Abstract
Objectives
We hypothesized that low-density lipoprotein (LDL) reduction regardless of mechanism would improve calf muscle perfusion, energetics, or walking performance in peripheral arterial disease (PAD) as measured by magnetic resonance imaging and magnetic resonance spectroscopy.
Background
Statins improve cardiovascular outcome in PAD, and some studies suggest improved walking performance.
Methods
Sixty-eight patients with mild to moderate symptomatic PAD (age 65 ± 11 years; ankle-brachial index [ABI] 0.69 ± 0.14) were studied at baseline and annually for 2 years after beginning simvastatin 40 mg (n = 20) or simvastatin 40 mg/ezetimibe 10 mg (n = 18) if statin naïve, or ezetimibe 10 mg (n = 30) if taking a statin. Phosphocreatine recovery time was measured by 31P magnetic resonance spectroscopy immediately after symptom-limited calf exercise on a 1.5-T scanner. Calf perfusion was measured using first-pass contrast-enhanced magnetic resonance imaging with 0.1 mM/kg gadolinium at peak exercise. Gadolinium-enhanced magnetic resonance angiography was graded. A 6-min walk and a standardized graded Skinner-Gardner exercise treadmill test with peak Vo2 were performed. A repeated-measures model compared changes over time.
Results
LDL reduction from baseline to year 2 was greater in the simvastatin 40 mg/ezetimibe 10 mg group (116 ± 42 mg/dl to 56 ± 21 mg/dl) than in the simvastatin 40 mg group (129 ± 40 mg/dl to 90 ± 30 mg/dl, p < 0.01). LDL also decreased in the ezetimibe 10 mg group (102 ± 28 mg/dl to 79 ± 27 mg/dl, p < 0.01). Despite this, there was no difference in perfusion, metabolism, or exercise parameters between groups or over time. Resting ABI did improve over time in the ezetimibe 10 mg group and the entire study group of patients.
Conclusions
Despite effective LDL reduction in PAD, neither tissue perfusion, metabolism, nor exercise parameters improved, although rest ABI did. Thus, LDL lowering does not improve calf muscle physiology or functional capacity in PAD. (Comprehensive Magnetic Resonance of Peripheral Arterial Disease; NCT00587678)
Keywords: lipids, magnetic resonance imaging, peripheral vascular disease
Peripheral arterial disease (PAD) is an independent predictor of cardiovascular disease morbidity and mortality (1) and is associated with a decrease in functional ability over time (2). Accordingly, for patients with PAD, it is critical to focus medical therapy on reducing their underlying cardiovascular risk as well as improving their functional status. Despite great strides in medical progress for the treatment of coronary artery disease, the therapeutic options and corresponding evidence base remain limited in PAD (3).
Lipid-lowering therapy with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) in PAD reduces all-cause and cardiac mortality (4), and the benefit is related to both the dose of statin and degree of low-density lipoprotein (LDL) lowering. As a result, it is recommended that patients with PAD (5) receive statins with a goal LDL level of <100 mg/dl. Statins have been shown to improve pain-free walking performance in patients with PAD (6); however, the mechanisms remain unclear. Additional studies have failed to corroborate an improvement in functional ability in patients with PAD treated with statins; however, there was evidence of less decline in walking performance in this group (7). Ezetimibe, a nonstatin drug, is effective in lowering LDL cholesterol when added to statin therapy (8) but is of uncertain value in PAD. Ezetimibe has been studied in atherosclerotic plaque regression trials involving carotid artery intimal medial thickness (9–11); however, it has not previously been evaluated in a clinical trial in lower extremity PAD.
Previous work by our group (12) used magnetic resonance imaging (MRI) techniques to demonstrate the multiple determinants of functional status in patients with PAD. We found that abnormal mitochondrial function is independent of calf muscle tissue perfusion; however, both correlate with impaired exercise capacity in patients with PAD and symptomatic claudication. Given the complexity of functional capacity in PAD and the pleiotropic (noncholesterol lowering) effects of statins (13), including improved endothelial function, stabilization of atherosclerotic plaque, and decreased vascular inflammation, they may have particular therapeutic benefit in this disease. Our study of superficial femoral artery plaque volume in this patient group demonstrated plaque regression with new statin therapy with or without ezetimibe, but progression when adding ezetimibe to ongoing statin therapy, although there was no placebo for the latter group (14). The potential benefits of LDL lowering with statins and/or ezetimibe on the underlying physiology in PAD remain incompletely understood.
Thus, we aimed to investigate the role of LDL lowering on the following parameters in PAD patients: 1) calf muscle perfusion with first-pass contrast-enhanced MRI; 2) calf muscle energetics with measurement of phosphocreatine (PCr) recovery time constant by 31P magnetic resonance spectroscopy (MRS); 3) macrovascular disease measured with magnetic resonance angiography (MRA); and 4) exercise capacity with functional testing. Two parallel studies were performed with the same primary endpoints. In the first study, statin-naïve patients were randomized to receive either simvastatin or a combination of simvastatin plus ezetimibe. The second part of the study evaluated patients already taking a statin with LDL >80 mg/dl who received open-label ezetimibe in addition to ongoing statin therapy.
Methods
Study design
Patients between the ages of 30 and 85 years with symptoms of intermittent claudication and an ankle-brachial index (ABI) between 0.4 and 0.9, based on vascular laboratory testing done during the screening period, were eligible. Exclusion criteria included rest pain, critical limb ischemia, contraindication to MRI, pregnancy, and comorbidities that severely limited the patient's ability to perform a walking treadmill test. Approximately 350 vascular laboratory patients were screened for participation over the 15-month recruitment period. Screened patients who did not enroll in the study refused to participate due to travel concerns, the extended nature of the study, or anticipated difficulty with functional testing due to musculoskeletal issues or frailty. Patients provided written informed consent before study enrollment. The study protocol was approved by the Human Investigation Committee at the University of Virginia. The present study was performed as a pre-specified secondary endpoint in a study of atherosclerotic plaque regression.
In the randomized study, statin-naïve patients (no statin therapy for at least the previous 6 months) regardless of baseline LDL cholesterol underwent a double-blind randomization to simvastatin 40 mg (S group) or a combination pill of simvastatin 40 mg + ezetimibe 10 mg (S + E group) daily using a block randomization scheme. For the parallel direct treatment study, patients were enrolled already on statin therapy but with LDL >80 mg/dl and had open-label ezetimibe 10 mg/day added (E group) to their usual statin. For the randomized study, the investigators and study subjects were blinded to therapy, and all results until follow-up studies and data analysis were complete.
Study protocol
As previously described, the study was divided over 2 days to allow sufficient time between exercise portions of the protocol to avoid fatigue and ischemic pre-conditioning. Patients were admitted overnight to the General Clinical Research Center. Due to imaging time constraints, only the more symptomatic leg was studied by MRI/MRS. Patients returned at yearly intervals twice and underwent repeat testing each year using the same protocols.
Magnetic resonance protocols
MRI protocols were completed on an Avanto 1.5-T scanner and MRS protocols on a Sonata 1.5-T scanner (both Siemens Healthcare, Erlangen, Germany) because MRS hardware and software were only available for the latter. Calf muscle perfusion was immediately measured at peak plantar flexion exercise using a magnetic resonance–compatible foot pedal ergometer at a rate of 10 to 12 rpm with first-pass gadolinium-enhanced imaging as previously described (15) (Fig. 1). Time-intensity curves were generated with Argus software (Siemens Healthcare) for a region of interest in the calf muscle with the greatest increase in signal intensity and the corresponding input artery (typically the popliteal). Tissue perfusion index methodology was shown to differentiate PAD from normal subjects. Likewise, PCr recovery kinetics by 31P MRS after peak plantar flexion exercise in the calf muscle was shown to reproducibly discriminate PAD from normal subjects (16). PCr recovery time constant was calculated using a monoexponential fit of PCr concentration versus time, immediately after peak exercise (Fig. 2). MRA was performed with a moving table/bolus chase technique in 3 stations from the abdominal aorta to the foot after administering gadopentetate dimeglumine (0.2 mmol/kg)–enhanced MRA with grading by 2 experienced observers by visual consensus using 2 different grading schemes, as previously described (9) (Fig. 3). For reference, values from normal subjects for the MRI measures are as follows: perfusion index = 0.69 ± 0.17 and PCr recovery time = 34 ± 16 s. Normal values for the MRA parameters are assumed (run off resistance index = 4 and MRA index = 0).
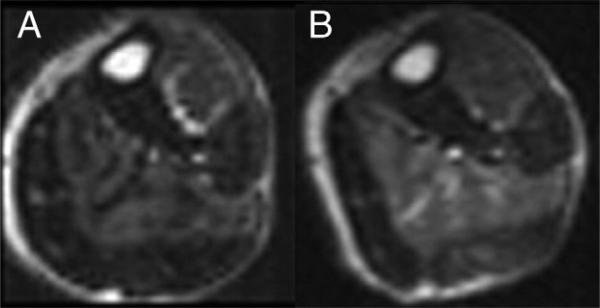
Figure 1. Post-Exercise Calf Muscle Perfusion.
First-pass contrast-enhanced calf muscle perfusion at baseline (A) and year 1 (B) in the same patient in the study group treated with simvastatin + ezetimibe. Increased signal intensity is seen in the anterior tibialis and soleus muscles at both time points (perfusion index: A = 0.37, B = 0.52).
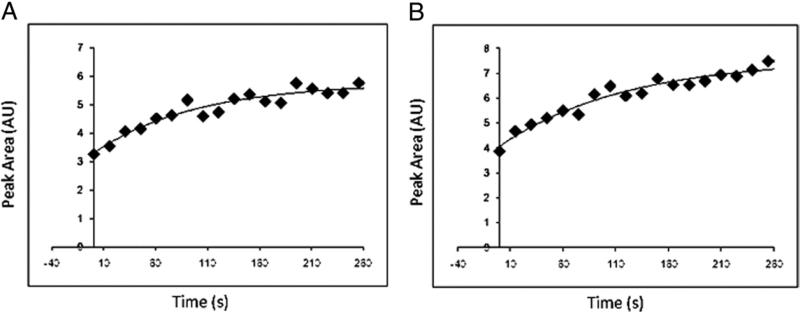
Figure 2. PCr Plots.
Phosphocreatine (PCr) recovery time plots at baseline (A) and year 1 (B) in a patient in the study group treated with simvastatin + ezetimibe with respective PCr recovery time constants of 98 and 121 s.
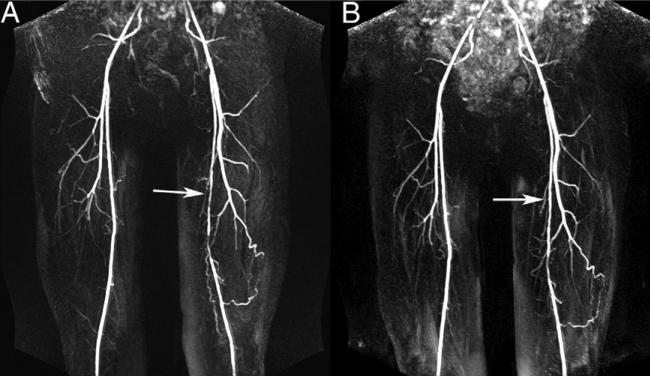
Figure 3. Representative MRA Images.
Contrast-enhanced magnetic resonance angiography (MRA) images at baseline (A) and year 1 (B) from a patient in the study group treated with simvastatin + ezetimibe showing improvement in the appearance of superficial femoral artery stenosis (arrows) over time (MRA index: A = 0.44, B = 0.38).
Functional measures
Subjects performed measures of exercise performance until symptom-limited claudication, exhaustion, or completion of study protocol: a 6-min walk and a standardized graded Skinner-Gardner exercise treadmill test (17) as previously described. Total treadmill exercise time, symptom-limited Vo2, and pre-/post-exercise ABI were measured during the treadmill test, as previously described. The time spent in physical activity at different intensities was assessed using the Aerobic Center Longitudinal Study's Physical Activity Questionnaire (18). The questionnaire was administered using an interview technique to increase accuracy (19). Total physical activity was calculated as MET·H·week−1 (1 MET = 3.5 ml·kg·min−1), using the Compendium of Physical Activities (20).
Lipids
Fasting lipids were measured at study entry and annually for 2 years. Patients were screened at 8 weeks via study coordinator verbal questioning and annually thereafter for symptoms of muscle pain, weakness, or tenderness, and blood was drawn for liver function tests at the same time points.
Statistical analysis
Primary outcomes were LDL cholesterol and change in perfusion index and PCr recovery time constant over time. All other lipid measures, MRA parameters, and exercise performance were secondary outcomes. All data and patient characteristics were presented as mean ± SD for continuous variables and number (%) for categorical variables. A p value ≤0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina). Comparisons of categorical variables between groups were performed with the chi-square test. Linear repeated-measures models were used to estimate changes in MRI parameters over time within each group and to compare changes in MRI parameters between S and S + E groups. The repeated-measures model includes all patients studied at baseline except for those with interval revascularization who were excluded from analysis. All lipid and MRI parameters are reported from the repeated-measures models, with Bonferroni adjustment for pairwise comparisons. Subjects with incomplete data were assumed to be missing at random, and imputation was not used due to the patient numbers. Due to skewness, log-transformation was used for several exercise and laboratory parameters for model fitting. Relationships between changes in lipid parameters and functional outcomes were examined using either a Pearson or Spearman correlation coefficient.
Results
Patients
Eighty-seven patients were initially enrolled, but 2 patients withdrew before completing baseline imaging due to claustrophobia. Thus, 85 patients were evaluated at baseline (S group, n = 22; S + E group, n = 22; E group, n = 41), and 68 were included in the study analysis after excluding 17 patients with interval revascularization of the study leg (S group, 3; S + E group, 4; E group, 10). An additional 4 patients died (1 in the S group, 1 in the S + E group, and 2 in the E group), and 1 received a pacemaker before the year 2 study, but their data were included in the final model. Other vascular events included interval revascularization of the nonstudy leg (S group, 1; S + E, 2), myocardial infarction (S + E group, 1; E group, 2), and transient ischemic attack/stroke (S + E group, 2; E group, 2). Patients with renal insufficiency (glomerular filtration rate, <45 ml/kg/min; n = 6) did not receive gadolinium due to concerns for nephrogenic systemic fibrosis that were raised after study initiation and were excluded from the MRA and perfusion portions of the study. The baseline patient characteristics for all groups are presented in Table 1. Two patients in the S group and 7 in S + E group had been taking statins previously for a mean of 65 ± 50 days. The mean time to the year 1 visit was 389 ± 58 days and 373 ± 74 days later for year 2.
Table 1.
Patient Characteristics at Enrollment According to Treatment Group
| Characteristic | All Patients (N = 68) | S Group (n = 20) | S + E Group (n = 18) | E Group (n = 30) |
|---|---|---|---|---|
| Age, yrs | 65 ± 11 | 59 ± 12 | 66 ± 8 | 68± 11 |
| ABI | 0.69 ± 0.14 | 0.68 ± 0.15 | 0.64 ± 0.14 | 0.72 ± 0.13 |
| Body mass index, kg/m2 | 29 ± 6 | 27± 5 | 28± 6 | 31± 6 |
| Male | 38 (56) | 14 (70) | 11 (61) | 13 (43) |
| Previous lower extremity revascularization | 12 (18) | 3 (15) | 4 (22) | 5 (17) |
| Leg of interest | 9 (13) | 3 (15) | 2 (11) | 4 (13) |
| Race | ||||
| White | 49 (72) | 14 (70) | 13 (72) | 22 (73) |
| Black | 15 (22) | 5 (25) | 4 (22) | 6 (20) |
| Native American | 4 (6) | 1 (5) | 1 (6) | 2 (7) |
| Risk factors | ||||
| Tobacco use | 30 (44) | 10 (50) | 12 (67) | 8 (27) |
| Diabetes mellitus | 22 (32) | 5 (25) | 7 (39) | 10 (33) |
| Hypertension | 58 (85) | 17 (85) | 13 (72) | 28 (93) |
| History of CAD | 42 (62) | 13 (65) | 11 (61) | 18 (60) |
| Hypercholesterolemia | 50 (73) | 11 (55) | 11 (61) | 28 (93) |
| Medications | ||||
| Aspirin/clopidogrel | 47 (69) | 14 (70) | 11 (61) | 22 (73) |
| ACE inhibitor | 31 (46) | 8 (40) | 6 (33) | 17 (57) |
| ARB | 9 (13) | 4 (20) | 3 (17) | 2 (7) |
| Beta-blocker | 29 (43) | 8 (40) | 7 (39) | 14 (47) |
| Cilastazol | 8 (12) | 2 (10) | 3 (17) | 3 (10) |
Values are mean ± SD or n (%). There were no differences in any demographic parameters in the S and S + E groups.
ABI = ankle-brachial index; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CAD = coronary artery disease; E group = study group treated with usual statin and open-label ezetimibe added; S group = study group treated with simvastatin; S + E group = study group treated with simvastatin + ezetimibe.
There was a low rate of study drug discontinuation. In the S group, 1 patient discontinued the study drug at year 1 and an additional patient reported study drug noncompliance at year 2. In the S + E group, 1 patient discontinued taking the study drug between years 1 and 2. In the E group, atorvastatin was the most commonly used statin (n = 16; mean dose, 33 mg) followed by simvastatin (n = 7; mean dose, 50 mg), and 1 patient each was taking lovastatin 40 mg, pravastatin 80 mg, and rosuvastatin 5 mg. Four patients in the E group were unable to tolerate continuing statin use due to nonspecific complaints; 1 additional patient self-discontinued taking the statin at year 1, as did another at year 2. All data were analyzed as intention-to-treat. Other lipid-lowering agents were used by a few study subjects and included niacin (S group, 1; E group, 2), fibrates (S + E group, 2; E group, 1), and fish oil (S group, 1; S + E group, 1; E group, 4).
Laboratory results
All groups had significant decreases in LDL and total cholesterol from baseline to year 1, which were sustained at year 2 (Table 2). The baseline LDL was similar in the S + E and S groups (116 ± 42 mg/dl and 129 ± 40 mg/dl, respectively). The year 1 LDL was less in the S + E group than in the S group (59 ± 23 mg/dl and 96 ± 34 mg/dl, respectively, p < 0.01), and the difference was sustained at year 2 (56 ± 21 mg/dl and 90 ± 30 mg/dl, respectively, p < 0.01). There was no change in high-density lipoprotein or triglycerides over the study period for any group. In the E group, LDL decreased from baseline to year 1 (102 ± 28 mg/dl to 76 ± 29 mg/dl, p < 0.01) and was stable at year 2 (79 ± 27 mg/dl, p < 0.01 vs. baseline).
Table 2.
Lipid Parameters Over Time
| Group | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| Total cholesterol, mg/dl | |||
| S group | 203 ± 47 | 166 ± 44* | 157 ± 35* |
| S + E group | 189 ± 44 | 129 ± 30*† | 123 ± 26* |
| E group | 171 ± 35 | 138 ± 33* | 146 ± 28* |
| All patients | 184 ± 43 | 143 ± 37* | 142 ± 32* |
| LDL, mg/dl | |||
| S group | 129 ± 40 | 96 ± 34* | 90 ± 30* |
| S + E group | 116 ± 42 | 59 ± 23*† | 56 ± 21*† |
| E group | 102 ± 28 | 76 ± 29* | 79 ± 27* |
| All patients | 113 ± 37 | 76 ± 31* | 75 ± 29* |
| HDL, mg/dl | |||
| S group | 44 ± 14 | 45 ± 13 | 44 ± 14 |
| S + E group | 46 ± 12 | 42 ± 13 | 44 ± 13 |
| E group | 42 ± 12 | 41 ± 12 | 43 ± 12 |
| All patients | 44 ± 12 | 43 ± 12 | 44 ± 13 |
| Triglycerides, mg/dl | |||
| S group | 175 ± 118 | 139 ± 87 | 125 ± 71 |
| S + E group | 135 ± 63 | 190 ± 192 | 165 ± 203 |
| E group | 149 ± 72 | 130 ± 52 | 152 ± 70 |
| All patients | 152 ± 84 | 153 ± 120 | 152 ± 116 |
Values are mean ± SD.
p ≤ 0.05 versus baseline.
p ≤ 0.01 versus S group.
HDL = high-density lipoprotein; LDL = low-density lipoprotein; other abbreviations as in Table 1.
MRI parameters
Change in perfusion index and PCr recovery time constant were primary outcome measures (Table 3). For the calf muscle perfusion index, there was no difference between the S and S + E groups or change over time for any group. There was no difference within groups for PCr recovery time. For all patients, the PCr recovery time remained unchanged between baseline (92 ± 64 s), year 1 (88 ± 64 s), and year 2 (78 ± 55 s, p = 0.67 vs. baseline). There was no change in the MRA runoff resistance index between any group or in the study as a whole. However, the MRA index was significantly higher in the S + E group than the S group at baseline and every subsequent time point (Table 3). There was no statistically significant change over time for any group or in the patients as a whole. There was no correlation between the change in LDL on calf muscle perfusion index or PCr recovery time constant.
Table 3.
Magnetic Resonance Parameters Over Time
| Group | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| Perfusion index | |||
| S group | 0.48 ± 0.18 | 0.42 ± 0.44 | 0.34 ± 0.19 |
| S + E group | 0.42 ± 0.13 | 0.44 ± 0.23 | 0.37 ± 0.13 |
| E group | 0.51 ± 0.16 | 0.46 ± 0.21 | 0.54 ± 0.20 |
| All patients | 0.48 ± 0.16 | 0.45 ± 0.21 | 0.44 ± 0.20 |
| PCr recovery time constant, s | |||
| S group | 94 ± 77 | 85 ± 80 | 84 ± 68 |
| S + E group | 104 ± 56 | 93 ± 46 | 73 ± 35 |
| E group | 83 ± 62 | 86 ± 65 | 74 ± 58 |
| All patients | 92 ± 64 | 88 ± 64 | 78 ± 55 |
| MRA index | |||
| S group | 0.73 ± 0.50* | 0.59 ± 0.38* | 0.71 ± 0.46* |
| S + E group | 1.10 ± 0.72 | 1.26 ± 0.73 | 1.32 ± 0.86 |
| E group | 0.65 ± 0.62 | 0.73 ± 0.72 | 0.63 ± 0.64 |
| All patients | 0.81 ± 0.64 | 0.86 ± 0.71 | 0.88 ± 0.79 |
| MRA ROR index | |||
| S group | 15.4 ± 7.2 | 13.7 ± 7.9 | 13.9 ± 6.2 |
| S + E group | 15.0 ± 6.1 | 15.9 ± 5.4 | 14.7 ± 6.2 |
| E group | 11.2 ± 5.8 | 11.3 ± 6.4 | 9.5 ± 4.4 |
| All patients | 13.5 ± 6.5 | 13.6 ± 6.5 | 12.7 ± 6.2 |
Values are mean ± SD.
p < 0.05 versus S + E group.
MRA = magnetic resonance angiography; PCr = phosphocreatine; ROR = runoff resistance; other abbreviations as in Table 1.
Functional testing
Results are reported in Table 4. The resting ABI was similar between the S and S + E groups at baseline (0.71 ± 0.23 and 0.65 ± 0.24, respectively) and year 1 (0.70 ± 0.23 and 0.66 ± 0.29, respectively). However, it was higher in the S group at year 2 (0.85 ± 0.19) than in the S + E group (0.66 ± 0.12, p < 0.03). The resting ABI increased in the E group and in the study population as a whole. In contrast, there was no change in post-exercise ABI over time for any group. There was no change in total treadmill exercise time or time to claudication (Table 4). Likewise, the 6-min walk for total distance and onset of claudication were unchanged. There was no correlation between the change in LDL with peak exercise Vo2 or 6-min walk parameters.
Table 4.
Exercise Parameters Over Time
| Group | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| Log (treadmill exercise time) | |||
| S group | 6.08 ± 0.84 | 5.90 ± 0.93 | 5.92 ± 1.08 |
| S + E group | 5.82 ± 0.67 | 5.94 ± 0.70 | 5.79 ± 0.67 |
| E group | 5.94 ± 0.78 | 5.89 ± 1.04 | 5.78 ± 0.63 |
| All patients | 5.95 ± 0.76 | 5.92 ± 0.91 | 5.82 ± 0.77 |
| Log (treadmill time to claudication) | |||
| S group | 5.2 ± 0.8 | 4.9 ± 0.8 | 5.0 ± 0.9 |
| S + E group | 4.9 ± 0.6 | 5.5 ± 0.7 | 5.1 ± 0.6 |
| E group | 5.2 ± 0.9 | 5.5 ± 0.7 | 5.2 ± 0.6 |
| All patients | 5.1 ± 0.8 | 5.3 ± 0.7 | 5.1 ± 0.7 |
| 6-min walk distance, ft | |||
| S group | 1,103 ± 407 | 1,189 ± 388 | 1,078 ± 485 |
| S + E group | 976 ± 324 | 922 ± 398 | 1,038 ± 248 |
| E group | 1,007 ± 435 | 1,057 ± 460 | 1,099 ± 394 |
| All patients | 1,024 ± 397 | 1,036 ± 434 | 1,075 ± 374 |
| 6-min claudication distance, ft | |||
| S group | 455 ± 197 | 532 ± 206 | 417 ± 83 |
| S + E group | 448 ± 187 | 413 ± 185 | 461 ± 161 |
| E group | 476 ± 244 | 525 ± 272 | 597 ± 318 |
| All patients | 465 ± 213 | 483 ± 232 | 518 ± 251 |
| Vo2, ml/kg/min | |||
| S group | 14.6 ± 4.6 | 12.8 ± 3.7 | 14.8 ± 4.6 |
| S + E group | 12.4 ± 3.2 | 12.4 ± 3.9 | 12.1 ± 2.9 |
| E group | 12.1 ± 3.8 | 11.6 ± 3.8 | 12.3 ± 4.6 |
| All patients | 12.8 ± 4.0 | 12.2 ± 3.7 | 12.9 ± 4.2 |
| Resting ABI | |||
| S group | 0.71 ± 0.23 | 0.70 ± 0.23 | 0.85 ± 0.19‡ |
| S + E group | 0.65 ± 0.24 | 0.66 ± 0.29 | 0.66 ± 0.12 |
| E group | 0.77 ± 0.23 | 0.79 ± 0.24 | 0.85 ± 0.26† |
| All patients | 0.72 ± 0.23 | 0.73 ± 0.25 | 0.79 ± 0.23*† |
| Post-exercise ABI | |||
| S group | 0.53 ± 0.23 | 0.54 ± 0.23 | 0.51 ± 0.22 |
| S + E group | 0.47 ± 0.29 | 0.37 ± 0.29 | 0.41 ± 0.28 |
| E group | 0.51 ± 0.31 | 0.53 ± 0.25 | 0.61 ± 0.39 |
| All patients | 0.51 ± 0.28 | 0.48 ± 0.26 | 0.52 ± 0.33 |
| Log (energy expenditure/week, kcal) | |||
| S group | 8.55 ± 1.19 | 8.65 ± 0.86 | 8.69 ± 1.19 |
| S + E group | 8.72 ± 1.61 | 8.49 ± 1.03 | 8.19 ± 1.66 |
| E group | 8.43 ± 1.18 | 8.51 ± 0.75 | 8.84 ± 1.21 |
| All patients | 8.54 ± 1.30 | 8.54 ± 0.86 | 8.43 ± 1.33 |
| Log (metabolic hours equivalent/wk) | |||
| S group | 4.16 ± 1.25 | 4.22 ± 0.96 | 4.26 ± 1.32 |
| S + E group | 4.20 ± 1.50 | 4.06 ± 0.89 | 3.75 ± 1.47 |
| E group | 4.00 ± 1.27 | 4.10 ± 0.78 | 4.45 ± 0.69 |
| All patients | 4.10 ± 1.31 | 4.12 ± 0.85 | 4.06 ± 1.15 |
Discussion
This study used complementary MRI techniques and exercise studies to comprehensively evaluate functional changes in patients with PAD who were treated with lipid-lowering therapy for 2 years. Statin-naïve patients randomized to simvastatin or simvastatin + ezetimibe demonstrated no change in calf muscle perfusion or metabolism, and there was no difference between groups in these MRI parameters despite greater initial LDL lowering in the S + E group. Similar findings were seen in the parallel study of patients previously on a statin with ezetimibe added at study enrollment and when looking at the study population as a whole. Thus, effective LDL lowering did not improve lower extremity functional capacity in PAD. However, resting ABI did improve in the E group and in the patients as a whole, whereas exercise ABI did not.
There is an increasing evidence base underlying the benefits of statins on morbidity and mortality in PAD. In a study of 1,374 patients with PAD, higher doses of statins were associated with lower incidence of all-cause mortality and cardiac death (4). Statin therapy reduced coronary events in elderly patients with PAD and LDL ≥125 mg/dl (21). Analysis of data from the Scandinavian Simvastatin Survival Study demonstrated a 38% reduction in the risk of the development of new or worsening claudication symptoms over a follow-up period of 5.4 years (22). In more than 6,700 patients in the Heart Protection Study, first major vascular events were reduced from 32.7% with placebo to 26.4% with simvastatin. There also was a 16% relative reduction in the rate of first peripheral vascular event, independent of baseline LDL, primarily due to a 20% relative reduction in noncoronary revascularization procedures (23). The latter study suggests that it may be the pleiotropic effects of statins that are responsible for benefit in PAD (24). In general, the mechanisms of benefit remain incompletely understood (25). Despite guideline-recommended use, statins are underused in the PAD population (26).
Several studies suggest benefits of statins on walking performance, although there is some lack of consensus in the literature. A nonrandomized retrospective study demonstrated that, when adjusted for comorbidities, patients with PAD and an ABI <0.9 who were taking statins demonstrated better 6-min walk performance, walking velocity, and overall performance score. A similar patient group studied by the same investigators showed less of a decline in annual walking performance when taking statins (27). Mohler et al. (6) studied 354 patients with symptomatic PAD randomized to either placebo or atorvastatin 10 mg or atorvastatin 80 mg and found an increase in pain-free walking time, a secondary endpoint, after 12 months in the atorvastatin 80-mg group (p = 0.025). However, the primary endpoints were negative. A recent multicenter double-blind trial found no increase in treadmill walking time compared with dietary intervention alone after 28 weeks of lipid lowering with either high- or low-dose niacin + lovastatin (28). In addition, a randomized study of atorvastatin 80 mg for 6 months in PAD demonstrated no effect on brachial artery flow-mediated dilation, carotid intimal-medial thickness, or ABI (29).
Despite achieving significant LDL and total cholesterol lowering, no improvement in functional performance was found in the present study. There are several potential explanations for this. For one, nearly one-half of the study population were already taking statins at the time of entry into the study and may have already received maximal benefit. However, even the group of statin-naïve patients did not show an improvement in exercise parameters. This may reflect a difference in power between studies, although Mohler et al (6). likewise did not find an increase in treadmill walking time. Another potential explanation is that a decline in walking performance over time is expected in PAD. A prospective cohort of PAD patients demonstrated a decrease in 6-min walk distance over 2 years of follow-up (2). Thus, the absence of a functional decline or decrease in perfusion or energetics in the present study may represent a positive finding. Thus, a limitation of the present study is the absence of a placebo group without active treatment to better understand the natural history. Due to the known morbidity and mortality benefits with statins in PAD, a placebo group was not included in the statin-naïve patients.
An improvement in resting ABI was noted in the E group and the patient group as a whole. Although this is encouraging and underscores the simplicity and importance of ABI as a measure, it is less clear as to why this benefit did not translate into any improvement in calf muscle physiology or exercise capacity. In our baseline study of this patient population, we found a correlation between ABI and per-fusion index of r = 0.33, p < 0.01 (12). More subtle measures of physiological changes are needed in this patient population to test the potential benefits of novel therapies.
In the same patient population, we showed plaque regression in the superficial femoral artery in statin-naïve patients treated with either simvastatin or simvastatin/ezetimibe (14). It may be that this effect of the initiation of statin therapy is responsible for much of the therapeutic benefit in PAD. Despite the plaque regression in the statin-naïve patients begun on statins, no improvement in tissue perfusion, cellular metabolism, or functional status was seen. This suggests that the vascular benefits of statins in PAD may primarily be at the level of macrovascular atherosclerotic plaque and not at the microvascular or cellular level. It may be that improvement in tissue perfusion and cellular metabolism is necessary for improvement in functional performance. This speaks against the benefit of lipid lowering in PAD being pleiotropic. This also points out the need for therapies focused on improving skeletal muscle perfusion and metabolism in PAD.
In coronary artery disease, high-dose statin therapy has been shown to reduce atherosclerotic plaque progression by intravascular ultrasound (30) and also to have an impact on angina and time to ischemic events (31). The mechanisms of skeletal muscle dysfunction and claudication in PAD may be quite different from those of myocardial dysfunction and angina in CAD.
Study limitations
The study was primarily powered to measure atherosclerotic plaque progression and not to detect changes in calf muscle perfusion or metabolism. As a result, it was not powered to detect differences in perfusion or metabolism among the groups. Given the relatively large SDs for PCr recovery time, an improvement in calf muscle energetics over time with LDL reduction may have been shown with a larger study population. It is possible that more than 2 years of statin therapy is needed to observe a difference in calf muscle perfusion or metabolism. Alternatively, a more potent statin or higher dose of simvastatin may have resulted in an improvement in calf muscle metabolism or perfusion. For example, an improvement in pain-free walking time was found in PAD patients treated with atorvastatin 80 mg.
We studied only the more symptomatic leg for calf muscle perfusion and energetics; however, the MRA and exercise parameters are certainly influenced by disease in both legs. We did not measure the extent of collateral blood vessels seen on MRI because the development of a signifi-cant collateral blood supply could alter the relationship between discrete stenosis seen on MRA and calf muscle measures of perfusion or metabolism.
A significant number of patients (n = 17) had to be excluded from study analysis due to interval revascularization of their study leg, which reduced overall power of the study. Due to the coexistence of renal disease in patients with PAD, additional patients were excluded from the gadolinium-based calf muscle perfusion and MRA due to concerns for nephrogenic systemic fibrosis. Nongadolinium-based techniques for measuring calf muscle perfusion and oxygenation are being developed. Arterial spin labeling (32) and blood oxygen level–dependent imaging are 2 such promising techniques (33).
Despite randomization of the statin-naïve patients, there remained a significant difference in the MRA index at each time point between S and S + E groups. Despite this difference in macrovascular disease, no differences in baseline physiological parameters were found. The relatively low LDL cholesterol at baseline for the study population as a whole may have contributed to the negative findings seen in this study. There was no placebo control in the statin-naïve patients for reasons stated previously. Without statins on board, many of these parameters may have worsened over the 2-year time period as has been demonstrated by others. The study would have been strengthened by the use of a placebo control in the E group.
Conclusions
In patients with peripheral arterial atherosclerosis, calf muscle perfusion and metabolism did not improve with lipid-lowering therapy despite a significant reduction in LDL and total cholesterol. There was no decline in exercise performance or MRI measurements of calf muscle perfusion or metabolism, which might be construed as a positive finding in PAD patients. However, the primary indication for lipid-lowering therapy in PAD remains the reduction of cardiovascular events and not specifically the improvement of calf muscle physiology, energetics, or walking performance. These findings point out the obvious need for improved medical and alternative therapies to improve these latter parameters as well as walking performance in these patients.
Acknowledgments
The authors are thankful for the contributions of David Isbell, MD, Jayne Missel, RN, and the University of Virginia Vascular Laboratory.
Supported by National Heart Lung Blood Institute R01 HL075792 (to Dr. Kramer), M01RR000847 from the National Center for Research Resources, and National Institute of Biomedical Imaging and Bioengineering T32 EB003841 (to Drs. West and Anderson). Study drugs were supplied by Merck/Schering-Plough. Drs. Epstein, Meyer, Hagspiel, and Kramer receive research support from Siemens Healthcare.
Abbreviations and Acronyms
- ABI
ankle-brachial index
- E group
study group treated with usual statin and open-label ezetimibe added
- LDL
low-density lipoprotein
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- PAD
peripheral arterial disease
- PCr
phosphocreatine
- S group
study group treated with simvastatin
- S + E group
study group treated with simvastatin + ezetimibe
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–21. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 4.Feringa HHH, Karagiannis SE, van Waning VH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–43. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol. 2006;47:1239–312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Mohler ER III, Hiatt WR, Creager MA, for the Study Investigators Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Guralnik JM, Greenland P, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107:757–61. doi: 10.1161/01.cir.0000050380.64025.07. [DOI] [PubMed] [Google Scholar]
- 8.Gagne C, Bays HE, Weiss SR, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–91. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 9.Kastelein JJP, Akdim F, Stroes ESG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–22. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 11.Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–205. doi: 10.1016/j.jacc.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JD, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–34. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 14.West AM, Anderson JD, Meyer CH, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon timing of statin initiation. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.04.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isbell DC, Epstein FH, Zhong X, et al. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2007;25:1013–20. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isbell DC, Berr SS, Toledano AY, et al. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47:2289–97. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 18.Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronefeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–39. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 19.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;21:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Aronow WS, Ahn C. Frequency of new coronary events in older persons with peripheral arterial disease and serum low-density lipo-protein cholesterol > 125 mg/dl treated with statins versus no lipid-lowering drug. Am J Cardiol. 2002;90:789–91. doi: 10.1016/s0002-9149(02)02616-4. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen TR, Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian Simvastatin Survival Study (4S). Am J Cardiol. 1998;81:333–5. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 23.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 24.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295:547–53. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 25.Regensteiner JG, Stewart KJ. Established and evolving medical therapies for claudication in patients with peripheral arterial disease. Nat Clin Pract Cardiovasc Med. 2006;3:604–10. doi: 10.1038/ncpcardio0660. [DOI] [PubMed] [Google Scholar]
- 26.Hoeks SE, Scholte op Reimer WJM, van Gestel YRBM, et al. Medication underuse during long-term follow-up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2:338–43. doi: 10.1161/CIRCOUTCOMES.109.868505. [DOI] [PubMed] [Google Scholar]
- 27.Giri J, McDermott MM, Greenland P, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47:998–1004. doi: 10.1016/j.jacc.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 28.Hiatt WR, Hirsch AT, Creager MA, et al. Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. 2010;15:171–9. doi: 10.1177/1358863X09360579. [DOI] [PubMed] [Google Scholar]
- 29.Spring S, Simon R, van der Loo B, et al. High-dose atorvastatin in peripheral arterial disease (PAD): effect on endothelial function, intima-media-thickness and local progression of PAD. An open randomized controlled pilot trial. J Thromb Haemost. 2008;99:182–9. doi: 10.1160/TH07-04-0265. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 31.Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med. 1999;341:70–6. doi: 10.1056/NEJM199907083410202. [DOI] [PubMed] [Google Scholar]
- 32.Wu WC, Mohler E III, Ratcliffe SJ, et al. Skeletal muscle microvascular flow in progressive peripheral artery disease: assessment with continuous arterial spin-labeling perfusion magnetic resonance imaging. J Am Coll Cardiol. 2009;53:2372–7. doi: 10.1016/j.jacc.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledermann H, Schulte AC, Heidecker HG, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation. 2006;113:2929–35. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]