Abstract
Even among asymptomatic people at low risk (<10%) by Framingham Risk Score (FRS), high coronary artery calcium (CAC) scores signify higher predicted risk of coronary heart disease (CHD) events. We sought to determine non-invasive factors (without radiation exposure) significantly associated with CAC in low-risk, asymptomatic persons. In a cross-sectional analysis, we studied 3046 participants from MESA at low 10-year predicted risk (FRS <10%) for CHD events. Multivariable logistic regression was used to assess the association of novel markers with presence of any CAC (CAC >0) and advanced CAC (CAC ≥ 300). CAC >0 and CAC ≥ 300 were present in 30% and 3.5% of participants, respectively. Factor VIIIc, fibrinogen and sICAM were each associated with CAC presence (P ≤ 0.02); and C-reactive protein, D-dimer and carotid intima-media thickness (CIMT) with advanced CAC (P ≤ 0.03). The base model combining traditional risk factors had excellent discrimination for advanced CAC (C-statistic, 0.808). Addition of the 2 best-fit models combining biomarkers plus/minus CIMT improved the c-statistics to 0.822 and 0.820, respectively. All 3 models calibrated well, but were similar in estimating individual risk probabilities for advanced CAC (prevalence = 9.97%, 10.63% and 10.10% in the highest quartiles of predicted probabilities versus 0.26%, 0.26% and 0.26% in the lowest quartiles, respectively). In conclusion, in low risk individuals, traditional risk factors alone predicted advanced CAC with high discrimination and calibration. Biomarker combinations +/− CIMT were also significantly associated with advanced CAC, but improvement in prediction and estimation of clinical risk were modest compared to traditional risk factors alone.
Keywords: coronary calcium, biomarkers, novel markers, low-risk, risk factors
Introduction
The Framingham Risk Score (FRS) has been validated as a useful tool in the estimation of coronary heart disease (CHD) risk.(1) Events, however, may still occur in those at low predicted 10-year CHD risk since the FRS provides only an average probability of event occurrence.(2, 3) One proposed method for improving identification of persons at risk for coronary events has been to implement widespread screening for coronary artery calcium (CAC) in intermediate risk adults.(4) CAC is highly correlated with plaque burden, plaque rupture(5, 6) and coronary events;(7–10) and CAC ≥ 300 predicts coronary events beyond traditional Framingham risk factors in intermediate and low risk persons.(11–13) Factors associated with CAC presence (CAC >0) and/or advanced CAC (CAC ≥ 300) in low-risk persons (which make up 75% of the general population(14)) have not been identified. The present study therefore aimed to assess novel markers significantly associated with the presence of CAC, or advanced CAC among low-risk, asymptomatic adults thereby avoiding radiation exposure, discovery of incidental findings requiring follow-up CT (computed tomography) scans, and potentially increased costs associated with CAC measurement in low-risk persons.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study examining measures of subclinical atherosclerosis, progression of subclinical atherosclerosis, and conversion to clinical events. Details of the study design, as well as inclusion and exclusion criteria and baseline characteristics have been described previously.(15) Briefly, at baseline the cohort included 6814 participants (3213 men and 3601 women) aged 45 to 84 years from four different racial/ethnic groups (38% white, 28% African American, 22% Hispanic and 12% Chinese) in six US communities including Baltimore, Maryland; Chicago, Illinois; Forsyth, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota. The participants were free of clinical CVD at first examination (July 2000 to August 2002).
For the current study, we included men and women aged ≤ 79 years at baseline, categorized as being at low 10-year risk for hard CHD events (10-year predicted risk <10%) based on the FRS(1). Individuals older than 79 years could not have a calculated FRS and were therefore excluded. Consistent with the ATP-III definitions of patients with coronary risk equivalents, the present analyses also excluded participants with a diagnosis of diabetes, peripheral arterial disease (ankle brachial index <0.9), carotid artery disease (≥50% carotid artery stenosis), history of abdominal aortic aneurysm, severe chronic renal insufficiency and end-stage renal disease (GFR <30 mL/min/1.73 m2 – based on the Modification of Diet in Renal Disease [MDRD](16) equation). We further excluded participants who were already receiving lipid-lowering therapy, since this may have affected their FRS estimate.
Baseline examination, laboratory data, cardiac CT and carotid ultrasonography have been described elsewhere.(12, 15) Body mass index (BMI) was defined as weight in kilograms (kg) divided by height in meters squared (m2). Medication use was derived from medication lists and clinical staff entry of prescribed medications. Aspirin use was defined as ≥3 days per week at baseline. Agatston CAC score >0 is defined as CAC presence, and CAC score ≥ 300 is termed advanced CAC. Carotid ultrasonography was performed by obtaining images of the right and left common carotid and internal carotid arteries (including near and far wall images), using high-resolution B-mode ultrasound.
All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). P-value of <0.05 was considered significant. Baseline characteristics were compared according to CAC classification using general linear models for continuous variables and cross-tabulations for categorical variables. For multivariate analyses, the associations of individual biomarker levels with the presence of CAC or advanced CAC burden were examined (separately) using logistic regression models, and the standardized estimates - multivariable-adjusted odds ratios (OR) and their 95% confidence intervals (CI) - were assessed. The Framingham 10-year risk scores for all subjects were calculated according to NCEP guidelines.(17)
Several different approaches were employed in developing the models for assessing association of novel marker combinations with CAC presence/advanced CAC including data-driven methodologies (combination of novel markers independently associated with CAC in MESA), clinical/mechanistic approaches (combination of novel markers either from each major group – lipoprotein, inflammatory and hemostatic factors; as well as measures of subclinical atherosclerosis, endothelial dysfunction and chronic kidney disease – or based on clinical perception), and backward stepwise selection techniques.
Novel markers were added individually to the base model to assess their independent associations with CAC presence. Using this approach, various models were fitted to estimate the associations of combinations of novel markers with the presence of CAC. The base model which included traditional risk factor covariates only (see table II footnotes), and the backward stepwise selection model used p <0.10 as the criterion for retention (beginning with all available covariates), were utilized for our analysis. Similarly, for associations with advanced CAC, several models with combination novel markers were fitted. The models employed for our analysis include the base model with covariates only, in addition to: CRP (high-sensitivity C-reactive protein), D-dimer and carotid intima-media thickness (CIMT) - model 1; CRP, D-dimer, LDLpn (LDL particle number), cystatin C and soluble intercellular adhesion molecule (s-ICAM) - model 2; and the unbiased approach using a backward stepwise selection model with p <0.10 as the criterion for retention.
Table II.
Backward Selection Model for Biomarker Prediction of Coronary Artery Calcium Presence (932 of 3046)
| Model | OR (95% CI) per 1 SD |
|---|---|
| Fibrinogen | 1.11 (1.01, 1.22) |
| Soluble intercellular adhesion molecule-1 | 1.16 (1.04, 1.298) |
| Factor VIIIc | 1.12 (1.03, 1.23) |
| Carotid intima media thickness | 1.08 (0.99, 1.18) |
| Age | 1.09 (1.07, 1.10) |
| Gender | 3.09 (2.47, 3.86) |
| Race | 0.80 (0.74, 0.86) |
| Systolic blood pressure | 1.01 (1.00, 1.01) |
| Total cholesterol | 1.01 (1.00, 1.01) |
| High density lipoprotein cholesterol | 0.99 (0.98, 1.00) |
| Current smoking | 1.40 (1.04, 1.89) |
| Hypertension treatment | 1.36 (1.10, 1.68) |
For tables II and III:
Base model, models 1 – 5 for advanced coronary artery calcium adjusted by age, gender, race, systolic blood pressure, total cholesterol, high density lipoprotein cholesterol, current smoking, hypertension treatment.
The backward stepwise selection models are selected from all biomarkers and all base model covariates.
All soluble intercellular adhesion molecule-1 values are imputed. Akaike information criteria and C-statistics are the average value of its five soluble intercellular adhesion molecule-1 imputations.
OR = odds ratio, CI = confidence interval, SD = standard deviation, AIC = Akaike information criterion
To avoid potential bias and power limitations due to missing cases in complete-case analyses, missing data for s-ICAM were replaced by multiple imputations,(18) with all covariates and novel markers taken as predictors. Likelihood ratio tests were used to obtain p-values to determine the level of significance of each model relative to the base model. Akaike information criteria (AIC) were used to assess the level of informativeness of each model with lower AIC depicting greater informativeness; and the C-statistic was used to measure the discrimination of each model with higher c-statistic reflecting greater discrimination. Receiver operator characteristic (ROC) curves were then plotted for the base model, as well as the combination models exhibiting the greatest levels of discrimination for advanced CAC (best-fit models) with and without CIMT.
Results
From a total of 6814 MESA participants, the study sample included 3046 persons (44.7%) aged ≤ 79 years predicted to be at low risk by FRS, who also underwent CT for CAC measurement. The mean age of the sample was 57±9 years, and 74% were women (mean ages were 59+/−9 for women and 52+/−6 for men). A total of 932 of 3046 participants had CAC (29% of women and 35% of men). Baseline characteristics stratified by presence of CAC or advanced CAC are shown in Table I. CAC presence was associated with adverse levels of most of the established CV risk factors. Of note, smoking and lipid-lowering medications were not associated with CAC presence in this lower risk sub-sample of the MESA cohort.
Table I.
Baseline Characteristics (N = 3046)
| Characteristics | Coronary Artery Calcium | Coronary Artery Calcium | ||||
|---|---|---|---|---|---|---|
| =0 (n = 2114) | >0 (n = 932) | P-value | <300 (n = 2938) | ≥ 300 (n = 108) | P-value | |
| Age (years) | 55.4+/−8.0 | 60.8+/−9.1 | <0.01 | 56.8+/−8.6 | 64.7+/−8.7 | <0.01 |
| Women | 75.3% | 70.2% | <0.01 | 74.0% | 66.7% | 0.09 |
| White | 37.3% | 46.6% | <0.01 | 39.3% | 61.0% | <0.01 |
| African American | 27.4% | 21.6% | 13.0% | 9.3% | ||
| Hispanic | 23.2% | 17.3% | 26.0% | 16.7% | ||
| Chinese | 12.1% | 14.6% | 21.7% | 13.0% | ||
| Current smoking | 10.2% | 9.9% | 0.91 | 10.0% | 12.0% | 0.5 |
| Systolic Blood Pressure (mmHg) | 118+/−19 | 123+/−19 | <0.01 | 120+/−19 | 125+/−17 | <0.01 |
| Diastolic Blood Pressure (mmHg) | 70+/−10 | 71+/−10 | 0.02 | 70+/−10 | 72+/−10 | 0.13 |
| Antihypertensive medications use | 18.1% | 25.1% | <0.01 | 19.8% | 31.5% | <0.01 |
| Body Mass Index (kg/m2) | 28.0+/−5.8 | 28.0+/−5.6 | 0.92 | 30.0+/−5.7 | 27.7+/−5.6 | 0.64 |
| Family history of premature CHD | 36.6% | 50.2% | <0.01 | 40.0% | 62.4% | <0.01 |
| Physical activity (MET-min/wk) | 896+/−2607 | 1031+/−2940 | 0.14 | 930+/−2724 | 1146+/−2400 | 0.42 |
| Total cholesterol (mg/dL) | 195+/−35 | 200+/−35 | <0.01 | 197+/−35 | 206+/−37 | <0.01 |
| HDL cholesterol (mg/dL) | 55+/−15 | 55+/−16 | 0.66 | 55+/−15 | 58+/−14 | 0.03 |
| LDL cholesterol (mg/dL) | 116+/−30 | 122+/−32 | <0.01 | 118+/−30 | 125+/−34 | 0.03 |
| Triglycerides (mg/dL) | 117+/−69 | 119+/−62 | 0.43 | 118+/−67 | 119+/−71 | 0.88 |
| Mean Framingham Risk Score (%) | 2.8+/−2.4 | 4.3+/−2.5 | <0.01 | 3.2+/−2.5 | 5.1+/−2.6 | <0.01 |
| Medications use | ||||||
| Estrogen use (in women) | 29.4% | 30.6% | 0.68 | 29.4% | 38.9% | 0.08 |
| Aspirin | 10.3% | 14.5% | <0.01 | 11.3% | 19.6% | 0.01 |
| ACE inhibitors/ARBs | 4.3% | 7.2% | <0.01 | 5.8% | 9.3% | 0.1 |
| Beta blockers | 4.7% | 5.8% | 0.15 | 5.3% | 6.5% | 0.61 |
| Nitrates | 0.1% | 0.1% | 0.92 | 0.07% | 0.0% | 0.79 |
| Calcium blockers | 6.6% | 8.4% | 0.06 | 6.9% | 13.9% | <0.01 |
CAC >0 denotes CAC presence; CAC ≥ 300 denotes advanced CAC
CAC = coronary artery calcium, CHD = coronary heart disease, ACE = angiotensin converting enzyme, ARBs = angiotensin receptor blockers, HDL = high density lipoprotein cholesterol, LDL = low density lipoprotein cholesterol,
Only 108 out of 3046 participants predicted to be at low risk by FRS had advanced CAC (3.0% of women and 4.5% of men). Many traditional risk factors were found to be significantly associated with advanced CAC. Race/ethnicity, antihypertensive medications use and aspirin use were related to both CAC presence and advanced CAC.
In univariate analysis (data not shown), most of the novel markers, with the exception of CRP and D-dimer, were significantly associated with CAC presence (all p <0.01) while cystatin-C, CRP, D-dimer, Factor VIIIc, homocysteine (tHcy) and CIMT were significantly associated with advanced CAC (all p <0.01). No significant interactions between sex and any of the novel markers were found. The multivariable analysis showed that factor VIIIc, fibrinogen and sICAM (imputed and unimputed) were significantly associated with CAC presence; while CRP, D-dimer, and CIMT were significantly related to advanced CAC.
Several biomarker/measure combination models were examined based on data-driven methodologies (from multivariable analysis), clinical/mechanistic approaches, and backward stepwise selection techniques. In the examination of the association of biomarker combinations with CAC presence, the backward stepwise selection process (Table II) provided the greatest informativeness (i.e. AIC) and discrimination for CAC presence with C-statistic = 0.731 (base model C-statistic = 0.725). sICAM-1, factor VIIIc, fibrinogen and CIMT, in addition to many of the traditional risk factors, were selected into the model.
Table III shows the biomarker combination model selection table for association of biomarker combinations with advanced CAC. All of the models provided excellent discrimination for advanced CAC with C-statistic in excess of 0.80. When compared with the base model (C-statistic = 0.808), model 1 (base model plus CRP, D-dimer and CIMT) showed modest improvement in discrimination for advanced CAC (C-statistic = 0.822; p <0.01). With the exclusion of CIMT, model 2 – which combined base model covariates with CRP, D-dimer, LDLpn, cystatin C and sICAM – exhibited the highest level of discrimination for advanced CAC, again with a slight improvement over the base model (c-statistic = 0.820, p = 0.04). Accordingly, the ROC curves for these 3 models substantially overlapped (Figure 1). Of note, many established risk factors (in addition to the same biomarker/measure combinations from model 1) were selected by the backward selection model which again exhibited the greatest informativeness with the lowest AIC. There were no age, gender or race interactions in the association between biomarker combinations and advanced CAC.
Table III.
Models for Biomarker Prediction of Advanced Coronary Artery Calcium (108 of 3046)
| Model Structure | OR (95% CI) per 1 SD | C-statistic | AIC | p-value§ | |
|---|---|---|---|---|---|
| Base model | 0.808 | 801.91 | |||
| Model 1 | C-reactive protein | 1.15 (0.99, 1.34) | 0.822 | 791.43 | <0.01 |
| D-dimer | 1.13 (1.00, 1.26) | ||||
| Carotid intima media thickness | 1.35 (1.12, 1.62) | ||||
| Model 2 | Low density lipoprotein particle number | 1.28 (0.91, 1.79) | 0.820 | 800.13 | 0.04 |
| C-reactive protein | 1.12 (0.96, 1.31) | ||||
| D-dimer | 1.12 (1.00, 1.26) | ||||
| Cystatin C | 0.90 (0.73, 1.12) | ||||
| Soluble intercellular adhesion molecule-1 | 1.15 (0.88, 1.50) | ||||
| Backward stepwise selection model | D-dimer | 1.13 (1.00, 1.26) | 0.820 | 788.39 | |
| C-reactive protein | 1.16 (1.00, 1.35) | ||||
| Carotid intima media thickness | 1.37 (1.15, 1.65) | ||||
| Age | 1.12 (1.09, 1.15) | ||||
| Gender | 6.44 (3.70, 11.23) | ||||
| Race | 0.71 (0.59, 0.83) | ||||
| Total cholesterol | 1.01 (1.00, 1.02) | ||||
| Current smoking | 2.42 (1.28, 4.60) | ||||
| Hypertension treatment | 2.00 (1.26, 3.19) | ||||
Figure 1.
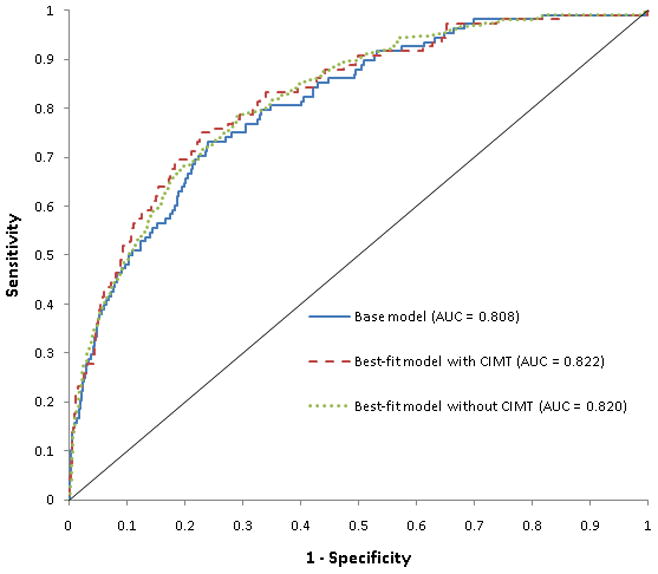
Area under the receiver operator characteristic curve for base model and best-fit models (with and without CIMT) in the association of biomarker combinations with advanced coronary artery calcium.
AUC = Area under the curve
CIMT = carotid intima media thickness
These 2 best-fit models (with and without CIMT) were then compared to the base model for their applicability in the assessment of an individual’s risk for advanced CAC in the population. This was done by comparing observed advanced CAC in the study population to estimated probabilities of advanced CAC (as determined from the models) divided into quartiles (Figure 2). This showed that for the base model and the 2 best-fit models with and without CIMT, participants with the highest estimated probabilities (the 4th quartile groups) had very high prevalence of advanced CAC when compared with the lowest quartile groups (9.97%, 10.63% and 10.10% versus 0.26%, 0.26% and 0.26%, respectively). Thus, there were minimal differences between all 3 model-estimated probabilities for an individual’s risk assessment for advanced CAC (calibration). Of note, most of the advanced CAC participants were from the highest probability quartile groups (70.4%, 75% and 71.3%, respectively).
Figure 2.
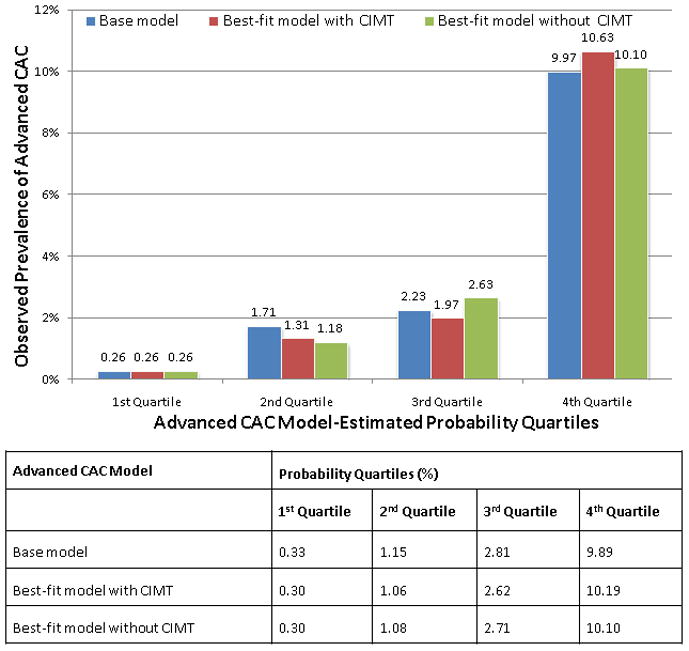
Best-fit (with and without CIMT) and base model-estimated probability quartiles for advanced coronary artery calcium compared to observed prevalence of advanced coronary artery calcium in the study sample.
CIMT = carotid intima media thickness
FRS = Framingham Risk Score
* Base model: traditional risk factors (see table 4 footnote)
† Best-fit model with CIMT: C-reactive protein, d-dimer plus CIMT
‡ Best-fit model without CIMT: C-reactive protein, d-dimer, low density lipoprotein particle number, cystatin C and soluble intercellular adhesion molecule-1
Discussion
Even among a group of asymptomatic adults selected for low-risk status by 10-year FRS <10% in this study, traditional risk factors were the foremost factors still significantly associated with presence of any CAC and advanced CAC. We found significant and independent associations of presence of CAC with factor VIIIc and fibrinogen; and of advanced CAC with CRP, D-dimer and CIMT. However, our combination models demonstrated better discrimination for advanced CAC than CAC presence. Interestingly, in these already low risk persons, the base model with traditional risk factors exhibited excellent discrimination for advanced CAC. As such, some of the models employing novel marker combinations [CRP, D-dimer (with CIMT); and CRP, D-dimer, LDLpn, cystatin C and sICAM (without CIMT)], although significantly different from the base model, showed modest improvement - beyond traditional risk factors - in their discrimination for advanced CAC. Furthermore, although they calibrated well, these models were very similar to the base model in the estimation of individual risk probabilities.
In asymptomatic persons, CAC score helps to predict coronary events beyond traditional Framingham risk factors;(7, 9, 11–13) with more severe CAC burden (CAC ≥ 300 or 400) being associated with the greatest risk.(11, 12) This intensified risk with increased CAC score has been established especially in intermediate risk participants,(7, 11) and has been suggested as an adjunct to risk assessment in some current guidelines for refinement of clinical risk prediction in asymptomatic, intermediate risk patients.(4, 19) Advanced CAC burden (CAC ≥ 300) is also associated with increased coronary events even in those predicted to be at low risk for clinical coronary events.(9, 11, 13)
Although our study includes only participants estimated to be at low predicted 10-year risk by FRS, a small but important subset of the cohort (3.5%) was found to have advanced CAC. These individuals represent an enriched sample of those likely to have events in the near term (either due to advanced age, sex, other risk factor abnormalities, or even genetic factors) despite being at “low risk” for CHD events by the FRS. Clinically, this is a subgroup of major interest since they represent patients who might benefit from, but might not receive, intensive therapy and risk factor modification in the reduction of future coronary events. This is especially relevant to women less than age 70 who are frequently classified as low risk by FRS.(14) Thus, the identification of factors associated with advanced CAC in these low predicted risk individuals could have far reaching clinical implications, and is therefore more crucial than the prediction of only CAC presence in the assessment of future CVD risk.
In our study, model selection for combination markers yielded better discrimination in the identification of advanced CAC than presence of CAC. Interestingly however, the models with the combinations of novel markers showed moderate improvement over traditional risk factors in their discrimination for advanced CAC. Furthermore, although they all calibrated well, the best-fit models were similar to the base model with traditional risk factors in risk estimation; suggesting that even in low risk persons, traditional risk factors remain useful in the clinical estimation of an individual’s risk. To our knowledge, no other study has examined associations of combinations of these novel markers with presence of CAC or advanced CAC. The findings of our study suggest that traditional risk factors still play a significant role in the atherosclerotic (and therefore coronary calcium formation) process, and reemphasizes the significance of the traditional risk factors even in individuals predicted to be at low FRS risk.
It is known that cardiovascular risk factors particularly those related to the metabolic syndrome - obesity, dyslipidemia, hypertension and insulin resistance - as well as diabetes and smoking, lead to vascular injury with endothelial damage, oxidized lipid accumulation and inflammation,(17, 20–23) which promote formation of atherosclerotic plaque.(20, 23, 24) This process is considerably amplified by interactions between co-existing risk factors.(23) Calcification, which represents an advanced stage of atherosclerosis/plaque formation, is formed and regulated by this inflammatory milieu and is an active process, similar to bone formation, in which pericyte-like cells secrete a matrix scaffold which later becomes calcified.(23) Thus, the ability of CAC burden to predict events probably lies in its capacity to predict patients likely to possess (in addition to stable calcified plaques) soft, non-calcified plaques prone to rupture and acute thrombosis.(5) CAC also correlates with total atherosclerotic burden.(5, 25) It is therefore not surprising that these risk factors which predict atherosclerosis and CV events, also predict advanced CAC.
Like CAC, CIMT is a subclinical measure of atherosclerosis, and exhibited the best single association with advanced CAC (more than the biomarkers). There was however, a fairly small difference in its discrimination when the best fit model with CIMT was compared to the best-fit model without CIMT, or importantly, the base model with traditional risk factors. This is particularly noteworthy since although a non-invasive test, CIMT measurement is dependent on technician and reader skills, and is more costly than traditional risk factors plus/minus biomarkers.
Our study suggests that in persons predicted to be at low 10-year risk for CHD events based on FRS, traditional risk factors should be employed in the identification of low risk persons who likely have advanced CAC, and are therefore at risk for future events. Further study is required to define the subset of low risk individuals who may benefit from undergoing CT evaluation. In addition, our study data suggests that the subset of individuals with low predicted risk (FRS <10%) but elevated levels of traditional risk factors might be particularly worthy of study
Several limitations of our study should be acknowledged. First, we could not stratify our analysis by gender or race due to the restricted number of participants in our study. Also, the age-range and selection criteria for FRS <10% limited the number of men in the study. Second, at the time the analysis for the present study was carried out, very few CVD events had occurred in this low risk population. We were therefore unable to assess prediction of clinical events relative to CAC. Third, other more novel biomarkers which could have been included in CAC assessment - including lipoprotein-associated phospholipase A2, brain natriuretic peptide, Von Willebrand Factor, lipoprotein (a), and others - were not available within the cohort at the time of the study. Fourth, because of the low-risk nature of the study participants, the prevalence of CAC (particularly that of advanced CAC burden), was relatively low. Finally, temporality could not be determined due to the cross-sectional nature of the study.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Source of Funding and Acknowledgements: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 3.Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92(12):1752–1759. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Taylor A, Farb A, Malcom GT, Virmani R. Coronary calcification: insights from sudden coronary death victims. Z Kardiol. 2000;89 (Suppl 2):49–53. doi: 10.1007/s003920070099. [DOI] [PubMed] [Google Scholar]
- 7.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107(20):2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Daviglus ML, Pirzada A, Liu K, Yan LL, Garside DB, Dyer AR, Hoff JA, Kondos GT, Greenland P, Stamler J. Comparison of low risk and higher risk profiles in middle age to frequency and quantity of coronary artery calcium years later. American Journal of Cardiology. 2004;94(3):367–369. doi: 10.1016/j.amjcard.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 12.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. [see comment] New England Journal of Medicine. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 13.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). [see comment] Archives of Internal Medicine. 2007;167(22):2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Giles WH, Mokdad AH. The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43(10):1791–1796. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Schafer JL. In: Analysis of Incomplete Multivariate Data. 1. Hall C, editor. London: CRC Press; 1997. (Monographs on Statistics and Applied Probability) [Google Scholar]
- 19.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo M, Rizvi AA, Rini GB, Berneis K. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: what is the clinical relevance? Acta Diabetologica. 2009;46(1):1–11. doi: 10.1007/s00592-008-0057-4. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Insulin resistance, haemostatic and inflammatory markers and coronary heart disease risk factors in Type 2 diabetic men with and without coronary heart disease. Diabetologia. 2004;47(9):1557–1565. doi: 10.1007/s00125-004-1491-7. [DOI] [PubMed] [Google Scholar]
- 22.Tracy RP. Inflammation, the metabolic syndrome and cardiovascular risk. Int J Clin Pract Suppl. 2003;(134):10–17. [PubMed] [Google Scholar]
- 23.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 25.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]


