these two phases is T3-elicited chromatin juxtaposition of the upstream T3 response element (TRE) with the GC box located in the 5′-flanking region of the transcription initiation site. Remodeling of this chromatin segment requires, along with other remodeling machinery, either the TRAP220(MED1)-containing Mediator complex in predifferentiation cells (6) or the corepressor receptor interacting protein 140 (RIP140) complex in postdifferentiating cells (7). Presumably, juxtaposition of this chromatin segment allows hormone signals to be more rapidly transmitted through the TRAP220(MED1)- or RIP140-containing holo-T3 receptor (TR) (binding to TRE) and specificity protein-1 (binding to the GC box basal promoter) complex, resulting in timely exchange of the Bramha-related gene-1- containing (during activation) with the Bramha-containing (during repression) chromatin remodeling complex on this promoter. During the T3-activated phase (in predifferentiating stages), nucleosomes spanning this promoter slide toward the 3′-end, pushing the nucleosome on the transcription initiation site (TIS) to disassemble and allowing full activation of this gene. In the course of the T3-repressed phase (in postdifferentiating stages), nucleosomes slide toward both the 5′-end and 3′-end of the promoter to cover the entire promoter and the TIS. Correspondingly, this chromatin segment becomes more compact and assembles into heterochromatin in fully differentiated adipocytes, where this gene becomes completely silenced. A diagram depicting our working model of these events is shown in Fig. 1A. For the transition of this promoter from T3-triggered activation to repression, the key seems to be the switch from the TRAP220(MED1) to the RIP140 complex as cultures undergo differentiation (6, 7). However, we have not validated whether there is indeed such a switch during differentiation, and if so, how RIP140 could replace TRAP220(MED1) in differentiating cultures.
Fig. 1.
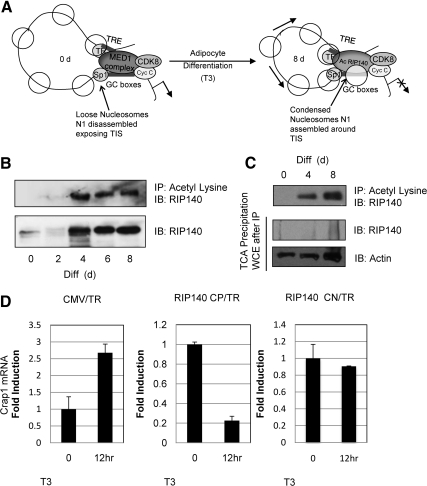
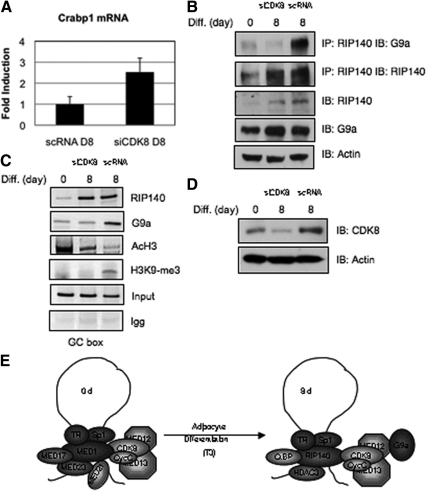
Acetylated RIP140 down-regulates Crabp1 mRNA levels in adipocyte differentiation. A, A model based upon our previous findings. In predifferentiated cultures, T3 initially activates crabp1 gene expression. The MED1 complex associated with factors CDK8 and cyclin C maintains the juxtaposed chromatin conformation between the distal TRE region through TRα, and through specificity protein-1 at the GC boxes. During this period of T3 activation, nucleosomes slide toward 3′-end resulting in disassembly of the nucleosome covering the TIS, allowing activation of the Crabp1 gene. After differentiation, MED1 and associated coactivators are no longer detected, instead, acetylated RIP140 and associated corepressors along with CDK8 and cyclin C are detected at the promoter and maintain the juxtaposed conformation. Arrows depict nucleosomes sliding bidirectionally toward the 5′-end and 3′-end of the loop to cover the entire promoter, the chromatin then adopts a more compact conformation, and the gene becomes silenced. B, Increase in lysine-acetylated RIP140 during adipocyte differentiation. Co-IP with acetyl lysine in differentiating 3T3-L1 cells to monitor acetylated RIP140 levels. RIP140 protein expression levels shown throughout differentiation in 3T3-L1. C, Ratio of acetylated/unacetylated RIP140 at differentiation d 0, 4, and 8 in 3T3-L1 cells. Co-IP with acetyl lysine (top panel) shows RIP140 acetylation status. TCA precipitation of the remaining whole cell extract (WCE) (after IP with acetyl lysine) reveals deacatylated pool, lacking RIP140 (middle panel). D, qPCR Crabp1 mRNA levels in undifferentiated 3T3-L1 transfected with human TRα and cytomegalovirus (CMV) promoter driven empty vector (left panel) RIP140 CP acetylation mutant (K158/287Q) (middle panel) or RIP140 CN acetylation mutant (K158/287A) with and without T3 treatment (12 h, 1 μm). IB, Immunoblot; CN, constitutive negative; CP, constitutive positive; Diff., differentiation.
RIP140 is a coregulator for a wide spectrum of NR and transcription factors (8, 9). It elicits mostly gene-repressive activities by recruiting histone-modifying enzymes like histone deacetylases (10) and other coregulators, such as c-terminal binding protein (CtBP) (11). Its gene-repressive activity can also be associated with its subnuclear distribution (12). In many gene regulatory contexts, coregulator modification plays a central role in facilitating gene activation or repression (13). To understand the dynamics of the remodeling complex containing RIP140, we have exploited insights gathered from studies of posttranslational modification (PTM) of RIP140 (14). The PTM of RIP140 that have a functional consequence include phosphorylation (15, 16), acetylation (17), methylation (18, 19), vitamin B6 conjugation (20), and sumoylation (21). Protein kinase C epsilon-triggered phosphorylation stimulates its arginine methylation and subsequent nuclear export to carry out functions in the cytoplasm (22). Critical to RIP140's gene regulatory activity in adipocyte differentiation potentially includes lysine acetylation on K158 and K287 and threonine phosphorylation on Thr202 and Thr207 (23). We have found that lysine acetylation enhances its gene-repressive activity (17) and is preceded by MAPK-mediated transient threonine phosphorylation on Thr202/Thr207 (23). In these earlier studies, we generated RIP140 mutants that mimic either hyperacetylation (to enhance its repressive activity, K158/287Q), or hypoacetylation (to reduce its repressive activity, K158/287A) (23). However, we have not yet determined the specific physiological context where lysine acetylation of RIP140 could be important. It was also unclear why acetylated RIP140 could become more repressive.
Another question raised in our previous study of the T3-repressive phase (7) relates to the composition of the TRAP220(MED1)-containing Mediator complex on this promoter. In the course of adipocyte differentiation, the TRAP220(MED1) subunit initially occupies the CrabpI promoter in the predifferentiating stage (6). In differentiated cells, RIP140 occupies this promoter, and concurrently, several coactivators, such as glucocorticoid receptor binding protein 1 and p300/CBP-associated factor, are absent from this promoter (7). Nonetheless, it was unclear as to the dynamics of the Mediator components on this promoter in the T3-repressive phase, in particular those components could possibly be involved in gene repression, such as the cyclin-dependent kinase (CDK)8 module that contains CDK8 and cyclin C. The CDK8 module has been implicated in both gene activation and repression (24). For gene repression, as a part of the Mediator complex, CDK8 can sterically block Mediator interaction with RNA polymerase II (pol II) necessary for gene activation (25), or it can phosphorylate pol II carboxy terminal domain (CTD) to disrupt pol II Mediator association (26). In addition, CDK8 can negatively regulate gene expression by inactivating transcription factor II H, which targets pol II CTD for activation (27). CDK8 has also been known to phosphorylate specific gene transcriptional activators, leading to their polyubiquitination and proteosome degradation (28). However, the role of the CDK8 module in T3-triggered repression of the CrabpI gene was not clear.
In this current study, we find RIP140 replacing the TRAP220(MED1) component in differentiating adipocytes and uncover direct interaction of RIP140 with CDK8, which is specifically enhanced by lysine acetylation on RIP140. This underlies, at least partially, the T3-triggered repression of the CrabpI gene. RIP140-CDK8 interaction represents a new gene-repressive mechanism of RIP140, enabling it to more directly affect transcription machinery. This is different from those previously reported activities of RIP140 that primarily involve the recruitment of chromatin modifying enzymes.
Results
Lysine acetylation of RIP140 is important for its gene-repressive activity
RIP140 activity can be extensively regulated by various PTM (14). Lysine acetylation of RIP140 on Lys158/Lys287 renders RIP140 more repressive as demonstrated using a transrepressive reporter (23). To determine the physiological context of this modification in the adipocyte differentiation model, we monitored the kinetics of RIP140 lysine acetylation throughout adipocyte differentiation (Fig. 1B). The data reveal RIP140 acetylation on differentiation d 4, when its protein level is also significantly elevated. The very similar kinetic patterns of RIP140 protein level and its acetylation indicate that in this experimental model, lysine acetylation readily occurs as soon as RIP140 protein level is elevated. This is consistent with our previous data showing that the upstream enzyme ERK/MAPK that stimulates RIP140's lysine acetylation is significantly activated on d 3 of differentiation (23). Considering the importance of the acetylation status of RIP140, we then quantified the ratio of acetylated/deacetylated RIP140 by differentially detecting the two forms of RIP140 in differentiating adipocyte cultures. This was done by using an acetyl lysine-specific antibody to immunoprecipitate all the endogenous lysine-acetylated proteins on d 0, 4, and 8 in 3T3-L1 cells, followed by a trichloroacetic acid (TCA) precipitation of the remaining whole-cell extract. Afterwards, we then conducted Western blot analyses to detect RIP140 in these two fractions (lysine-acetylated protein pool vs. remaining nonacetylated pool). The data show (Fig. 1C) that all endogenous RIP140 are readily acetylated, because its expression is elevated and becomes detectable, such as on d 4 and 8, because RIP140 is not detected in the nonacetylated pool. Western blot analysis of actin serves as the control for proteins in the nonacetylated pool. This data indicates that most, if not all, endogenous RIP140 are readily acetylated, because its expression is increased in the process of adipocyte differentiation.
Previously, we reported that RIP140 plays a functional role in repressing Crabp1. In this study, we wanted to determine whether RIP140's lysine acetylation contributes to its repressive activity of the wild-type protein used in this particular experimental system (7). To do so, we used previously generated hyper- and hypoacetylation mutants of RIP140 (RIP140 CP and RIP CN, respectively) to conduct gain-of-function studies in the predifferentiated cultures where RIP140 level is negligible and the CrabpI gene is typically activated by T3. As shown in Fig. 1D, left, in this culture where the endogenous RIP140 level is negligible, the endogenous Crabp1 mRNA level is induced by T3 (∼2.5-fold induction). Although ectopic expression of the constitutive acetylation mimetic mutant RIP140 K158/K287Q (RIP140 CP) in this culture down-regulates its endogenous Crabp1 mRNA levels (∼80% fold reduction) only in the presence of T3 (Fig. 1D, middle panel). However, ectopic expression of the acetyl negative RIP140 K158/287A (RIP140 CN) has no significant effect. These data show that mimicking acetylation with glutamine substitution at amino acid sites 158/287, (RIP140 CP) results in repression comparable with the effect of wild-type RIP140 as we previously reported, whereas modifying these sites with alanine (RIP140 CN) results in diminished repressive function of RIP140. Furthermore, these data support that acetylation of RIP140 is crucial for its repressive activity on its target gene, Crabp1, in this experimental system.
Acetylated RIP140 is recruited to Crabp1 promoter and recruits repressive components
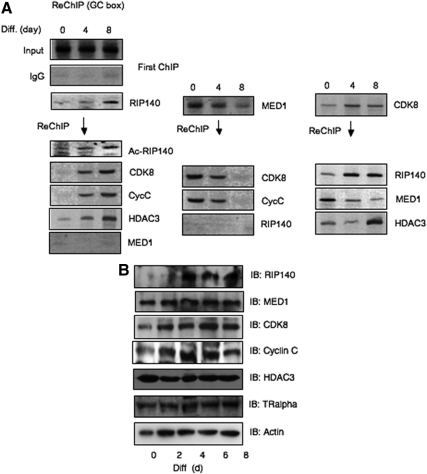
To understand how acetylated RIP140 is more repressive, we monitored the recruitment of RIP140, as well as other cofactors, to the Crabp1 basal promoter containing the GC box during adipocyte differentiation using chromatin immunoprecipitation (ChIP). To differentiate the activating and repressive complexes, we monitored the endogenous components associated with either mediator subunit 1 (MED1), RIP140, or CDK8 on the Crabp1 promoter region using repeated ChIP (Re-ChIP). The primary ChIP experiments (Fig. 2A, top) show RIP140 recruitment to the GC box region in more differentiated cells, whereas MED1 leaves the promoter in these cells. CDK8 is present throughout differentiation. Re-ChIP reveals that the recruited RIP140 is lysine-acetylated (Ac-RIP140) and associates with other repressive components, such as histone deacetylase 3 (HDAC3). In addition, acetylated RIP140 increasingly associates with CDK8 and the submodule component cyclin C but does not associate with MED1. Re-ChIP of MED1 data show that the CDK8 submodule associates with MED1 during early differentiation, which gradually leaves this promoter in later stages of differentiation. Consistently, RIP140 is not a part of this MED1 complex. Re-Chip of CDK8 further validates shifting of the CDK8 module from the activating MED1 complex in predifferentiation cultures to the repressive RIP140 complex in differentiated cultures. Western blot analysis in differentiating 3T3-L1 cells (Fig. 2B) indicates that there is no significant change in the expression of these endogenous components to account for the changes in their promoter occupancy.
Fig. 2.
CDK8 shifts association from mediator to RIP140 during differentiation. A, ChIP/Re-ChIP to monitor endogenous protein complexes associated with RIP140, MED1, and CDK8 on Crabp1 basal promoter on d 0, 4, and 8 of 3T3-L1 differentiation. These ChIP data are quantified as shown in Supplemental Fig. 1 and Supplemental data, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. B, Western blot analysis to monitor relevant endogenous protein levels throughout differentiating 3T3-L1 cells. IB, Immunoblot; Cyc C, Cyclin C; Diff., differentiation.
Ac-RIP140 and MED1 compete for association with TRα
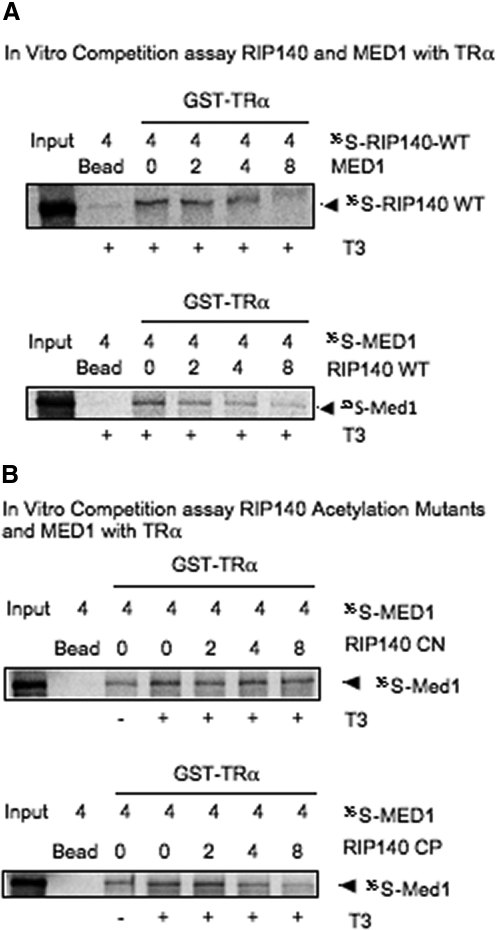
We detected a shifting of association of the CDK8 module from MED1 to RIP140 plausibly due to increased RIP140 recruitment to the promoter and simultaneously decreased MED1 on this genomic region. We then hypothesized that RIP140 could compete with MED1 for interaction with TRα. To test this theory, we performed in vitro direct competition assays. Figure 3A shows an in vitro pull down competition assay with glutathione S-transferase (GST)-TRα and 35S-labeled RIP140 wild type (WT) and titrated in cold MED1 in the presence of T3. The data (Fig. 3A) show that MED1 can compete out RIP140 for direct interaction with GST-TRα (Fig. 3A, upper), and RIP140 WT competes out MED1 for interaction with GST-TRα (Fig. 3A, lower). To determine the effect of RIP140 acetylation on its ability to compete out MED1, we did the same experiment with the acetyl negative (RIP140 CN) and acetyl positive mutants (RIP140 CP). The data (Fig. 3B) show that the acetylated RIP140 (CP), but not the acetyl negative RIP140 (CN), is able to effectively compete with MED1 for direct binding to gst-TRα. These data provide direct evidence for competition between acetylated RIP140 with MED1 for occupancy on the Crabp1 promoter, through interacting with TRα, in differentiated cultures.
Fig. 3.
RIP140 and MED1 compete for association with TRα. A, In vitro competition assay of RIP140 and MED1 with regards to binding to TRα. In vitro pull down assay between GST-TRα and 35S-RIP140 WT, with titration of unlabeled MED1 in vitro transcribed and translated (TNT) protein (upper). In vitro pull down assay between GST-TRα and 35S-MED1, with titration of unlabeled RIP140 WT TNT protein (lower). B, In vitro pull down assay between GST-TRα and 35S-RIP140 CN, with titration of unlabeled MED1 TNT protein (upper). In vitro pull down assay between GST-TRα and 35S-RIP140 CP, with titration of unlabeled MED1 TNT protein (lower).
Acetylated RIP140 directly interacts with CDK8 and associates in vivo
We have previously detected the association of endogenous RIP140 on the GC box region, which confers its repressive activity to the Crabp1 gene (7). To assess the repressive activity of acetylated RIP140, we ectopically expressed the acetyl mimetic (RIP140 CP) and acetyl negative (RIP140 CN) mutants in predifferentiated cultures, and monitored the GC box region of Crabp1 gene (Fig. 4). As suspected, the expression of acetyl mimetic RIP140 reduces the CrabpI promoter association with MED1 and dramatically increases its recruitment of HDAC3, G9a, and CDK8. In addition, one epigenetic mark of active transcription, such as acetylated histone H3, is decreased, whereas that of repressive mark, such as H3K9me3, is increased. However, expression of the acetyl-negative RIP140 elicits no obvious effects on the recruitment of repressive components and only marginally affects the recruitment of MED1 and the level of H3K9me3. Considering these very different effects of lysine modification mutants of RIP140, it is clear that PTM plays an important role in the function of RIP140, at least in its repressive activity on the Crabp1 promoter, possibly through regulating its association with other regulatory proteins.
Fig. 4.

Acetyl mimetic RIP140 associates with repressive cofactors on promoter ChIP monitoring proteins associated with acetyl mimetic RIP140 CP (K158/287Q) and acetyl negative RIP140 CN (K158/287A) mutants transfected in undifferentiated 3T3-L1 cells, and detection of various epigenetic markers on GC box region. CP, Constitutive positive; CN, constititive negative; Cyc C, Cyclin C; CMV, cytomegalovirus.
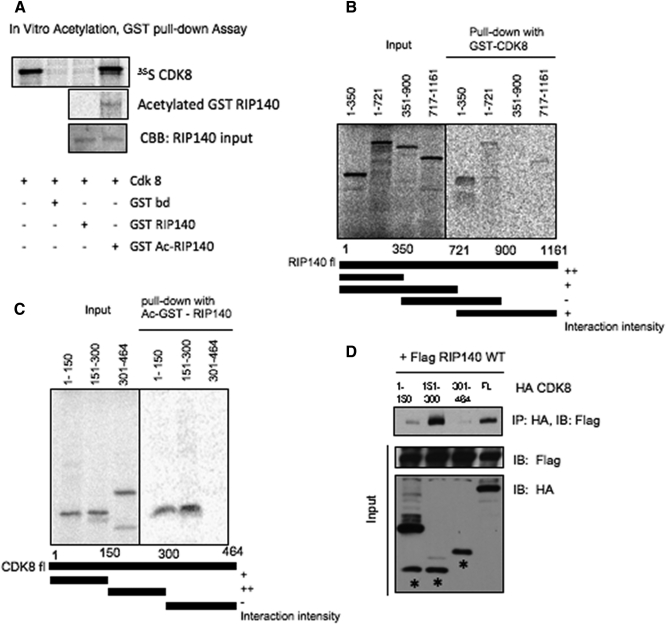
To further validate acetylated RIP140-CDK8 as a repressive complex, we ectopically expressed acetyl mimetic (RIP140 CP) and acetyl-negative (RIP140 CN) in predifferentiated cells and monitored their in vivo complexes. As shown in Fig. 5, the acetyl mimetic RIP140 associates with CDK8 and HDAC3 much stronger than the acetyl negative RIP140 in vivo. Because CDK8 more strongly associates with hyperacetylated RIP140 than with hypoacetylated RIP140 (Fig. 5), it is possible that the two proteins directly interact, and the interaction is regulated by acetylation on RIP140. Previously, we reported ERK-MAPK mediated phosphorylation of RIP140 on Thr202/Thr207 as a stimulus for recruitment of p300 to facilitate lysine acetylation of RIP140 (23). We then generated in vitro acetylated GST-RIP140 using p300 as the enzyme source and then used the acetylated RIP140 to conduct GST-pull down assays to test its direct interaction with 35S-labeled CDK8. As shown in Fig. 6A, only the acetylated, but not the unmodified, RIP140 interacts directly with CDK8. Next, we map the principal CDK8-interaction domain of RIP140. Using reciprocal pull down with GST-CDK8 and truncated 35S-labeled RIP140 fragments, we have determined the CDK8-interacting interface to be located within the N-terminal domain of RIP140 encompassing amino acids 1–350 (Fig. 6B). Interestingly, the two essential lysine residues of RIP140 for acetylation, K158 and K287, are located within this N-terminal domain. The carboxyl-terminal domain of RIP140 also seems to interact with CDK8, albeit very weakly.
Fig. 5.
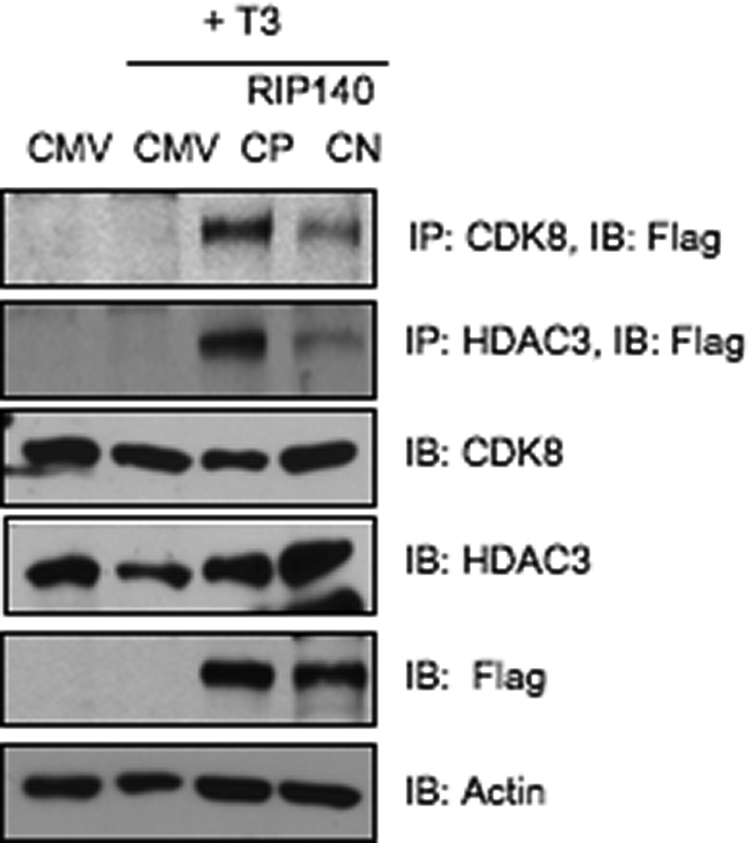
Acetyl mimetic RIP140 associates with repressive cofactors in vivo. Co-IP of acetyl mimetic Flag-RIP140 CP (K158/287Q) and acetyl negative Flag-RIP140 CN (K158/287A) with endogenous CDK8, and HDAC3 in undifferentiated 3T3-L1 cells in presence of T3 (1 μm, 12 h). CP, Constitutive positive; CN, constitutive negative; CMV, cytomegalovirus promoter driven empty vector; IB, immunoblot.
Fig. 6.
Acetylation required for RIP140 and CDK8 direct interaction. A, In vitro acetylation of GST-RIP140, followed by GST-pull down with 35S methionine labeled in vitro transcribed and translated (TNT) CDK8. B, RIP140 interaction domain mapping. GST-pull down with GST-CDK8 and truncated 35S methionine-labeled TNT RIP140 domain fragments. C, In vitro pull down assay with in vitro acetylated GST-RIP140 with 35S methionine-labeled TNT CDK8 domain fragments. D, In vivo association of RIP140 with CDK8 domains. Co-IP of Flag-RIP140 WT with HA-CDK8 domain fragments. IB, Immunoblot; CBB, coomassie brilliant blue.
We also mapped the RIP140-interacting domain of CDK8 (Fig. 6C). The data show that the acetylated RIP140 interacts with the amino terminal (1–150 residues) and more strongly with the central (151–300 residues) segments of CDK8. This is also supported by Co-IP of overexpressed Flag-RIP140 wild type with HA-CDK8 domain fragments from the cells (Fig. 6D). The central domain of CDK8 most strongly associates with RIP140 in vivo. These data show that lysine-acetylated RIP140 directly interacts with CDK8.
CDK8 is a necessary component in regulating RIP140 repressive functions
To confirm the role of CDK8 in mediating repression of the Crabp1 gene, we performed loss of function experiments. We silenced CDK8 in differentiated adipocytes and monitored the endogenous Crabp1 mRNA levels by quantitative PCR (qPCR). As shown in Fig. 7A, silencing CDK8 rescues the otherwise down-regulation of Crabp1 mRNA levels. Previously, we showed RIP140's repressive complex to be inclusive of G9a, heterochromatin protein 1α, and heterochromatin protein 1γ and noted the presence of repressive chromatin marks, such as H3K9, on the Crabp1 promoter during differentiation (7). In recent years, new roles of repression have been implicated for Mediator submodule, such as its ability to recruit H3K9 methyl transferase G9a to target genes (29). Considering this possibility, we hypothesized whether CDK8 module could regulate RIP140 association with G9a. Indeed, although RIP140 does not directly interact with G9a, it associates with G9a in vivo in differentiated adipocyte cultures (Fig. 7B). Moreover, silencing CDK8 abrogates RIP140's association with G9a in vivo. These data show that CDK8, known to interact with G9a through its MED12 subunit, is responsible for recruiting G9a to be associated with the RIP140 complex. To corroborate CDK8's functional role in recruiting G9a, we monitor recruitment of G9a to the Crabp1 promoter under silencing CDK8 (Fig. 7C). It appears that G9a is not as efficiently recruited to the promoter under CDK8 silencing, and concurrently, there is a decrease in H3K9 methylation on this promoter. CDK8 silencing efficiency is shown in Fig. 7D. Together, these data show that RIP140 and CDK8 jointly act as a repressive complex for Crabp1 gene in differentiated adipocytes.
Fig. 7.
CDK8 facilitates repression of Crabp1 gene. A, qPCR monitoring Crabp1 mRNA levels under silencing of CDK8 in differentiated 3T3-L1. B, Co-IP of RIP140 complex with G9a in differentiated 3T3-L1 under silencing of CDK8. C, ChIP of GC box region under silencing of CDK8. D, CDK8 silencing efficiency in differentiated 3T3-L1 cells. E, A mechanism model. In predifferentiated cultures, T3 initially activates Crabp1 gene expression. MED1 maintains juxtaposed chromatin conformation between the distal TRE region and GC boxes and associates with coactivators and CDK8 module. During differentiation, RIP140 is increasingly expressed and acetylated. Acetylated RIP140 replaces MED1 on the Crabp1 promoter and maintains juxtaposed chromatin conformation while recruiting other repressive cofactors, such as G9a through the CDK8 submodule, which is constitutively associated with the Crabp1 promoter. Diff., Differentiation; IB, immunoblot; sc, scramble RNA; si, silencing RNA.
Discussion
In this study, we elucidate the importance of RIP140's PTM in its gene-regulatory activity and provide evidence for a new mechanism underlying its gene-repressive activity, which is by direct interaction with CDK8. For its target gene, Crabp1, in the context of adipocyte differentiation, this mode of action takes place in the T3-triggered gene-repressive phase. In differentiating adipocyte cultures, RIP140 protein level is elevated, and the protein is increasingly acetylated on lysine residues. Elevation in the RIP140 level allows RIP140 to effectively compete with TRAP220(MED1) for binding to liganded TR, as supported by the in vitro competition assays of Fig. 3. Furthermore, lysine acetylation of RIP140 increases its interaction with CDK8, which remains on the promoter and can recruit other repressive remodeling components, such as G9a. Interestingly, CDK8 is required for G9a recruitment. Together, these form a more repressive remodeling complex to eventually silence the Crabp1 gene in differentiated cells. This working model is presented in Fig. 7E. Previously, we have shown RIP140 mediating the repression of the Crabp1 gene during adipocyte differentiation, with corresponding corecruitment of several repressive chromatin remodeling components, such as CtBP, HDAC3, and G9a (7). RIP140 is known to directly interact with CtBP (11) and HDAC3 (10), but we have failed to detect its direct interaction with G9a; therefore, it was unclear how could G9a be recruited to this promoter in the phase of T3-triggered repression. Data shown in this current study provide the explanation for G9a recruitment, which is mediated by RIP140's direct interaction with CDK8 that is known to recruit G9a (29). In past studies, we have shown that the RIP140's gene-repressive activity can also be enhanced by direct conjugation with pyridoxal 5′-phosphate to Lys613 (20) or lysine methylation on Lys591, Lys653, and Lys757 (18). It would be of great interest to understand these repressive modifications of RIP140 and whether these various mechanisms act synergistically with this pathway to mediate its gene-repressive activity for all target genes or if any of these elicits effects in a gene-specific manner.
In terms of gene regulation of other target genes, CDK8 has been found to be responsible for the activation of TR-regulated Diol gene (30). In this case, the CDK8 submodule acts to facilitate interaction between pol II and the Mediator. Conceivably, CDK8 acts similarly in the T3-triggered Crabp1 gene activation in predifferentiating cells. Although CDK8 is constitutively present at the Crabp1 promoter throughout differentiation (Fig. 2A), G9a is not detected on this promoter during T3-triggered activation in predifferentiating cells (7). Presumably, the Mediator complex in predifferentiating cells recruits the CD8 submodule complex alone without G9a (6). However, in the T3-triggered CrabpI gene repression, it is plausible that CDK8 recruits chromatin remodeling enzyme G9a through its MED12 subunit. G9a may trigger H3K9 methylation and provide docking sites for heterochromatin proteins. It remains to be determined how CDK8 recruits G9a in the T3-repressive phase. For example, there might be differential MED12 recruitment to the promoter during adipocyte differentiation. CDK8 may also regulate pol II activity. Whether or not RIP140 interaction has any effect on CDK8 kinase activity in directly phosphorylating pol II CTD, or simple occlusion of pol II from gene promoters, during the repressive phase remains to be investigated. The direct interaction of RIP140 with CDK8 would represent a new mechanism of RIP140's gene regulatory activity and, presumably, can have a more direct effect on gene transcription.
The direct interaction of RIP140 and CDK8 is crucial to the overall gene silencing of the CrabpI gene. Importantly, the dynamics of this interaction seems to be regulated, at least partially, by lysine acetylation of RIP140. Lysine acetylation of RIP140 is stimulated by ERK/MAPK-triggered phosphorylation on Thr202/Thr207 of RIP140, which also facilitates RIP140's interaction with HDAC3 (16). In this earlier study, we observed significant ERK/MAPK activation on d 2, which stimulates lysine acetylation on RIP140. Thus, ERK/MAPK-triggered phosphorylation on RIP140 can facilitate two events leading to its gene-repressive activity, one by interacting with HDAC3 and the other by interacting with CDK8-G9a. Our previous studies of its relationship with HDAC3 have shown that inhibiting HDAC3 with TSA could only partially relieve the repressive activity of RIP140, suggesting that RIP140 modulates multiple pathways to repress gene expression (10). This current study provides an explanation for this dilemma. Another feature of this culture model is the gradual elevation of RIP140 protein level. Although we have not examined regulation of RIP140 expression in this model, we have found that RIP140 protein level can be elevated by down-regulation of microRNA-33 in macrophages (31). Whether or not microRNA-33 plays a role in regulating RIP140 protein level in this adipocyte differentiation model system remains to be examined.
Materials and Methods
Cell culture, silencing of CDK8, and transfection
3T3-L1 cell differentiation was induced by a cocktail (50 μm 3-isobutyl-1-methylxanthine, 170 μm insulin, and 250 nm dexamethasone) plus 0.1 μm T3. For CDK8-silencing, 3T3-L1 cells were transfected on differentiation d 5 with 10 nm small interfering RNA (Mm_CDK8_1 and Mm_CDK8_2 small interfering RNA; QIAGEN, Valencia, CA) or scramble RNA (Thermo Scientific, Inc., Rockford, IL) by HiPerfect (301704; QIAGEN) for 72 h, and then mRNA or protein was collected for RT-qPCR or Western blot analysis.
IP and Western blot analysis
Three hundred micrograms of whole-cell extracts were subjected to IP with the indicated antibodies, and the precipitated protein complex was analyzed by Western blotting. Western blot analyses were performed as previously described with indicated antibodies (6).
ChIP and Re-ChIP
ChIP assays were performed as described. In brief, a total of approximately 2.5 × 107 cells was used for ChIP assay and were cross-linked with 1% formaldehyde for 10 min at room temperature. Cells were collected, and sonicated cell extracts, which were normalized as the same amount of proteins, were precipitated with 2 μg of the following antibodies: TRα1 (sc772) and CtBP1 (sc17759) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), acetylated histone H3 (06-599), G9a (07-551), HDAC3 (05-813), and H3K9-me3 (17-625) (Upstate Biotechnology, Waltham, MA), and RIP140 (ab42126), Cdk8 (ab2955), TRAP220 (ab60950), and cyclin c (ab2950) (Abcam, Inc., Cambridge, MA) at 4 C overnight, followed by the addition of protein G beads for 1 h. Fifteen percent of cell extracts was used as input. Protein G beads were washed extensively, and the captured DNA was eluted twice with 250 μl of elution buffer (1% sodium dodecyl sulfate and 0.1 m NaHCO3), which were reverse cross-linked at 65 C for 4 h. Samples were digested with proteinase K for 1 h at 45 C. DNA was precipitated and analyzed by PCR for GC box region (sense, 5′-TAC CCT AGC GAC TCA AGG CGC TTG-3′ and antisense, 5′-TGG CAG TGG TGT TGG CTG TAG CG-3′). For Re-ChIP assays, immunoprecipitated complex was eluted with 10 mm dithiothreitol, diluted in 20 volumes of Re-ChIP dilution buffer [1% Triton X-100, 2 mm EDTA, 150 mm NaCl, and 20 mm Tris-HCl (pH 8.1)], and subjected to ChIP procedures.
Real-time PCR
Total RNA was extracted from 3T3-L1 cells using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA was reverse transcribed by using the Omniscript RT kit (205113; QIAGEN). For real-time PCR analysis, 2X Brilliant II Master Mix (600804; Agilent Technologies, Santa Clara, CA) was used, and PCR was performed on an MX3000P Stratagene thermocycler (Stratagene, La Jolla, CA). The relative values were normalized to β-actin and presented as ΔΔCt methods.
In vitro acetylation assay
p300 immunoprecipitates were prepared as described (32). p300 immunoprecipitates were thawed on ice and spun down at 4 C. Beads were drained completely using a syringe and needle and incubated with 5× HAT assay buffer, 2 μg of GST-RIP140, and 2 μl of [14C] acetyl-coenzyme A at 30 C with shaking for 2 h. After reaction, beads were spun down, and supernatant transferred to new tube and incubated with Co-IP buffer for GST-pull down of RIP140.
In vitro pull down assay
Two micrograms of GST proteins were immobilized on GST agarose (Sigma-Aldrich, St. Louis, MO) and incubated o/n at 4 C with 35S proteins synthesized in a transcription-translation system (Promega, Madison, WI) in Co-IP buffer (a 500-μl reaction). Beads were washed three times with Co-IP buffer, samples run on SDS-PAGE, gel dried, and exposed for autoradiography.
In vitro competition assay
In brief, 2 μg of GST-TRα was immobilized on GST agarose and incubated overnight at 4 C with the presence of 35S-Protein. Sequential reactions were prepared with increasing amounts of unlabeled protein synthesized in a transcription-translation system present in reaction. In vitro pull down assay was then performed.
TCA precipitation
One volume of 100% TCA wt/vol was added to four volumes of protein sample, and incubated on ice for 10 min. Sample was centrifuged at 14,000 rpm for 5 min. Supernatant was decanted, and pellet was washed two times with 200 μl of cold acetone. Pellet was dried at 95 C on heating block for 10 min; 4× sample buffer was added to pellet and boiled for 10 min and subjected to SDS-PAGE and Western blot analysis.
Acknowledgments
We thank Dr. S. Flaisher-Grinberg and Dr. H. Hiasa for help with data analyses.
This work was supported by National Institutes of Health Grants DK60521, DK060521-07S1, DA11190, DK54733, DK054733-09S1, and K02-DA13926 and the Distinguished McKnight University Professorship (L.-N.W.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Coregulators: RIP140 | TRAP220;
Ligands: Thyroid hormone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- CDK
- Cyclin-dependent kinase
- ChIP
- chromatin immunoprecipitation
- Crabp1
- cellular retinoic acid binding protein 1
- CtBP
- c-terminal binding protein
- CTD
- carboxy terminal domain
- GST
- glutathione S-transferase
- HDAC3
- histone deacetylase 3
- MED1
- mediator subunit 1
- NR
- nuclear receptor
- pol II
- RNA polymerase II
- PTM
- posttranslational modification
- qPCR
- quantitative PCR
- Re-ChIP
- repeated ChIP
- RIP140
- receptor interacting protein 140
- TCA
- trichloroacetic acid
- TIS
- transcription initiation site
- TR
- T3 receptor
- TRE
- T3 response element
- WT
- wild type.
References
- 1. Lonard DM, O'Malley BW. 2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 2. Privalsky ML. 2004. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol 66:315–360 [DOI] [PubMed] [Google Scholar]
- 3. Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei LN. 22. March 2011 Chromatin remodeling and epigenetic regulation of the CrabpI gene in adipocyte differentiation. Biochim Biophys Acta 10.1016/j.bbalip.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei LN, Hu X. 2004. Receptor interacting protein 140 as a thyroid hormone-dependent, negative co-regulator for the induction of cellular retinoic acid binding protein I gene. Mol Cell Endocrinol 218:39–48 [DOI] [PubMed] [Google Scholar]
- 6. Park SW, Li G, Lin YP, Barrero MJ, Ge K, Roeder RG, Wei LN. 2005. Thyroid hormone-induced juxtaposition of regulatory elements/factors and chromatin remodeling of Crabp1 dependent on MED1/TRAP220. Mol Cell 19:643–653 [DOI] [PubMed] [Google Scholar]
- 7. Park SW, Huang WH, Persaud SD, Wei LN. 2009. RIP140 in thyroid hormone-repression and chromatin remodeling of Crabp1 gene during adipocyte differentiation. Nucleic Acids Res 37:7085–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavaillès V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. 1995. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 14:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee CH, Chinpaisal C, Wei LN. 1998. Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol Cell Biol 18:6745–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei LN, Hu X, Chandra D, Seto E, Farooqui M. 2000. Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J Biol Chem 275:40782–40787 [DOI] [PubMed] [Google Scholar]
- 11. Vo N, Fjeld C, Goodman RH. 2001. Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol Cell Biol 21:6181–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tazawa H, Osman W, Shoji Y, Treuter E, Gustafsson JA, Zilliacus J. 2003. Regulation of subnuclear localization is associated with a mechanism for nuclear receptor corepression by RIP140. Mol Cell Biol 23:4187–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenfeld MG, Lunyak VV, Glass CK. 2006. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Gene Dev 20:1405–1428 [DOI] [PubMed] [Google Scholar]
- 14. Mostaqul Huq MD, Gupta P, Wei LN. 2008. N, Post-translational modifications of nuclear co-repressor RIP140: a therapeutic target for metabolic diseases. Curr Med Chem 15:386–392 [DOI] [PubMed] [Google Scholar]
- 15. Huq MD, Khan SA, Park SW, Wei LN. 2005. Mapping of phosphorylation sites of nuclear corepressor receptor interacting protein 140 by liquid chromatography-tandem mass spectroscopy. Proteomics 5:2157–2166 [DOI] [PubMed] [Google Scholar]
- 16. Gupta P, Huq MD, Khan SA, Tsai NP, Wei LN. 2005. Regulation of co-repressive activity of and HDAC recruitment to RIP140 by site-specific phosphorylation. Mol Cell Proteomics 4:1776–1784 [DOI] [PubMed] [Google Scholar]
- 17. Huq MD, Wei LN. 2005. Post-translational modification of nuclear co-repressor receptor-interacting protein 140 by acetylation. Mol Cell Proteomics 4:975–983 [DOI] [PubMed] [Google Scholar]
- 18. Huq MD, Ha SG, Barcelona H, Wei LN. 2009. Lysine methylation of nuclear co-repressor receptor interacting protein 140. J Proteome Res 8:1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mostaqul Huq MD, Gupta P, Tsai NP, White R, Parker MG, Wei LN. 2006. Suppression of receptor interacting protein 140 repressive activity by protein arginine methylation. EMBO J 25:5094–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huq MD, Tsai NP, Lin YP, Higgins L, Wei LN. 2007. Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat Chem Biol 3:161–165 [DOI] [PubMed] [Google Scholar]
- 21. Rytinki MM, Palvimo JJ. 2008. SUMOylation modulates the transcription repressor function of RIP140. J Biol Chem 283:11586–11595 [DOI] [PubMed] [Google Scholar]
- 22. Ho PC, Lin YW, Tsui YC, Gupta P, Wei LN. 2009. A negative regulatory pathway of GLUT4 trafficking in adipocyte: new function of RIP140 in the cytoplasm via AS160. Cell Metab 10:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho PC, Gupta P, Tsui YC, Ha SG, Huq M, Wei LN. 2008. Modulation of lysine acetylation-stimulated repressive activity by Erk2-mediated phosphorylation of RIP140 in adipocyte differentiation. Cell Signal 20:1911–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knuesel MT, Meyer KD, Bernecky C, Taatjes DJ. 2009. The human CDK8 subcomplex is a molecular switch that controls mediator coactivator function. Gene Dev 23:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elmlund H, Baraznenok V, Lindahl M, Samuelsen CO, Koeck PJ, Holmberg S, Hebert H, Gustafsson CM. 2006. The cyclin-dependent kinase 8 module sterically blocks mediator interactions with RNA polymerase II. Proc Natl Acad Sci USA 103:15788–15793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA. 1998. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell 2:43–53 [DOI] [PubMed] [Google Scholar]
- 27. Akoulitchev S, Chuikov S, Reinberg D. 2000. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407:102–106 [DOI] [PubMed] [Google Scholar]
- 28. Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ. 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Gene Dev 15:1078–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding N, Zhou H, Esteve PO, Chin HG, Kim S, Xu X, Joseph SM, Friez MJ, Schwartz CE, Pradhan S, Boyer TG. 2008. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell 31:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belakavadi M, Fondell JD. 2010. Cyclin-dependent kinase 8 positively cooperates with mediator to promote thyroid hormone receptor-dependent transcriptional activation. Mol Cell Biol 30:2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho PC, Chang KC, Chuang YS, Wei LN. 2011. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. FASEB J 25:1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen LF, Greene WC. 2005. Assessing acetylation of NF-κB. Methods 36:368–375 [DOI] [PubMed] [Google Scholar]