Abstract
The Carney complex is an inherited tumor predisposition caused by activation of the cAMP-dependent protein kinase [protein kinase A (PKA)] resulting from mutation of the PKA-regulatory subunit gene PRKAR1A. Myxomas and tumors in cAMP-responsive tissues are cardinal features of this syndrome, which is unsurprising given the important role played by PKA in modulating cell growth and function. Previous studies demonstrated that cardiac-specific knockout of Prkar1a causes embryonic heart failure and myxomatous degeneration in the heart, whereas limited Schwann cell-specific knockout of the gene causes schwannoma formation. In this study, we sought to determine the role of PKA activation in this phenotype by using genetic means to reduce PKA enzymatic activity. To accomplish this goal, we introduced null alleles of the PKA catalytic subunits Prkaca (Ca) or Prkacb (Cb) into the Prkar1a-cardiac knockout (R1a-CKO) or limited Schwann cell knockout (R1a-TEC3KO) line. Heterozygosity for Prkaca rescued the embryonic lethality of the R1a-CKO, although mice had a shorter than normal lifespan and died from cardiac failure with atrial thrombosis. In contrast, heterozygosity for Prkacb only enabled the mice to survive 1 extra day during embryogenesis. Biochemical analysis indicated that reduction of Ca markedly reduced PKA activity in embryonic hearts, whereas reduction of Cb had minimal effects. In R1a-TEC3KO mice, tumorigenesis was completely suppressed by a heterozygosity for Prkaca, and by more than 80% by heterozygosity for Prkacb. These data suggest that both developmental and tumor phenotypes caused by Prkar1a mutation result from excess PKA activity due to PKA-Ca.
Carney complex (CNC) is a form of multiple endocrine neoplasia clinically comprised of multiple endocrine gland tumors in the setting of spotty skin pigmentation, myxomas, and pigmented schwannomas (1). This syndrome is caused by inactivating mutations in PRKAR1A in approximately 75% cases, as determined by a recent analysis of a cohort of more than 350 patients (2). Although a second locus has been identified on chromosome 2p, a second causative gene has not yet been identified (3).
Mice heterozygous for mutations in Prkar1a are tumor prone (4–5) and thus are an appropriate model for studying the human disease. To study tissue-specific tumorigenesis, we have previously described mice carrying tissue-specific knockouts (KO) of Prkar1a in Pit1+ pituitary cells (6), the heart (7), and in the neural crest, both diffusely (8) and in a limited subset of cells (9).
In mice lacking Prkar1a in the heart [R1a cardiac KO (CKO) mice], animals die around d 11.5 of embryogenesis (e11.5) from a thinned and dilated myocardium, which exhibits myxomatous degeneration (7). At the cellular level, there was decreased cardiomyocyte proliferation and marked down-regulation of cardiac-specific transcription factors. In contrast, mice with a limited Schwann cell-specific KO of Prkar1a (R1a-TEC3KO mice) exhibit Schwann cell tumors with high penetrance through a mechanism that may involve altered production of the neurofibromatosis proteins (9).
To determine whether the effects of Prkar1a ablation were due to excess PKA signaling or to alternative mechanisms, we used genetic crosses to reduce PKA signaling. In the cardiac KO model, we report that genetic reduction of the PKA-Ca subunit rescued both the embryonic lethality and the increase in PKA activity, indicating that excess PKA activity is the underlying defect in this model system. In contrast, reduction of PKA-Cb subunits provided only a minimal effect on the phenotype, which reflects the fact that PKA-Ca is the predominant PKA-C subunit in developing cardiomyocytes. In Schwann cells, reduction of PKA-Ca completely suppressed the tumor phenotype, whereas PKA-Cb had effects that were similar but not as strong. These data suggest that the phenotypes associated with Prkar1a mutations are due predominantly to excess PKA activity and do not require other mechanisms. Further, in the tissues studied, these effects appear to be mediated through PKA-Ca, likely due to the fact that this is the most highly expressed PKA-C subunit.
Results
Genetic reduction of Prkaca but not Prkacb rescues the embryonic lethality of R1a-CKO mice
We previously reported that R1a-CKO mice died from cardiac defects at e11.5 and exhibited increased PKA activity (7). In this model, cre-mediated recombination occurs in approximately 80% of cardiomyocytes (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). To determine whether the depletion of PKA-Ca or PKA-Cb could rescue cardiac dysfunction in R1a-CKO animals, we crossed the R1a-CKO mice to strains carrying mutations in genes encoding either PKA-Ca (Prkaca+/−) or PKA-Cb (Prkacb+/−).
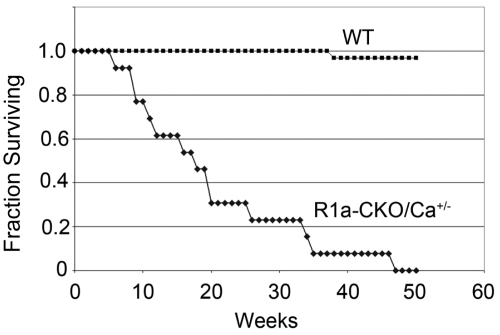
R1a-CKO carrying a null allele of Prkaca (MHCcre;Prkar1aloxP/loxP;Prkaca+/−, henceforth R1a-CKO/Ca+/−) mice were born at Mendelian ratios and were indistinguishable from their littermates. In contrast, R1a-CKO/Cb+/− mice were not observed at the time of weaning. To determine the timing of the demise of R1a-CKO/Cb+/− animals, sets of litters were collected at e11.5, e12.5, e13.5, e15.5, and e17.5. As we had previously observed, R1a-CKO mice were observed at normal frequency at e11.5, but were absent or exhibited near-complete resorption by e12.5. This timing corresponds to the time when the fetal cardiac circulation becomes necessary for maintenance of the embryonic circulation, and demise at this time is characteristic of mutations causing embryonic heart failure (10). R1a-CKO/Cb+/− embryos were detected at an expected distribution at e11.5 and e12.5, but exhibited demise by e13.5; thus, reduction of PKA-Cb increased embryo survival by 1 d (data not shown). To determine whether R1a-CKO/Ca+/− mice demonstrated complete or partial reversion of the phenotype, we allowed a cohort of these normal appearing mice (n = 14) to age undisturbed to assess overall morbidity and mortality. In contrast to wild-type (WT) littermates, R1a-CKO/Ca+/− mice exhibited a median survival of 18 wk, and none survived beyond 48 wk of age. Kaplan-Meier analysis (Fig. 1) confirmed that this was significantly reduced compared with control littermates (P < 0.001, Fig. 1).
Fig. 1.
Survival analysis in R1a-CKO/Ca+/− mice (n = 14) compared with WT littermate controls. Animals were monitored since birth and were killed when morbid. Note that R1a-CKO or R1a-CKO/Cb+/− do not survive embryogenesis.
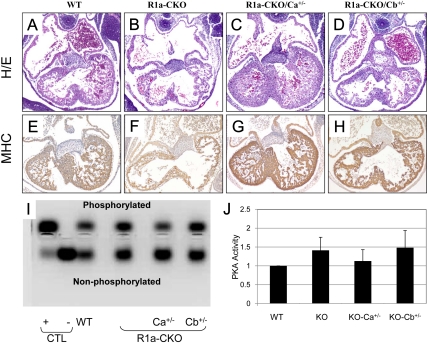
To study the structural effects of these manipulations, we collected embryonic hearts from mice at e11.5, before the onset of embryonic demise (Fig. 2, A–H). As described previously (7), R1a-CKO hearts exhibited marked thinning of the ventricular walls and dilation of the atria. Genetic reduction of Ca restored left ventricle (LV) thickness and trabeculae, making the hearts indistinguishable from normal. In contrast, the morphology of R1a-CKO/Cb+/− hearts was mildly improved, with an appearance intermediate between WT and R1a-CKO.
Fig. 2.
Analysis of embryonic hearts at e11.5. A–D, Midlevel sections from WT, R1a-CKO, R1a-CKO/Ca+/−, and R1a-CKO/Cb+/− hearts stained with hematoxylin and eosin (H/E). E–H, Sections immunostained for myosin heavy chain (MHC). Note that hearts are all oriented the same, with the LV in the bottom right corner of each photomicrograph. I and J, PKA assay from control samples and e11.5 hearts of the indicated genotypes. I, Transilluminator photograph of representative PKA assay showing the resolution of phosphorylated and nonphosphorylated PKA substrate. Control (CTL) lanes show PKA-C incubated in vitro with (+) or without (−) exogenous cAMP in the assay. J, Quantitation of the phosphorylated substrate bands from three independent assays. Each assay was normalized to WT PKA activity, which was assigned a value of 1.
To compare phenotypic and biochemical changes, we measured PKA activity in e11.5 hearts of each genotype (Fig. 2, I and J). As reported previously (7), R1a-CKO hearts exhibited an increase in PKA activity of 41% compared with WT hearts. Introduction of a Prkaca null allele reduced PKA activity to near baseline (12% above WT) whereas introduction of a Prkacb null allele produced PKA levels similar to the R1a-CKO alone (48% above WT). These results did not reach statistical significance owing to the small sample numbers (n = 3), but the trend was consistent across all replicates.
Thus, from morphological, functional, and biochemical standpoints, overall PKA activity appears to be the major factor determining the outcome of cardiac development in this model.
Characterization of adult R1a-CKO/Ca+/− animals
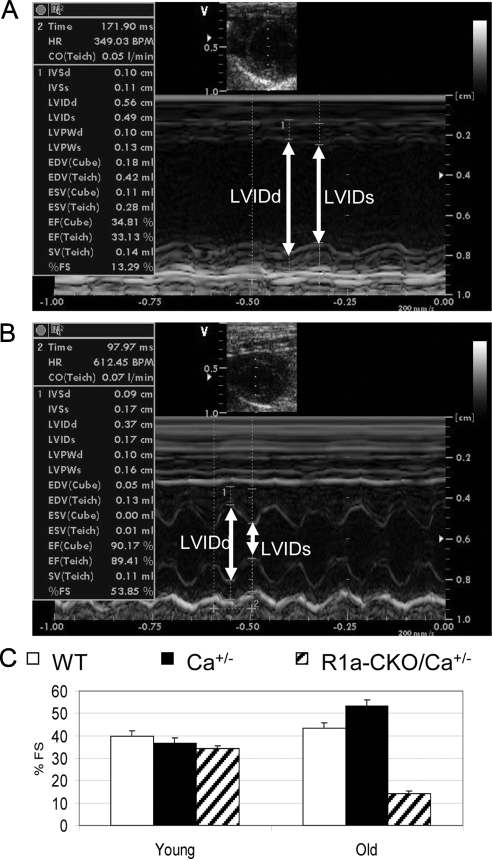
To access the impact of these genetic manipulations on cardiac function throughout the animal's life, we used serial echocardiography to monitor the hearts of a set of surviving R1a-CKO/Ca+/− mice and their littermate controls. Surprisingly, echocardiography of young R1a-CKO/Ca+/− animals at 8, 20, and 26 wk of age, showed virtually normal cardiac function, with percent fractional shortening (% FS) values similar to WT and other littermate controls (Fig. 3). This observation suggests that deletion of one copy of Prkaca can rescue Prkar1a-CKO cardiac function in developing mice and is consistent with the normal appearance of the hearts in utero (Fig. 2). However, as the animals aged, R1a-CKO/Ca+/− mice developed late-onset cardiac dysfunction. The LV became dilated with significant decreases in % FS, with accompanying increases in mean LV internal dimension during systole (LVIDs) and during diastole (LVIDd) and the respective pressures (data not shown). Consistent with echocardiography, the histological examination of the hearts of morbid mice revealed dilated LV in R1a-CKO/Ca+/− mice at this stage. Strikingly, they displayed extremely thin ventricular walls with large organized thrombi in both atrial chambers or in the left atrium alone (Fig. 4). These results indicate that depletion of Prkaca (but not Prkacb) can reverse the heart failure of R1a-CKO mice, although this reversal is incomplete.
Fig. 3.
Depletion of Prkaca transiently rescued the cardiac dysfunction of R1a-CKO mice. A, Representive M-mode echocardiogram from a 47-wk-old preterminal R1a-CKO/Ca+/− mouse. B, Representive M-mode echocardiogram from age-matched WT control. Note that LVID were significantly increased during both diastole (LVIDd) and systole (LVIDs) in failing R1a-CKO/Ca+/− hearts. C, Percent FS values in young R1a-CKO/Ca+/− mice were similar to that in WT and Ca+/− littermates, whereas old R1a-CKO/Ca+/− mice exhibited cardiac dysfunction.
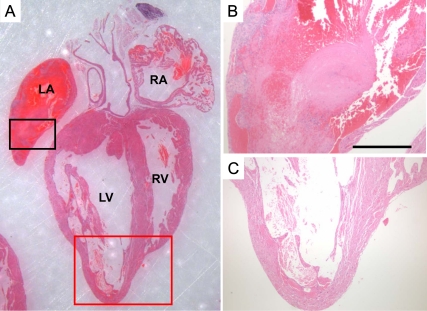
Fig. 4.
Histology of a preterminal heart from R1a-CKO/Ca+/− mice. This heart was taken from a 9.1-wk-old mouse exhibiting agonal breathing (representative of n = 14 animals). The heart was harvested before death and stained with hematoxylin and eosin (panel A). A large organized thrombus filled the left atrium (black square, shown magnified in panel B) and replaced the atrial muscles with fibrous tissue in most places. Analysis of the LV (red square shown magnified in panel C) demonstrated marked thinning of the myocardium. LA, left atrium; RA, right atrium; RV, right ventricle.
Differential expression of PKA-C subunits in the heart
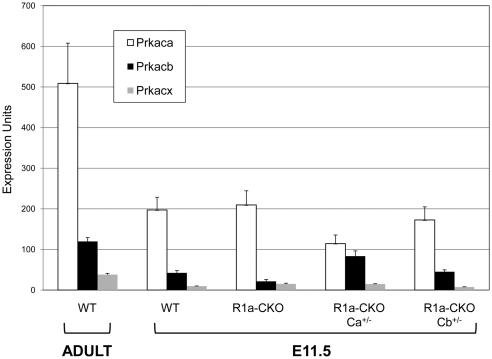
Prkaca and Prkacb have been described to have similar biochemical function, although they may exhibit different patterns of tissue-specific expression (11). To determine whether differential subunit expression could explain the differential effect of Ca vs. Cb reduction, we studied expression of the PKA-C isoforms in hearts from WT adult mice and from e11.5 embryos of each of the respective genotypes. These results (Fig. 5) demonstrate that PKA-Ca is the predominant isoform in normal mouse hearts, with 4.3-fold greater expression than Cb. Prkx is an additional PKA-C-like kinase that can also bind to PKA-regulatory subunits (12). Because it has been shown to be expressed in the heart and in the brain (13), we also measured expression levels of this transcript in mice. As shown in Fig. 5, levels of Prkx were low in normal adult hearts, approximately 13.5-fold lower than Ca and about 3.1-fold lower than Cb. In normal embryonic hearts, levels of each of these catalytic subunits (i.e. Ca, Cb, and Prkx) decreased around 3-fold, although the differential expression remained similar to that seen in adults. The fact that PKA-C subunit expression was higher in adults than in embryos suggests that haploid reduction of PKA-Ca may reduce PKA activity sufficiently to allow normal cardiac development and function through early life. However, as the animal ages and expression level increases, PKA-Ca eventually supersedes a threshold level, at which point the mice begin to develop PKA-mediated cardiac dysfunction, leading eventually to heart failure and death.
Fig. 5.
Expression of PKA-C subunits in embryonic and adult mouse hearts. Hearts were harvested from WT adult mice at 3 months of age or from e11.5 embryos of the indicated genotypes. Transcript levels for Prkaca, Prkacb, and Prkx were determined using quantitative RT-PCR, with expression levels normalized to Gapdh, which was assigned to have arbitrary expression units of 10,000. See Materials and Methods for details of the calculations.
In the R1a-CKO hearts, the levels of expression of PKA-Ca and Prkx did not change markedly, whereas a 50% reduction of PKA-Cb was observed. It is unclear whether this represents a significant change, because the small sample sizes (n = 3) do not support rigorous statistical analysis. In the R1a-CKO/Ca+/− mice, levels of PKA-Ca were reduced 50% as expected. Interestingly, levels of PKA-Cb were noted to double compared with R1a-CKO alone, although expression levels still appear to be lower than PKA-Ca itself. Finally, R1a-CKO/Cb +/− mice exhibited expression levels of all three subunits most similar to the WT e11.5 hearts. PKA-Cb levels were no different, indicating either that haploinsufficiency for Cb does not affect mRNA levels, or that our assays are not accurate enough at this low level of mRNA expression.
Genetic Deletion of PKA catalytic subunits in the R1a-TEC3KO mice also suppresses the phenotype
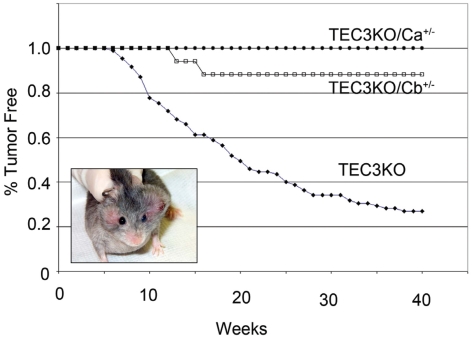
To determine whether the reduction of PKA catalytic subunits could suppress the tumorigenic phenotype of Prkar1a KO, we took advantage of our previously described R1a-TEC3KO model, which develops readily visible facial schwannomas (Fig. 6, inset) with high penetrance (62/85, 73%) (9). After 40 wk of observation (Fig. 6), we did not observe any tumors in R1a-TEC3KO/Ca+/− mice (0/15, 0%, P < 0.01 compared with R1a-TEC3KO). Although they were uncommon, we did observe tumors in R1a-TEC3KO/Cb+/− mice (2/17, 12%, P < 0.01 compared with R1a-TEC3KO). These data indicate that genetic reduction of PKA catalytic activity could also suppress the tumor phenotype; there was a suggestion that PKA-Ca was more potent in this regard than PKA-Cb, but we did not study enough mice to make a valid statistical comparison between the two groups.
Fig. 6.
Tumor incidence in R1a-TEC3KO mice with reduction of PKA-C subunits. Animals were monitored for tumors (shown in inset as a mouse with bilateral facial schwannomas) from birth up to a maximum of 40 wk. Tumor onset was defined as the time when a tumor reached 0.5 cm in greatest dimension.
Discussion
The inherited tumor syndrome CNC is caused by inactivating mutations in PRKAR1A, an integral regulator of PKA catalytic activity. Tumors from patients with CNC have elevated PKA activity (14), as do mouse cells (15) and tissues (16) engineered to lack this PKA subunit. More recently, mutations in phosphodiesterase genes, which caused increased PKA signaling by reduced cAMP degradation, have also been reported as causes of a similar phenotype (17, 18). Thus, there is good biological evidence to link the phenotype of CNC (and similar sporadic tumors) to excess and unregulated PKA activity. However, this concept has been challenged recently by the observation that Prkar1a;Prkaca double heterozygotes (Prkar1a+/−;Prkaca+/− mice) have an increased frequency of bone tumors compared with single Prkar1a heterozygotes (Prkar1a+/− mice) (19).
In the heart, proper regulation of PKA is known to be essential for cardiac development and function (20), although analysis of the role of PRKAR1A/Prkar1a itself has been more complex. Mice heterozygous for Prkar1a mutations exhibit decreased heart rate variability, although cardiac function is normal (4), and there is no evidence for enhanced cardiac death (4–5). In contrast, patients with (heterozygous) PRKAR1A mutations develop cardiac myxomas, which mouse studies have suggested may represent an aberrant response to an injured myocardium (21).
Mice lacking Prkar1a in the heart die before e12.5 (7), making further study of cardiac function impossible. In this study, we attempted to use genetic means to prove that the effects of Prkar1a loss are due to excess PKA activity. We report here that reduction of the PKA-Ca subunit, but not the PKA-Cb subunit, provides significant rescue of the phenotype. This is accompanied by the expected reduction in PKA activity observed in the embryonic hearts, indicating that cardiac failure is a direct consequence of PKA overactivity. Our studies suggest that the alternative PKA catalytic subunit Prkx is likely not a factor in these observations, consistent with the observation that PRKX protein was not detectable when studied by Western blotting in embryonic or adult human hearts (13).
Interestingly, when PKA-Ca was genetically reduced, there was up-regulation of PKA-Cb at the mRNA level, similar to what has previously been observed at the protein level in Prkar1a+/−; Prkaca+/− bone tumors (19). It has been suggested that up-regulation of PKA-Cb may explain the rescue of phenotypes caused by PKA-Ca down-regulation. Although this possibility cannot be excluded by our data, we think it unlikely based on the fact that PKA activity (Fig. 2) correlates with PKA-Ca reduction and phenotypic rescue. If up-regulation of PKA-Cb were driving these effects, we would predict that PKA activity would be unchanged; however, this is not the case.
In Schwann cell-derived tumors, we observed similar findings, with complete suppression of the Prkar1a-associated tumor phenotype up to 40 wk after reduction of PKA-Ca, and more than 80% suppression of the phenotype after reduction of PKA-Cb. These neural crest-derived tumors tend to undergo mesenchymal-to-epithelial transition (22), and blockade of this phenomenon may contribute to the reduced rate of tumor formation. Of note, the ability of genetic reduction of PKA-C to suppress the phenotype caused by Prkar1a ablation was also observed in a neural crest development model, in which reduction of PKA-Ca suppressed abnormal bone differentiation of mesenchymal cells (8).
The discrepancy between the phenotypic rescue observed in both the models described here (cardiac KO and limited Schwann cell KO) and the neural crest KO model (8) and the enhanced bone tumor phenotype in the Prkar1a+/−;Prkaca+/− model (19) has a few possible explanations. First, there may be significant tissue specificity to the observed effects, such that bone and heart respond differently to increased PKA activity. In fact, the Prkar1a+/−;Prkaca+/− mice had fewer tumors in other organs than the Prkar1a+/− mice, suggesting this concept may play a role in understanding these observations. Arguing against this theory is the fact that the neural crest KO model (8) also involved a bone phenotype (defective intramembranous ossification). Second, the role of Prkar1a may differ between embryonic phenotypes (either cardiac or neural crest differentiation) and the adult tumorigenesis phenotype. Data presented here indicate that expression levels of PKA subunits may change over time; thus, the effects of specific collections of mutations may exhibit different temporal patterns. Third, one significant difference between the tissue-specific KO models and the double heterozygote model is that the tissue-specific KO completely lack Prkar1a, whereas the double heterozygote only has haploinsufficiency. Because these models may require secondary hits in other genes/pathways to occur before tumors are seen, the long-term effect of Prkar1a heterozygosity may alter the intracellular milieu such that the phenotypic effects are more complex compared with the KO. Finally, we cannot discount strain-specific effects in each of these mouse models. Most of the work in both models involves a mixed genetic background, although the predominant strains appear to have been different in the models. Although addressing this last possibility is technically straightforward, it would take a significant amount of inbreeding to bring each of these lines into a common strain and thus eliminate strain effects, a set of experiments that is not likely to be accomplished.
When the R1a-CKO/Ca+/− mice die, it is the result of a dilated cardiomyopathy accompanied by striking intracardiac thrombosis. At this time, we are unable to determine whether this arises from poor forward flow and intracardiac pooling, or represents the effects of a cardiac arrhythmia (e.g., atrial fibrillation). Based on our previous data, we hypothesize that proper regulation of PKA is required for normal cardiac remodeling, which occurs over the lifespan of the mouse, such that interference with this system leads to cardiac decompensation; however, direct experimental proof is still lacking.
It is interesting to compare the data from R1a-CKO/Ca+/− mice to the phenotype of a previously described model in which a dominant-negative S133A isoform of the cAMP response element binding (CREB) transcription factor was introduced into the heart by placing it under the control of the α-myosin heavy chain promoter (23). These mice developed a phenotype remarkably similar to that described here, with dilated cardiomyopathy and intracardiac thrombi. The mice exhibited abnormalities of the conduction system and inducible ventricular arrhythmias of a potentially lethal nature (24). Although seemingly contradictory, the data may indicate that chronic PKA stimulation, caused by loss of Prkar1a, leads to desensitization of downstream PKA effectors, including the CREB transcription factor. With chronic PKA activation, CREB mRNA levels decrease, and cAMP-responsive promoters lose responsiveness to CREB even when protein levels do not change (25). Thus, mechanisms exist to suppress chronic PKA signaling at the postreceptor level, mimicking the effect of the dominant-negative CREB mutant. These observations suggest that inhibitors of PKA signaling (including those that work at the postreceptor level) may provide benefit in heart failure patients beyond that observed with β-blockers alone, which function at the prereceptor level. Further studies will be necessary to clarify this issue.
These observations also point out an important conundrum for studies of the effects of manipulation of the PKA system, such as those associated with Prkar1a mutation. Namely, the effects of chronic PKA stimulation may be quite different from those observed via acute stimulation of the system. Given that much of the prior data on the function of the PKA system in altering growth, cytoskeletal components, and other cellular functions was carried out using acute (hours to days) pharmacological stimulation in cultured cells, many of the functions ascribed to PKA activation may not be applicable in studies of human patients with mutations of PRKAR1A (and by extension, phosphodiesterase family members). Patients with such mutations have long-term, chronic overactivity of the PKA system, the effects of which are only now beginning to be appreciated.
Elucidation of the biological and biochemical effects of genetic manipulation of the PKA system, such as seen in CNC patients and its corresponding mouse models, remains a complex and interesting topic that will continue to shed light not only on PKA biology, but also on how modulation of this system can produce effects in both genetic and nongenetic human diseases, particularly those that affect endocrine and other cAMP-responsive tissues.
Materials and Methods
Animals used in this study
All animal studies in this report were conducted under the highest standards of animal care. Cardiac-specific Prkar1a KO mice (hereafter R1a-CKO) and the limited Schwann cell-specific KO mice (hereafter R1a-TEC3KO) were generated and genotyped as described previously (5, 7, 9). Prkaca+/− and Prkacb+/− mice (26–27) were obtained from the Mutant Mouse Regional Resource Centers (MMRRC). tdTomato cre reporter mice (28) were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in 12-h light, 12-h dark cycles in microisolator racks. Genotyping of the WT allele and KO allele of Ca and Cb has been described previously (26, 29). For timed matings, mice were monitored daily. The morning that a vaginal plug was detected in a female mouse was considered as d 0.5 of gestation.
Molecular analyses
Histological and immunohistochemical analyses on embryonic hearts were carried out as described elsewhere (7). Real-time PCR analysis was performed in triplicate using i-Script cDNA synthesis and iQ SYBR Green Supermix reagents (Bio-Rad Laboratories, Inc., Hercules, CA) on a Stratagene (La Jolla, CA) Mx3500 real-time thermocycler. Primers for PCR of PKA-C subunits were as follows:
Prkaca F847 GCA AAG GCT ACA ACA AGG C
R1126 ATG GCA ATC CAG TCA GTC G
Prkacb F1319 GGG AGG AGA AAG ATA GCC
R1554 AAA CCA AAC CAG GAG GAC
Prkx F672 GCC GAT TCT CCT CAG TTG CT
R830 TGG CGA AGC CGA AGT CTG TC
Primers for Gapdh amplification have been described elsewhere (7).
To calculate a value for arbitrary expression units as determined by quantitative RT-PCR, the expression level of the Gapdh standard was set to 105 U. Expression levels for each gene are then calculated as 105/2ΔCt, where ΔCt = Ct(test mRNA) − Ct(Gapdh).
Measurement of cAMP-dependent protein kinase activity
PKA activity was measured by using the PepTag Assay for cAMP-dependent protein kinase (Promega Corp., Madison, WI) following the manufacturer's instructions. Briefly, embryonic hearts at e11.5 (n = 3) were collected and pooled for protein isolation. The proteins were then incubated with 2 μg of PepTag A1 peptide (L-R-R-A-S-L-G, kemptide) in a final volume of 25 μl for 30 min at room temperature. The reactions were stopped by heating to 95 C for 10 min. The samples were separated on a 0.8% agarose gel at 100 V for 20 min, and the gel was photographed on transilluminator. PKA activity was determined by measurement of the production of the phosphorylated peptide, as measured densitometrically using GeneTools 4.01 (Syngene, Cambridge, UK). This assay was repeated three times. Data was normalized to WT PKA activity, which was assigned a value of 1.0 for each experiment.
Echocardiography
M-mode echocardiography was performed under isoflurane anesthesia using the Vingmed Ultrasound System (GE Healthcare, Piscataway, NJ) equipped with a 10-MHz linear array transducer. LV wall thickness was evaluated in the interventricular septum and the posterior wall. Other parameters included LVIDd and LVIDs from the M-mode tracings. Percent FS, a measure of LV systolic function, was calculated from M-mode-derived LV dimensions using the formula (LVIDd − LVIDs)/LVIDd × 100 (30).
Statistical analysis
All quantitative assays were analyzed by a two-sided t test as implemented by StatCrunch (http://www.statcrunch.com).
Acknowledgments
We thank Dr. Yoshinori Nishijima (College of Pharmacy, The Ohio State University, Columbus, OH, 43210) for his assistance with echocardiography.
This work was supported by National Institutes of Health Grant CA112268 (to L.S.K.) and by National Institutes of Health Grant CA16058 to the Ohio State University Comprehensive Cancer Center. G.N.J. was supported by Children's Tumor Foundation Young Investigator Award 2006-01-026.
Current address for Z.Y. : Department of Otolaryngology, Vanderbilt University, Nashville, Tennessee 37232.
Current address for G.N.J.: Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702.
Current address for K.M.K.: Department of Pharmaceutical Systems and Policy, West Virginia University, Morgantown, West Virginia 26506.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CKO
- cardiac knockout
- CNC
- Carney complex
- CREB
- cAMP response element binding
- FS
- fractional shortening
- KO
- knockout
- LV
- left ventricle
- LVIDd
- LV internal dimension during diastole
- LVIDs
- LV internal dimension during systole
- PKA
- protein kinase A
- WT
- wild type.
References
- 1. Stratakis CA, Kirschner LS, Carney JA. 2001. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86:4041–4046 [DOI] [PubMed] [Google Scholar]
- 2. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. 2000. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet 9:3037–3046 [DOI] [PubMed] [Google Scholar]
- 4. Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, La Perle K, Vaughan CJ, O'Hagan A, Bennett KR, Meyer BJ, Legius E, Karttunen M, Norio R, Kaariainen H, Lavyne M, Neau JP, Richter G, Kirali K, Farnsworth A, Stapleton K, Morelli P, Takanashi Y, Bamforth JS, Eitelberger F, et al. 2004. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci USA 101:14222–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, II, Carney JA, Westphal H, Stratakis CA. 2005. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65:4506–4514 [DOI] [PubMed] [Google Scholar]
- 6. Yin Z, Williams-Simons L, Parlow AF, Asa S, Kirschner LS. 2008. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol 22:380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yin Z, Jones GN, Towns WH, II, Zhang X, Abel ED, Binkley PF, Jarjoura D, Kirschner LS. 2008. Heart-specific ablation of Prkar1a causes failure of heart development and myxomagenesis. Circulation 117:1414–1422 [DOI] [PubMed] [Google Scholar]
- 8. Jones GN, Pringle DR, Yin Z, Carlton MM, Powell KA, Weinstein MB, Toribio RE, La Perle KM, Kirschner LS. 2010. Neural crest-specific loss of Prkar1a causes perinatal lethality resulting from defects in intramembranous ossification. Mol Endocrinol 24:1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones GN, Tep C, Towns WH, II, Mihai G, Tonks ID, Kay GF, Schmalbrock PM, Stemmer-Rachamimov AO, Yoon SO, Kirschner LS. 2008. Tissue-specific ablation of Prkar1a causes schwannomas by suppressing neurofibromatosis protein production. Neoplasia 10:1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. 2003. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis 35:1–21 [DOI] [PubMed] [Google Scholar]
- 11. Kirschner LS, Yin Z, Jones GN, Mahoney E. 2009. Mouse models of altered protein kinase A signaling. Endocr Relat Cancer 16:773–793 [DOI] [PubMed] [Google Scholar]
- 12. Pearce LR, Komander D, Alessi DR. 2010. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11:9–22 [DOI] [PubMed] [Google Scholar]
- 13. Li W, Yu ZX, Kotin RM. 2005. Profiles of PrKX expression in developmental mouse embryo and human tissues. J Histochem Cytochem 53:1003–1009 [DOI] [PubMed] [Google Scholar]
- 14. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. 2000. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- 15. Nadella KS, Kirschner LS. 2005. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65:10307–10315 [DOI] [PubMed] [Google Scholar]
- 16. Kirschner LS. 2009. Use of mouse models to understand the molecular basis of tissue-specific tumorigenesis in the Carney complex. J Intern Med 266:60–68 [DOI] [PubMed] [Google Scholar]
- 17. Horvath A, Mericq V, Stratakis CA. 2008. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med 358:750–752 [DOI] [PubMed] [Google Scholar]
- 18. Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. 2006. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- 19. Tsang KM, Starost MF, Nesterova M, Boikos SA, Watkins T, Almeida MQ, Harran M, Li A, Collins MT, Cheadle C, Mertz EL, Leikin S, Kirschner LS, Robey P, Stratakis CA. 2010. Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone. Proc Natl Acad Sci USA 107:8683–8688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lohse MJ, Engelhardt S. 2001. Protein kinase a transgenes: the many faces of cAMP. Circ Res 89:938–940 [PubMed] [Google Scholar]
- 21. Yin Z, Kirschner LS. 2009. The Carney complex gene PRKAR1A plays an essential role in cardiac development and myxomagenesis. Trends Cardiovasc Med 19:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadella KS, Jones GN, Trimboli A, Stratakis CA, Leone G, Kirschner LS. 2008. Targeted deletion of Prkar1a reveals a role for protein kinase A in mesenchymal-to-epithelial transition. Cancer Res 68:2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. 1998. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J Clin Invest 101:2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu W, Saba S. 2003. Cardiac electrophysiologic abnormalities in the CREBA133 transgenic mouse model of idiopathic dilated cardiomyopathy. J Cardiovasc Electrophysiol 14:982–989 [DOI] [PubMed] [Google Scholar]
- 25. Müller FU, Neumann J, Schmitz W. 2000. Transcriptional regulation by cAMP in the heart. Mol Cell Biochem 212:11–17 [PubMed] [Google Scholar]
- 26. Skålhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, Burton KA. 2002. Mutation of the Cα subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol 16:630–639 [DOI] [PubMed] [Google Scholar]
- 27. Howe DG, Wiley JC, McKnight GS. 2002. Molecular and behavioral effects of a null mutation in all PKA C β isoforms. Mol Cell Neurosci 20:515–524 [DOI] [PubMed] [Google Scholar]
- 28. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qi M, Zhuo M, Skålhegg BS, Brandon EP, Kandel ER, McKnight GS, Idzerda RL. 1996. Impaired hippocampal plasticity in mice lacking the Cbeta1 catalytic subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA 93:1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. 1999. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol 277:H1967–H1974 [DOI] [PubMed] [Google Scholar]