Abstract
Mesothelin has been implicated as a potential ideal target antigen for the development of antigen-specific cancer immunotherapy for the control of mesothelin-expressing cancers such as ovarian cancer, mesothelioma and pancreatic adenocarcinoma. In the current study, we utilized a DNA vaccine encoding human mesothelin (pcDNA3-Hmeso) to treat C57BL/6 mice challenged with luciferase-expressing, Hmeso-expressing ovarian cancer cell line, Defb29 Vegf-luc/Hmeso. The therapeutic effect of the tumor-challenged mice was followed by noninvasive bioluminescence imaging systems. The mechanism of the antitumor effect was characterized by depletion of subsets of lymphocytes as well as adopted transfer of serum from pcDNA3-Hmeso-vaccinated mice. We found that vaccination with pcDNA3-Hmeso DNA vaccine generates a significant antitumor effect and promotes survival in mice challenged with Defb29 Vegf-luc/Hmeso. Furthermore, we found CD4+ and CD8+ T-cell immune responses as well as the humoral immune responses are important for the observed antitumor effects in vaccinated mice. Our data indicated that vaccination with DNA vaccine targeting Hmeso could generate potent antitumor effects against mesothelin-expressing tumors through both T cell-mediated immunity as well as antibody-mediated immunity.
Keywords: ovarian cancer, adoptive serum transfer, human mesothelin-specific antibodies, DNA vaccine
Introduction
Intraperitoneal tumors such as ovarian cancer, malignant mesothelioma and pancreatic cancer represent serious diseases in humans. Ovarian cancer is the sixth most common malignancy in women and the leading cause of death from all gynecological cancers in the United States.1 Malignant mesothelioma is rarely noticed at its early stages and therefore little is known of the establishment and progression of the disease (for review, see references Zellos and Sugarbaker2 and Nowak et al.3). Pancreatic carcinoma is the fourth leading cause of cancer-associated deaths in the United States.4 Current therapies such as surgery, chemotherapy and radio-therapy usually fail to control advanced stages of these diseases. Therefore, alternative approaches such as immunotherapy may serve as an important method to control these intraperitoneal tumors.
Antigen-specific immunotherapy is an attractive approach for the treatment of cancers since it has the potency to specifically eradicate systemic tumors and control metastases without damaging normal cells. The immune system has multiple collaborative effector mechanisms capable of killing target cells through two major response pathways: T cell-mediated immunity and the humoral response. T cells can generate tumor-specific immune responses by recognizing tumor-specific antigens (as peptide fragments) via a vast array of clonally distributed antigen receptors. Thus, identification of tumor-associated antigens expressed uniquely in intra-peritoneal tumors is important for the development of antigen-specific cancer immunotherapy. B cells can also elicit a tumor-specific humoral response when activated by helper T cells to produce immunoglobulin G (IgG) antibodies specific to antigens. The antibodies will then respond by neutralization, opsonization or complement activation.
DNA vaccines have emerged as a potent antigen-specific immunotherapy since they have the ability to activate both T cell-mediated responses and humoral responses. Furthermore, DNA vaccines are a favorable form of vaccine for the control of infectious diseases and cancers since they offer many advantages over conventional vaccines, such as peptide or attenuated live pathogens (for review, see references5–8). For instance, DNA vaccines can be administered time after time without adverse effects and are relatively safe. In addition, DNA vaccines are comparatively easy to produce on a large scale and are able to yield products with high purity and stability. Most importantly, effective DNA vaccine delivery systems, such as direct intradermal administration of DNA vaccines via gene gun to professional antigen-presenting cells (APCs), have been well established. Using this delivery method, we have previously developed several innovative strategies to enhance DNA vaccine potency by modifying the properties of DNA-transfected APCs (for reviews, see references Hung and Wu9 and Boyd et al.10).
DNA vaccines targeting mesothelin as a tumor antigen may serve as an important form of vaccine against mesothelin-expressing intraperitoneal tumors, such as ovarian cancer, mesothelioma and pancreatic adenocarcinoma. Mesothelin has been found to be highly overexpressed in these intraperitoneal tumors.11–15 Furthermore, it is absent or present in low levels in normal tissues and other types of cancer.11 In addition, it has been suggested that mesothelin is a highly immunogenic protein in cancers with high mesothelin expression. For example, Ho et al.16 showed that a high percentage of antimesothelin antibodies was found in ovarian cancer and mesothelioma patient sera and was associated with high expression of the antigen in tumors. In comparison, antibodies to mesothelin were found in only 4% of pharynx and larynx squamous cell carcinoma patients in a study done by Suaraez-Alverez et al.17 Therefore, mesothelin represents a potentially ideal target antigen for the development of cancer immunotherapy using DNA vaccines against mesothelin-expressing tumors.
In the current study, we have generated a murine ovarian cancer cell line, Defb29 Vegf-luc/Hmeso that expresses human mesothelin (Hmeso). We found that treatment of mice challenged with Defb29 Vegf-luc/Hmeso tumor cells with mesothelin DNA vaccine inhibits tumor growth and promotes survival. We have shown that protective antitumor effect generated by the mesothelin DNA vaccine is dependent in part on CD8+ and CD4+ lymphocytes. Furthermore, we found that serum obtained from mesothelin DNA-immunized mice can kill tumor cells in vitro through rabbit complement and binds to mesothelin-expressing cancer cells. We also found that serum from mesothelin DNA-immunized mice produces an antitumor effect and leads to long-term survival in both immunocompetent and immunocompromised mice using adoptive serum transfer experiments. Therefore, employment of DNA vaccine encoding Hmeso in addition to the anti-Hmeso antibody containing serum obtained from vaccinating mice with mesothelin DNA vaccine serves as a potent antigen-specific cancer immunotherapy.
Results
Murine ovarian cancer cells transfected with DNA encoding Hmeso led to expression of Hmeso
We generated a Hmeso-expressing ovarian cancer cell line by transducing Defb29 Vegf-luciferase (Defb29 Vegf-luc) cells18 with retrovirus encoding full-length Hmeso (Defb29 Vegf-luc/Hmeso). To characterize the Hmeso expression of the transduced cells, we performed flow cytometry analysis using Hmeso-specific mouse monoclonal antibody, CAK-1. As shown in Figure 1, Defb29 Vegf-luc/Hmeso cells expressed Hmeso (left panel). In comparison, Defb29 Vegf-luc cells without transduction showed no expression of Hmeso (right panel). Thus, our data indicate that transduction of Defb29 Vegf-luc cells with retrovirus encoding Hmeso leads to expression of Hmeso.
Figure 1.
Flow cytometry analysis to characterize the expression of human mesothelin (Hmeso) in Defb29 Vegf-luc/Hmeso cell line. Characterization of Hmeso expression was performed in Defb29 Vegf-luc/Hmeso and Defb29 Vegf-luc cells using flow cytometry analysis. The cell lines were stained with the Hmeso-specific mouse monoclonal antibody CAK-1, followed by flow cytometry analysis. Mouse IgG1 isotype was used as a control.
Treatment with pcDNA3-Hmeso DNA vaccine inhibits tumor growth and promotes survival in mice challenged with Defb29 Vegf-luc/Hmeso tumor cells
To characterize the therapeutic effects of treatment with Hmeso DNA vaccine, we first challenged C57BL/6 mice with 5 × 105/mouse of Defb29 Vegf-luc/Hmeso cells. Three days later, tumor-challenged mice were treated with empty vector DNA (pcDNA3) or human-mesothelin DNA (pcDNA3-Hmeso) vaccines. Tumor growth in challenged mice was then monitored using bioluminescent imaging systems. As shown in Figure 2a, we observed a significant reduction in luciferase activity in Defb29 Vegf-luc/Hmeso tumor-bearing mice treated with pcDNA3-Hmeso compared to tumor-challenged mice treated with pcDNA3 (*P = 0.585). A graphical representation of the luminescent activity data is depicted in Figure 2b. We also characterized the survival of the treated mice using the Kaplan–Meier survival analysis. As shown in Figure 2c, prolonged survival was observed in tumor-challenged mice treated with pcDNA3-Hmeso compared to mice treated with pcDNA3 (*P<0.001). Thus, our data indicate that treatment with pcDNA3-Hmeso DNA leads to significant antitumor effects and prolonged survival in mice bearing mesothelin-expressing Defb29 Vegf-luc/Hmeso tumors.
Figure 2.
Characterization of antitumor effects generated by treatment with human mesothelin (Hmeso)-expressing DNA vaccine. C57BL/6 mice (five per group) were challenged with 5 × 105/mouse of Defb29 Vegf-luc/Hmeso cells (day 0). Three days after tumor challenge, mice with established Defb29 Vegf-luc/Hmeso tumors were treated with DNA vaccine encoding Hmeso (pcDNA3-Hmeso) via gene gun. An empty vector vaccine (pcDNA3) was used as a control. Mice were imaged using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for 1 min. (a) Luminescence images of representative Defb29 Vegf-luc/Hmeso-challenged mice treated with pcDNA3-Hmeso or pcDNA3 DNA vaccines from day 3 and 60 after tumor challenge. (b) Bar graph depicting the luminescence activity (tumor load) of tumor-bearing mice treated with pcDNA3-Hmeso DNA or pcDNA3 DNA from day 3 and 60 after tumor challenge. (c) Kaplan–Meier survival analysis of the tumor-challenged mice treated with pcDNA3-Hmeso or pcDNA3 DNA vaccines. The mice were tumor challenged on day 0.
CD8+ and CD4+ T lymphocytes are important for the protective antitumor effects generated by pcDNA3-Hmeso DNA vaccine
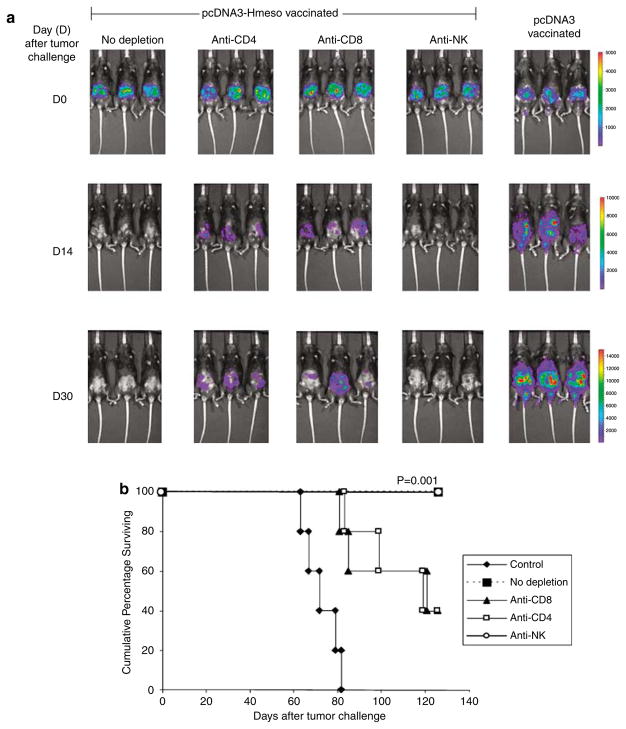
To determine the subset of lymphocytes that are important for the antitumor effect, we performed in vivo depletion experiments using monoclonal antibodies specific for CD4+ T cells, CD8+ T cells or natural killer (NK) cells. C57BL/6 mice were immunized with the pcDNA3-Hmeso DNA vaccine. One week after vaccination, depletion was initiated of pcDNA3-Hmeso-immunized groups. Depletion occurred every other day for 1 week and then once a week through follow-up imaging. Two weeks after the last vaccination, all mice were challenged with Defb29 Vegf-luc/Hmeso tumor cells. Another group of C57BL/6 mice were vaccinated with pcDNA3 as a tumor growth control. Tumor growth was monitored using bioluminescent imaging systems. As shown in Figure 3a, we observed a significant decrease in luciferase activity in pcDNA3-Hmeso-vaccinated mice compared to pcDNA3-vaccinated mice, indicating preventive antitumor effects of pcDNA3-Hmeso DNA vaccination (*P = 0.05). In addition, a significant increase in luciferase activity was observed in pcDNA3-Hmeso-vaccinated mice depleted of CD8+ or CD4+ T cells compared to pcDNA3-Hmeso-vaccinated mice without depletion or with NK depletion. Furthermore, as shown in Figure 3b, prolonged survival was observed in 100% of the pcDNA3-Hmeso DNA-vaccinated mice without lymphocyte depletion and with NK depletion as compared to only 40% of the pcDNA3-Hmeso DNA-vaccinated mice depleted of CD8+ and CD4+ cells. Our data suggest that immunization of mice with pcDNA3-Hmeso leads to significant protective antitumor effect and prolonged survival in mice challenged with Defb29 Vegf-luc/Hmeso tumor cells. Furthermore, our results suggest that, CD8+ T cells and CD4+ T cells but not NK cells contribute to the observed protective antitumor effects.
Figure 3.
In vivo antibody depletion experiment. C57BL/6 mice (five per group) were intraperitoneally (i.p.) immunized with pcDNA3-Hmeso twice at a 1-week interval via gene gun. One week after the last vaccination, the pcDNA3-Hmeso-vaccinated mice were depleted of either CD8, CD4 or NK cells using relevant antibodies every other day for 1 week and then once every week, as described in the Materials and methods section. A group of nondepleted pcDNA3-Hmeso-vaccinated mice was used as a control. Two weeks after vaccination, depleted and nondepleted mice were challenged with 1 × 106/mouse of Defb29 Vegf-luc/Hmeso tumor cells (day 0). Mice were imaged using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for 1 min. pcDNA3-vaccinated mice challenged with Defb29 Vegf-luc/Hmeso cells were used as a control. (a) Luminescence images of representative mice challenged with Defb29 Vegf-luc/Hmeso cells without depletion or with CD4 depletion, CD8 depletion or NK depletion from days 0, 14 and 30 after tumor challenge. (b) Kaplan–Meier survival analysis of the pcDNA3-Hmeso-vaccinated mice challenged with Defb29 Vegf-luc/Hmeso tumor cells without depletion or with CD4 depletion, CD8 depletion or NK depletion. The mice were tumor challenged on day 0. NK, natural killer.
Immunization of mice with pcDNA3-Hmeso elicits strong Hmeso-specific antibody responses
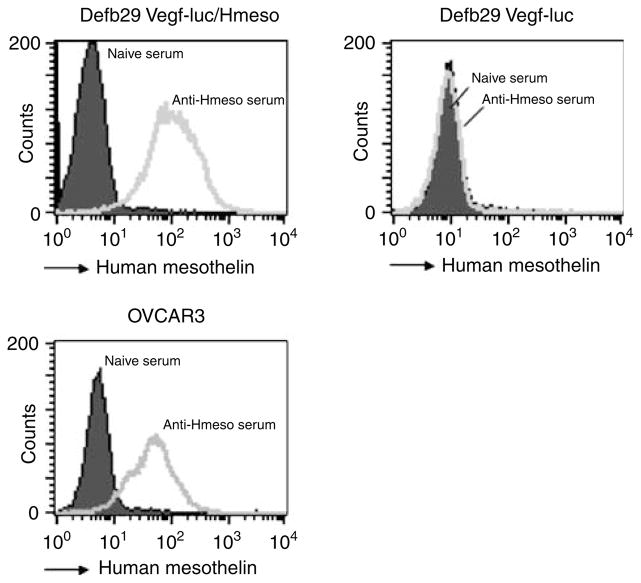
To characterize the antibody response in mice immunized with pcDNA3-Hmeso DNA vaccine, we performed flow cytometry analyses of mesothelin-expressing and non-mesothelin-expressing cell lines using sera from vaccinated mice. Sera were collected from pcDNA3-Hmeso-immunized C57BL/6 mice 1 week after the last immunization and used to stain the various ovarian cancer cell lines: Defb29 Vegf-luc/Hmeso (murine), Defb29 Vegf-luc (murine) and OVCAR3 (human). Sera from naive C57BL/6 mice were used as a control. As shown in Figure 4, Defb29 Vegf-luc/Hmeso and OVCAR3 cell lines, both known to express Hmeso, showed significant shifts of fluorescent signal. In comparison, no specific staining was observed in Defb29 Vegf-luc cells, which were used as a negative control. Furthermore, no specific staining was observed when staining mesothelin-expressing cell lines with sera collected from naive mice. These data suggest that immunization of C57BL/6 mice induces Hmeso-specific antibody responses in vaccinated mice.
Figure 4.
Flow cytometry analysis to characterize the expression of human mesothelin (Hmeso) in murine and human ovarian cancer cell lines. Hmeso-specific antibody containing serum was generated by immunization of C57BL/6 mice with pcDNA3-Hmeso DNA vaccine 3 times at 1-week intervals via gene gun. One week after vaccination, blood sera were collected from immunized mice and used to stain murine and human cancer cell lines. The characterization of Hmeso expression in Defb29 Vegf-luc/Hmeso, Defb29 Vegf-luc and OVCAR3 was performed with flow cytometry analysis using sera collected from pcDNA3-Hmeso-immunized mice. Sera from naive mice were used as a negative control.
Hmeso-specific antibodies from pcDNA3-Hmeso-immunized mice cause complement-mediated lysis of Defb29 Vegf-luc/Hmeso cells in vitro
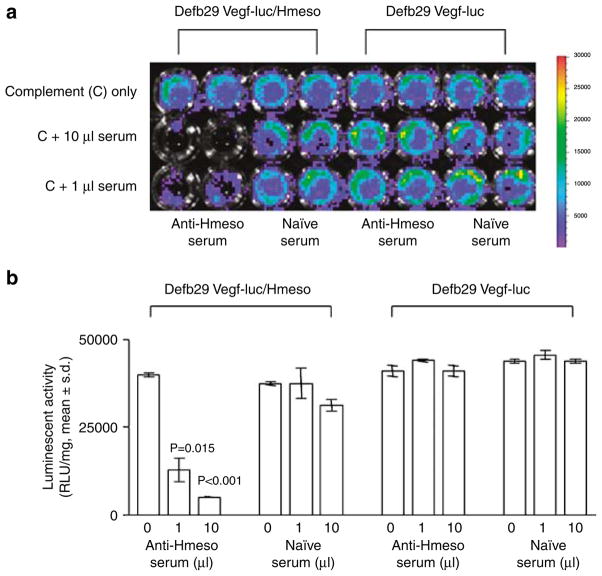
To determine whether the Hmeso-specific antibodies present in sera collected from pcDNA3-Hmeso-vaccinated mice can cause complement-mediated lysis of Hmeso-expressing tumor cells in vitro, we performed complement-dependent cytotoxicity experiments using Defb29 Vegf-luc/Hmeso or Defb Vegf-luc cell lines with rabbit sera for complement. As shown in Figure 5a, tumor cell lysis was observed specifically in Defb29 Vegf-luc/Hmeso cells incubated with sera collected from pcDNA3-Hmeso-vaccinated mice and complement but not with sera collected from naive mice and complement, as indicated by reduced luciferase expression (P<0.02). No specific lysis was observed when Defb29 Vegf-luc cells were incubated with sera collected from pcDNA3-Hmeso-vaccinated mice and complement or with sera collected from naive mice with complement. The luciferase activity in the wells was quantified in the form of bar graphs (Figure 5b). Our data indicate that Hmeso-specific antibodies in sera collected from pcDNA3-Hmeso-immunized mice can cause lysis of Hmeso-expressing tumor cells in the presence of complement in vitro.
Figure 5.
Complement-dependent cytotoxicity assay using human mesothelin (Hmeso)-specific antibodies from pcDNA3-Hmeso-immunized mice. 1 × 104 Defb29 Vegf-luc/Hmeso cells were seeded in 96-well plate. Defb29 Vegf-luc cells were used as a negative control. Cell viability was determined after adding serum and complement using the IVIS Imaging System Series 200. Sera obtained from either pcDNA3-Hmeso immunized mice or naive mice were added in amounts of 0, 1 and 10 μl/well to both cell lines. Rabbit serum (complement) was added to all wells at a 1:5 dilution. Bioluminescence signals were acquired for 1 min. (a) Representative figures of luminescence images of 96-well plates showing complement-mediated lysis effect on Defb29 Vegf-luc/Hmeso or Defb29 Vegf-luc cells. Note: significant lysis was demonstrated by decrease of luminescence activity. (b) Bar graph depicting the quantification of luminescence in Defb29 Vegf-luc/Hmeso or Defb29 Vegf-luc tumor cells mixed with sera from pcDNA3-Hmeso-immunized mice or sera from naive mice.
Adoptive transfer of Hmeso-specific antibodies leads to long-term survival of Defb29 Vegf-luc/Hmeso tumor-bearing immunocompetent mice
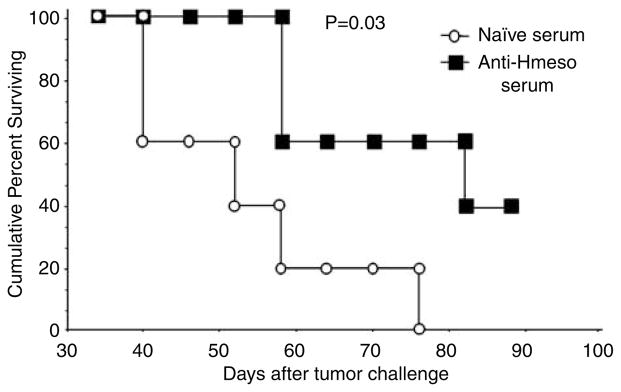
To characterize the antitumor effects generated by Hmeso-specific antibodies in serum from pcDNA3-Hmeso-immunized mice in the absence of T cells, we performed serum transfer experiments using Defb29 Vegf-luc/Hmeso tumor-bearing athymic nude mice. To characterize the influence of sera derived from pcDNA3-Hmeso-immunized mice on the survival of C57BL/6 mice challenged with Defb29 Vegf-luc/Hmeso cells, we performed serum transfer experiments using sera from immunized mice or naive mice. Since treatment with pcDNA3 showed no antitumor effect, serum from pcDNA3-immunized mice would not be significantly different from serum from naive mice. In fact, we have done the first experiment with naive mice challenged with Defb29 Vegf-luc/Hmeso cells and found no difference in luciferase expression from challenged mice treated with pcDNA3 (data not shown). Therefore, we have used serum from naive mice for the experiments involving treatment with serum. C57BL/6 mice with established Defb29 Vegf-luc/Hmeso tumors were intraperitoneally (i.p.) injected with sera collected from pcDNA3-Hmeso-immunized mice or naive mice. The survival of the tumor-challenged mice was characterized using Kaplan–Meier survival analysis. As shown in Figure 6, tumor-challenged mice that received anti-Hmeso serum showed significantly better survival compared to the survival of challenged mice that received sera from naive mice (*P = 0.03). Thus, these data indicate that Hmeso-specific antibodies in sera collected from pcDNA3-Hmeso-immunized mice are able to control Hmeso-expressing murine ovarian tumors in immunocompetent mice.
Figure 6.
Adoptive serum transfer experiments in tumor-bearing C57BL/6 mice. C57BL/6 mice (five per group) were challenged with 5 × 104/mouse of Defb29 Vegf-luc/Hmeso cells. Five days later, the tumor-bearing mice were treated with sera from pcDNA3-Hmeso-immunized mice or sera from naive mice intraperitoneally (i.p.) every 3 days for 4 times. Kaplan–Meier survival analysis of the tumor-bearing mice was performed. The days indicated follow from day 0 of tumor challenge. Hmeso, human mesothelin.
Adoptive transfer of Hmeso-specific antibodies leads to long-term survival of Defb29 Vegf-luc/Hmeso tumor-bearing immunocompromised mice
To characterize the antitumor effects generated by Hmeso-specific antibodies in sera from pcDNA3-Hme-so-immunized mice in the absence of T cells, we performed serum transfer experiments using Defb29 Vegf-luc/Hmeso tumor-bearing athymic nude mice. Recipient athymic nude mice were subcutaneously challenged with 5 × 104/mouse of Defb29 Vegf-luc/Hmeso cells. Equal tumor growth among mice was confirmed by bioluminescence imaging. One week after tumor challenge, tumor-bearing mice were i.p. injected with serum from pcDNA3-Hmeso-immunized mice or naive mice. Tumor growth among challenged mice was characterized by bioluminescence imaging. As shown in Figure 7a, tumor-bearing mice treated with sera from pcDNA3-Hmeso-immunized mice show significantly lower tumor volume over time than mice treated with sera from naive mice, as indicated by lower luciferase activity (*P = 0.013). A graphical representation of the tumor volume by quantification of luminescent activity is depicted in Figure 7b. We further characterized the survival of tumor-challenged mice following treatment with sera from pcDNA3-Hmeso-immunized mice using Kaplan–Meier survival analysis. As shown in Figure 7c, tumor-bearing mice that received sera from pcDNA3-Hmeso-immunized mice showed significantly better long-term survival compared to tumor-bearing mice that received sera from naive mice (*P = 0.011). Thus, these data indicate that treatment with Hmeso-specific antibody containing sera is capable of controlling Hmeso-expressing murine ovarian tumors in the absence of T cells.
Figure 7.
Serum transfer experiments in tumor-bearing immunocompromised mice. Athymic nude mice (five per group) were challenged with 5 × 104/mouse of Defb29 Vegf-luc/Hmeso cells (day 0). Three days later, the tumor-bearing mice were treated with sera from pcDNA3-Hmeso-immunized mice or sera from naive mice intraperitoneally (i.p.) every 3 days for 4 times. Tumor load in treated mice was monitored using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for 1 min. (a) Representative luminescence images of tumor-bearing athymic nude mice that received sera from naive mice or sera from pcDNA3-Hmeso immunized mice. (b) Bar graph depicting the luminescence activity (tumor load) on day 28 after tumor challenge of tumor-bearing athymic nude mice treated with sera from naive mice or sera from pcDNA3-Hmeso-immunized mice. (c) Kaplan–Meier survival analysis of tumor-bearing athymic nude mice that received sera from naive mice or sera from pcDNA3-Hmeso-immunized mice. Hmeso, human mesothelin.
Adoptive transfer of Hmeso-specific antibodies leads to long-term survival of OVCAR3-luc/GFP tumor-bearing immunocompromised mice
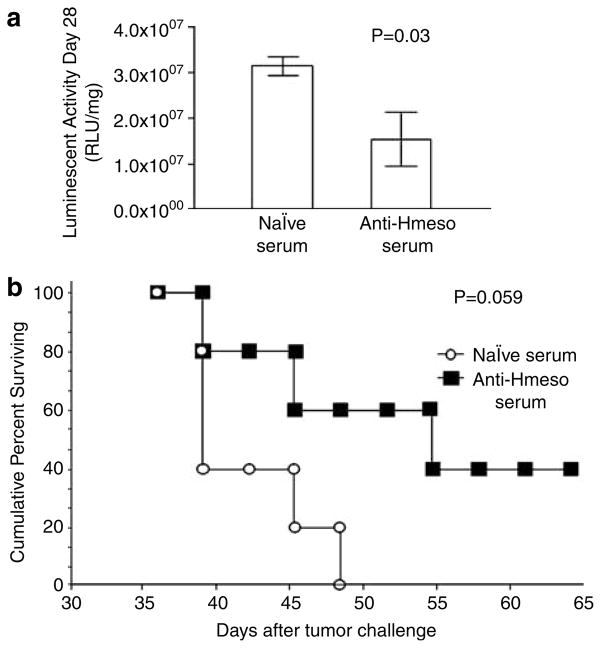
To determine if Hmeso-specific antibodies in sera from pcDNA3-Hmeso-immunized mice are capable of controlling mesothelin-expressing human ovarian cancer, we performed serum transfer experiments using OVCAR3-luc/GFP tumor-bearing athymic nude mice. Recipient athymic nude mice were subcutaneously challenged with 5 × 104/mouse of OVCAR3-luc/GFP cells. Equal tumor growth among mice was confirmed by bioluminescence imaging. One week after tumor challenge, OVCAR3-luc/GFP tumor-bearing mice were i.p. injected with sera from pcDNA3-Hmeso-immunized mice or naive mice. As shown in Figure 8a, OVCAR3-luc/GFP-challenged mice treated with sera from pcDNA3-Hmeso-immunized mice show significantly lower tumor volume over time than tumor-bearing mice treated with serum from naive mice (*P = 0.03). We further characterized the survival of tumor-challenged mice treated with sera from pcDNA3-Hmeso-immunized mice using Kaplan–Meier survival analysis. As shown in Figure 8b, tumor-challenged mice that received sera from pcDNA3-Hmeso-immunized mice showed better survival compared to challenged mice that received sera from naive mice, although the differences are not statistically significant (*P = 0.059). Thus, these data indicate that treatment of OVCAR3-luc/GFP-challenged athymic nude mice with anti-Hmeso serum induces therapeutic antitumor effects and borderline prolonged survival compared to treatment of challenged mice with naive serum.
Figure 8.
Serum transfer experiments in human ovarian cancer bearing immunocompromised mice. Athymic nude mice (five per group) were challenged with 5 × 104/mouse of OVCAR3-luc/Hmeso cells (day 0). Three days later, the tumor-bearing mice were treated with sera from pcDNA3-Hmeso-immunized mice or sera from naive mice intraperitoneally (i.p.) every 3 days for 4 times. Tumor load in treated mice was monitored using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for 1 min. (a) Bar graph depicting the luminescence activity (tumor load) on day 28 after tumor challenge of tumor-bearing athymic nude mice treated with sera from naive mice or sera from pcDNA3-Hmeso-immunized mice. (b) Kaplan–Meier survival analysis of tumor-bearing athymic nude mice that received sera from naive mice or sera from pcDNA3-Hmeso-immunized mice. The days indicated follow from day 0 of tumor challenge. Hmeso, human mesothelin.
Discussion
In the current study, we created a murine ovarian cancer cell line that expressed Hmeso for our DNA vaccine studies. We found that the Defb29 Vegf-luc/Hmeso tumor-bearing mice can be effectively controlled by treatment with Hmeso DNA vaccine, pcDNA3-Hmeso. In addition, we found that both CD4+ and CD8+ T cells but not NK cells contribute to the antitumor effects generated by vaccination with pcDNA3-Hmeso DNA vaccine. Furthermore, we found that the Hmeso-specific antibodies in sera collected from pcDNA3-Hmeso DNA-immunized mice are capable of controlling Hmeso-expressing murine and human ovarian cancer cell lines, resulting in prolonged survival of tumor-bearing mice. Our results serve as an important foundation for future clinical translation.
While our system demonstrated significant therapeutic effects against Hmeso-expressing ovarian cancer with pcDNA3-Hmeso DNA vaccine, it does not address the issue of tolerance. Hmeso is not normally expressed in the mouse, thus no tolerance against Hmeso is expected in mice. In fact, we have also performed similar experiments using a DNA vaccine encoding murine mesothelin in C57BL/6 mice challenged with murine mesothelin-expressing mouse ovarian surface epithelial cancer (MOSEC) cells. Treatment of mice challenged with MOSEC tumor cells with the murine mesothelin DNA vaccine failed to control tumor growth (data not shown). Thus, to extend our study to future clinical translation, we need to consider innovative strategies that are capable of breaking tolerance against endogenous antigens. For example, the employment of xenogeneic antigens for the DNA vaccine development has been shown to effectively break tolerance in some cancer models.19–28 Other strategies that are capable of breaking tolerance include the employment of suicidal DNA vectors and bacterial vectors.29–33 Thus, the use of tolerance-breaking methods in conjunction with therapeutic strategies targeting Hmeso may overcome this problem.
In the current study we observed that Hmeso-specific antibodies in sera collected from mice immunized with pcDNA3-Hmeso have therapeutic effects against Hmeso-expressing murine and ovarian tumors. The therapeutic effects translate into a better survival in tumor-bearing mice. Although the mechanism for the antitumor effects mediated by Hmeso-specific antibodies remains unclear, our in vitro data suggest that complement-mediated lysis may contribute to the antitumor effect (see Figure 5). The encouraging results from this preclinical study suggest that the further development of mesothelin-specific antibody-based immunotherapy may represent a potentially plausible approach for the control of intraperitoneal mesothelin-expressing tumors. Currently, there is one early phase of clinical trials using humanized monoclonal antibodies against Hmeso in patients with mesothelin-expressing pancreatic cancer (Dr Elizabeth Jaffe, personal communication) and ovarian cancers (Dr Deborah Armstrong, personal communication) at Johns Hopkins Hospital.
In summary, we have shown that DNA vaccine encoding Hmeso is capable of generating therapeutic antitumor effects against Hmeso-expressing tumors through both T cell-mediated and humoral-mediated immune responses. Further development of the DNA vaccine employing strategies that are capable of breaking tolerance to Hmeso may lead to eventual clinical translation.
Materials and methods
Mice
Female C57BL/6 and athymic nude mice were acquired from the National Cancer Institute. All animals were maintained under specific pathogen-free conditions, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Cell lines
A syngeneic mouse ovarian epithelial cancer cell line ID8 transfected with VEGF-A and β-defensin 29 (named Defb29 Vegf) was a generous gift from Dr Coukos.34 Defb29 Vegf-luc were generated by transducing Defb29 Vegf cells with the retrovirus containing luciferase pLuci-thy1.1 and flow cytometry sorting following the protocol described previously.18 For stable expression of Hmeso on this cell line, Defb29 Vegf-luc was further transduced with retrovirus containing full-length mesothelin cDNA and isolated as described previously.35 Growth rate of Defb29 Vegf-luc/Hmeso cells was comparable to those of Defb29 Vegf-luc cells (data not shown). Luciferase- and GFP-expressing OVCAR3 (OVCAR3-luc/GFP) was generated by transduction with a lentivirus containing luciferase and GFP. Lentiviral vector pCDH1-luc-EF1-GFP was transfected into Phoenix packaging cell line using lipofectamine (Invitrogen, Carlsbad, CA, USA) and the virion-containing supernatant was collected 48 h after transfection. The supernatant was then filtered through a 0.45 mm cellulose acetate syringe filter (Nalgene, Rochester, NY, USA) and used to infect OVCAR3 cells in the presence of 8 mg/ml Polybrene (Sigma-Aldrich, St Louis, MO, USA). Transduced cells were isolated using preparative flow cytometry with GFP signal.
Plasmid DNA constructs and DNA preparation
The generation of pcDNA3-Hmeso has been described previously.35 A lentiviral construct pCDH-Luc-EF1-GFP (System Biosciences, Mountain View, CA, USA) expressing both luciferase and GFP was made to transduce the OVCAR3 cells. Firefly luciferase was amplified by PCR from pGL3-basic (Promega, Madison, WI, USA) and cloned into pCDF1-MCS2-EF1-copGFP (System Biosciences). All the constructs were verified by restriction analysis and DNA sequencing using ABI 3730 DNA Analyzer at Johns Hopkins DNA analysis facility.
Tumor treatment
Naive C57BL/6 (five per group) mice were i.p. injected with 5 × 105 Defb29 Vegf-luc/Hmeso cells. After 3 days, mice were treated with 2 μg/mouse of pcDNA3-Hmeso or empty vector (pcDNA/myc-His) DNA vaccine through gene gun 3 times at 1-week interval. Tumor load in DNA-treated mice was evaluated by luminescence activity once per week for 8 weeks using IVIS Imaging System Series 200 (Xenogen, Cranbury, NJ, USA).
Tumor protection and depletion of lymphocyte subsets in vivo
C57BL/6 mice (five per group) were vaccinated with 2 μg/mouse of empty vector (group 1) or pcDNA3-Hmeso DNA (groups 2–5) by gene gun 2 times at 1-week interval. Of them, mice groups 3–5 vaccinated with pcDNA3-Hmeso were i.p. injected with blocking antibody using a protocol similar to one described previously.36 Mice were injected with 100 μg/mouse of purified rat monoclonal antibody GK1.5 (anti-CD4, group 3), mAb 2.43 (anti-CD8, group 4) or mAb PK136 (anti-NK1.1, group 5). Depletion was started 1 week after mesothelin DNA vaccination and continued every other day for 1 week and every week onwards. All the five groups of mice were then challenged i.p. with 1 × 106/mouse of Defb29 Vegf-luc/Hmeso cells 2 weeks after the last vaccination. Depletion was maintained by continuing the antibody injections weekly for the duration of the tumor-imaging follow-up. Differences in the luminescence activity of tumor growth were monitored once a week.
Antibody binding and flow cytometry analysis
Blood was obtained from C57BL/6 mice (five per group) vaccinated with 2 μg/mouse of pcDNA-Hmeso DNA vaccine 3 times at 1-week interval 1 week after the last vaccination. The presence of Hmeso-specific antibodies was characterized by staining the Defb29 Vegf-luc, Defb29 Vegf-luc/Hmeso and human OVCAR3 ovarian cancer cells using serum from pcDNA3-Hmeso DNA-vaccinated mice in a 1:200 dilution, followed by Phycoerythrin-conjugated antimouse IgG antibody (eBioscience, San Diego, CA, USA) staining. Serum from naive mice was used as control. Analysis of cell staining was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
Adoptive serum transfer experiment in C57BL/6 and athymic nude mice
Serum containing Hmeso-specific antibodies was prepared from C57BL/6 mice that were immunized with 2 μg/mouse pcDNA-Hmeso 3 times in 1-week interval. One week after last vaccination, sera obtained from these immunized mice or naive mice (control) were collected for adoptive therapy. C57BL/6 mice were challenged with 5 × 105/mouse of Defb29 Vegf-luc/Hmeso cells, and followed by IVIS bioluminescent imaging on day 3 to confirm equal amount of growing tumor in each mouse. Tumor-challenged mice (five per group) were subjected to adopted therapy with i.p. injection of serum from mesothelin-immunized mice or naive mice (100 μl/mouse every 3 days for 4 times). Athymic nude mice were injected with 2 × 105/per mouse of Defb29 Vegf-luc/Hmeso cells or OVCAR3-luc/GFP cells. Treatments with serum from immunized or naive mice were commenced after confirmation of equal amount of tumor growth on day 5 (for mice challenged with Defb29 Vegf-luc/Hmeso tumor cells) and day 3 (for mice challenged with OVCAR3-luc/GFP tumor cells). These mice were followed for their tumor growth by IVIS bioluminescent imaging every week and also for their survival.
Complement-dependent toxicity assay
Target cells Defb29 Vegf-luc/Hmeso were seeded in 96-well plate (1 × 104/well). Sera collected from mice immunized with Hmeso (pcDNA-Hmeso) or nonimmunized mice were added into the well in the following amounts: 10, 1 and 0 μl and followed by a naive rabbit serum in a final dilution of 1:5, used for complement (Sigma-Aldrich) with culture medium in total volume of 100 μl.37 Defb29 Vegf-luc cells were used as a negative control. After incubation for 6 h, cell viability was measured as bioluminescent activity by IVIS Imaging System Series 200.
Statistical analysis
All data expressed as means±standard deviation are representative of at least two different experiments. Comparisons between individual data points were made using a Student’s t-test. Differences in survival between experimental groups were analyzed using the Kaplan–Meier approach. The statistical significance of group differences will be assessed using the log-rank test.
Acknowledgments
We thank Dr Richard Roden for helpful discussions. We acknowledge Archana Monie and Talia Hoory for the preparation of the manuscript. This work was supported by ovarian cancer grants from the Alliance for Cancer Gene Therapy (ACGT) and the NCDGG (1U19 CA113341-01).
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Zellos LS, Sugarbaker DJ. Multimodality treatment of diffuse malignant pleural mesothelioma. Semin Oncol. 2002;29:41–50. doi: 10.1053/sonc.2002.30230. [DOI] [PubMed] [Google Scholar]
- 3.Nowak AK, Lake RA, Kindler HL, Robinson BW. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82–96. doi: 10.1053/sonc.2002.30234. [DOI] [PubMed] [Google Scholar]
- 4.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 5.Shedlock DJ, Weiner DB. DNA vaccination: antigen presentation and the induction of immunity. J Leukoc Biol. 2000;68:793–806. [PubMed] [Google Scholar]
- 6.Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 7.Moniz M, Ling M, Hung CF, Wu TC. HPV DNA vaccines. Front Biosci. 2003;8:d55–d68. doi: 10.2741/936. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–24. [PubMed] [Google Scholar]
- 10.Boyd D, Hung CF, Wu TC. DNA vaccines for cancer. Drugs. 2003;6:1155–1164. [PubMed] [Google Scholar]
- 11.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 13.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 14.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 15.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 16.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 17.Suarez-Alvarez B, Garcia Suarez MM, Arguelles ME, Sampedro A, Alvarez Marcos C, Mira E, et al. Circulating IgG response to stromelysin-3, collagenase-3, galectin-3 and mesothelin in patients with pharynx/larynx squamous cell carcinoma. Anticancer Res. 2001;21:3677–3684. [PubMed] [Google Scholar]
- 18.Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- 19.Mendiratta SK, Thai G, Eslahi NK, Thull NM, Matar M, Bronte V, et al. Therapeutic tumor immunity induced by polyimmunization with melanoma antigens gp100 and TRP-2. Cancer Res. 2001;61:859–863. [PubMed] [Google Scholar]
- 20.Steitz J, Bruck J, Steinbrink K, Enk A, Knop J, Tuting T. Genetic immunization of mice with human tyrosinase-related protein 2: implications for the immunotherapy of melanoma. Int J Cancer. 2000;86:89–94. doi: 10.1002/(sici)1097-0215(20000401)86:1<89::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wei YQ, Tian L, Zhao X, Yang L, Hu B, et al. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor. J Immunol. 2003;170:3162–3170. doi: 10.4049/jimmunol.170.6.3162. [DOI] [PubMed] [Google Scholar]
- 22.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA. 2001;98:11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forconi F, King CA, Sahota SS, Kennaway CK, Russell NH, Stevenson FK. Insight into the potential for DNA idiotypic fusion vaccines designed for patients by analysing xenogeneic anti-idiotypic antibody responses. Immunology. 2002;107:39–45. doi: 10.1046/j.1365-2567.2002.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102:1258–1264. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steitz J, Bruck J, Gambotto A, Knop J, Tuting T. Genetic immunization with a melanocytic self-antigen linked to foreign helper sequences breaks tolerance and induces autoimmunity and tumor immunity. Gene Ther. 2002;9:208–213. doi: 10.1038/sj.gt.3301634. [DOI] [PubMed] [Google Scholar]
- 26.Johnen H, Kulbe H, Pecher G. Long-term tumor growth suppression in mice immunized with naked DNA of the human tumor antigen mucin (MUC1) Cancer Immunol Immunother. 2001;50:356–360. doi: 10.1007/s002620100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su JM, Wei YQ, Tian L, Zhao X, Yang L, He QM, et al. Active immunogene therapy of cancer with vaccine on the basis of chicken homologous matrix metalloproteinase-2. Cancer Res. 2003;63:600–607. [PubMed] [Google Scholar]
- 28.Hawkins WG, Gold JS, Dyall R, Wolchok JD, Hoos A, Bowne WB, et al. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery. 2000;128:273–280. doi: 10.1067/msy.2000.107421. [DOI] [PubMed] [Google Scholar]
- 29.Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BR, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9:33–39. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y, Zhou H, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. Transcription factor Fos-related antigen 1 is an effective target for a breast cancer vaccine. Proc Natl Acad Sci USA. 2003;100:8850–8855. doi: 10.1073/pnas.1033132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niethammer AG, Primus FJ, Xiang R, Dolman CS, Ruehlmann JM, Ba Y, et al. An oral DNA vaccine against human carcinoembryonic antigen (CEA) prevents growth and dissemination of Lewis lung carcinoma in CEA transgenic mice. Vaccine. 2001;20:421–429. doi: 10.1016/s0264-410x(01)00362-0. [DOI] [PubMed] [Google Scholar]
- 33.Xiang R, Lode HN, Chao TH, Ruehlmann JM, Dolman CS, Rodriguez F, et al. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492–5497. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 35.Hung CF, Calizo R, Tsai YC, He L, Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 36.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 37.Jasinska J, Wagner S, Radauer C, Sedivy R, Brodowicz T, Wiltschke C, et al. Inhibition of tumor cell growth by antibodies induced after vaccination with peptides derived from the extracellular domain of Her-2/neu. Int J Cancer. 2003;107:976–983. doi: 10.1002/ijc.11485. [DOI] [PubMed] [Google Scholar]