Abstract
Aim
CCL20, also known as MIP-3 alpha, is a chemokine that participates in chemotaxis of immature dendritic cells, effector/memory T-cells, and B-lymphocytes. The objectives of this study were to determine whether CCL20 can be detected in amniotic fluid (AF) and if AF concentration of this chemokine changes with advancing gestational age, parturition (term and preterm), and intra-amniotic infection/inflammation (IAI).
Methods
A cross-sectional study was conducted including the following groups: 1) midtrimester of pregnancy (n=65); 2) term not in labor (TNL; n=22); 3) term in labor (TIL; n=47); 4) spontaneous preterm labor (PTL) who delivered at term (n=57); 5) spontaneous PTL without IAI who delivered preterm (n=71); and 6) spontaneous PTL with IAI (n=38). AF CCL20 concentrations were determined using ELISA.
Results
1) The median AF CCL20 concentration in TNL was higher than that of midtrimester patients; 2) Women in spontaneous labor at term had a higher median AF concentration of CCL20 than patients at term not in labor; 3) Patients with spontaneous PTL and IAI had a significantly higher median AF concentration of CCL20 than those without IAI who delivered preterm and those who delivered at term. Moreover, women with spontaneous PTL without IAI who delivered preterm had a significantly higher median AF concentration than those with PTL who subsequently delivered at term.
Conclusions
1) CCL20 is a physiologic constituent of AF and its concentration increases as term approaches; 2) spontaneous labor (term and preterm) in the absence of IAI is associated with an increased bioavailability of AF CCL20 suggesting that an increase in CCL20 is part of the common pathway of human parturition; 3) patients with IAI had dramatic elevations in the AF CCL20 concentrations suggesting that this chemokine participates in the host response to infection or other stimuli associated with intra-amniotic infection.
Keywords: Amniotic fluid, cytokine, inflammation, LARC, macrophage inflammatory protein 3 alpha, parturition, pregnancy
INTRODUCTION
Intra-amniotic infection/inflammation (IAI) is causally linked to spontaneous preterm labor and delivery [102] and it is associated with both maternal and neonatal morbidity and mortality [120]. Amniotic fluid is traditionally considered to be sterile [102]. The first line of defense against ascending infection is composed of the cervical mucus plug [27,47,48,103] and chorioamniotic membranes [72,128]; as well as cellular components of the innate immune system including neutrophils, macrophages, natural killer cells, and trophoblasts [42,102]. Microorganisms, their products, and other stimuli elicit an inflammatory response which leads to an increased production of pro-inflammatory cytokines by gestational tissues [25,71] amniotic fluid [12,14,15,24,71,100,101,104-106,136], maternal serum [90], and fetal cord blood [15,41,105,136]. It has been proposed that pro-inflammatory cytokines and chemokines participate in the mechanisms responsible for the initiation of labor in the setting of infection, but also in spontaneous labor at term [101]. One mechanism through which inflammation is produced involves increased leukocyte trafficking which is induced by chemokines [18,30,31,64,66,70].
Chemokines are a group of structurally related chemotactic proteins implicated in the recruitment of cells to sites of inflammation. They also participate in homeostatic cellular trafficking [118]. Exodus-1, also known as CC chemokine ligand 20 (CCL20), is the only chemokine known to interact with the CC chemokine receptor 6 (CCR6) [6], a property shared with some antimicrobial peptides: the β-defensins [123,135]. Together, CCL20 and CCR6 play a role in both homeostatic and inflammatory states. The evidence in support of this is: 1) CCL20 and CCR6 are involved in recruiting immature dendritic cells to sites where antigen entry may occur [10,17,21,22,40,62,93,114,133,135]; 2) mediate effector/memory T-cell interactions with endothelial cells [26,78,93,113,135]; and 3) participate in the migration of memory B-lymphocytes [9,11,23,73,79,118].
There is a paucity of information about CCL20 in human pregnancy. In vitro, CCL20 mRNA is expressed in fetal lung and liver [108]. In vivo, one study with a small sample size suggests no change in maternal serum CCL20 concentrations in women with preterm labor and delivery compared to either preterm controls or women at term in labor [77]. A second study demonstrates increased CCL20 protein levels in decidual biopsies in patients with preeclampsia compared to normal controls at the time of delivery [55]. So far, the concentration of CCL20 in amniotic fluid has not been reported. The objective of this study was to determine whether CCL20 can be detected in the amniotic fluid and if the amniotic fluid concentration of this chemokine changes with gestational age, in the presence of spontaneous labor (term and preterm), or with intra-amniotic infection/inflammation.
METHODS
A cross-sectional study was designed by searching our clinical database and bank of biological samples, including women in the following groups: Group 1 consisted of women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered normal infants at term (n=65). Group 2 included women with normal term gestations. This group was subdivided into women not in labor (TNL; n=22) and women in spontaneous labor (TIL; n=47). Group 3 included women with spontaneous preterm labor (PTL) and intact membranes that were subdivided into the following categories: 3A) patients with PTL who delivered at term (n=57); 3B) patients with PTL who delivered preterm without IAI (n=71); 3C) patients with PTL who delivered preterm with IAI (n=38). Patients with preterm premature rupture of membranes, multi-fetal gestation, chromosomal abnormalities, and fetal congenital malformations were excluded.
All women provided written informed consent prior to the collection of amniotic fluid. The collection of amniotic fluid samples was approved by the institutional review boards of Wayne State University, Pennsylvania Hospital, Sotero del Rio Hospital and the National Institutes of Child Health and Human Development. Many of these samples have been used previously in studies of cytokines, chemokines, growth factors, arachidonic acid metabolites, and antimicrobial peptides in amniotic fluid.
Definitions
Women were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complications of pregnancy, and delivered a term neonate of appropriate birth weight for gestational age without congenital anomalies or complications. Preterm labor was diagnosed by the presence of at least two regular uterine contractions occurring every 10 minutes associated with cervical changes requiring hospitalization before 37 weeks of gestation. Preterm delivery was defined as delivery before 37 weeks of gestation. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was defined as an amniotic fluid white blood cell (WBC) count >100 cells/mm3.
Sample collection
Amniotic fluid was obtained by transabdominal amniocentesis under ultrasonographic guidance for genetic indications, to assess the microbial state of the amniotic cavity, and to determine fetal lung maturity in patients approaching term. Immediately upon retrieval, amniotic fluid was transported to the laboratory in a sterile capped syringe and cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas. White blood cell count, glucose concentration and Gram-stain were also performed shortly after collection. The results of these tests were used for subsequent clinical management. Amniotic fluid not required for clinical purposes was centrifuged and stored frozen at −70°C until analysis.
CCL20 immunoassay
Amniotic fluid concentrations of CCL20 were determined by the use of specific and sensitive enzyme-linked immunoassays. CCL20 immunoassays were purchased from R&D Systems (Minneapolis, MN, USA) and were validated in our laboratory for use with human amniotic fluid before the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents (matrix) did not interfere with antigen-antibody binding in this assay system. Amniotic fluid samples were incubated in duplicate wells of the micro titer plates, which have been pre-coated with a monoclonal antibody specific for CCL20. During this incubation any CCL20 present in the standards or amniotic fluid samples was bound by the immobilized antibodies in the assay plates. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for CCL20 was added to the wells. Unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates and color developed in proportion to the amount of CCL20 bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of CCL20 in amniotic fluid samples were determined by interpolation from individual standard curves composed of recombinant human CCL20. The calculated inter- and intra-assay coefficients of variation for CCL20 immunoassays in our laboratory were 5.1% and 2.3%. The lower limit of detection (sensitivity) was calculated to be 3.33 pg/ml.
Statistical analysis
The Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Since a normal distribution was not achieved after logarithmic transformation, Kruskal-Wallis with post-hoc tests (Mann-Whitney U tests) were used to determine the differences of the median among groups. Spearman rank correlation was utilized to assess correlations. SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. A p-value <0.05 was considered statistically significant.
RESULTS
Table 1 displays the demographic and clinical characteristics of patients with spontaneous preterm labor. There were no significant differences in the median gestational age at amniocentesis between groups.
Table I.
Demographic and clinical characteristics of the study population
| Characteristics | PTL with Term Delivery (n=57) | PTL and PTD without IAI (n=71) | p-value | PTL and PTD with IAI (n=38) | p-value |
|---|---|---|---|---|---|
| Maternal age (years)† | 22 (15 - 39) | 22 (16 - 44) | NS | 23 (15 - 41) | NS |
| Parity† | 2 (1 - 6) | 2 (1 - 9) | NS | 2 (1 - 9) | NS |
| Prior history of Preterm delivery | 24.6% 14/57 | 38.0% 27/71 | NS | 26.3% 10/38 | NS |
| Gestational age at amniocentesis (weeks)† | 30.6 (19 - 35) | 29.5 (19 - 34) | NS | 26.1 (20 - 34) | NS |
| Gestational age at delivery (weeks) | 38.6 (37 - 42) | 32.9 (21 - 36) | <0.05 † | 28.2 (20 - 36.9) | <0.05¶ |
| Birth weight (gms) | 2948 (2390 - 4080) | 1765 (360 - 2920) | <0.05 † | 1085 (280 - 2950) | < 0.05¶ |
The values are given as median (ranges) or percentages (number).
PTL; preterm labor, PTD; preterm delivery, IAI; intra-amniotic infection/inflammation.
Kruskal – Wallis with posthoc analysis.
Kruskal – Wallis with posthoc analysis, significance only between PTL and PTD with IAI vs. PTL with Term Delivery
Detectable Immunoreactive CCL20 in Amniotic Fluid
CCL20 was detectable in 88.3% (265/300) of amniotic fluid samples. CCL20 was below the limit of detection for the following: midtrimester 41.5% (27/65), term not in labor 22.7% (5/22), term in spontaneous labor 2.1% (1/47), PTL without IAI 1.4% (1/71), and PTL with term delivery 1.8% (1/57).
AF CCL20 Concentration is Higher at Term than in the Midtrimester
The median amniotic fluid concentration of CCL20 was higher in women at term not in labor than in women in the midtrimester of pregnancy (TNL: 34.6 pg/ml, range: 0.0 – 134.0 vs. midtrimester: 15.3 pg/ml, range: 0 – 158.3; p=0.04; Figure I).
Figure I.
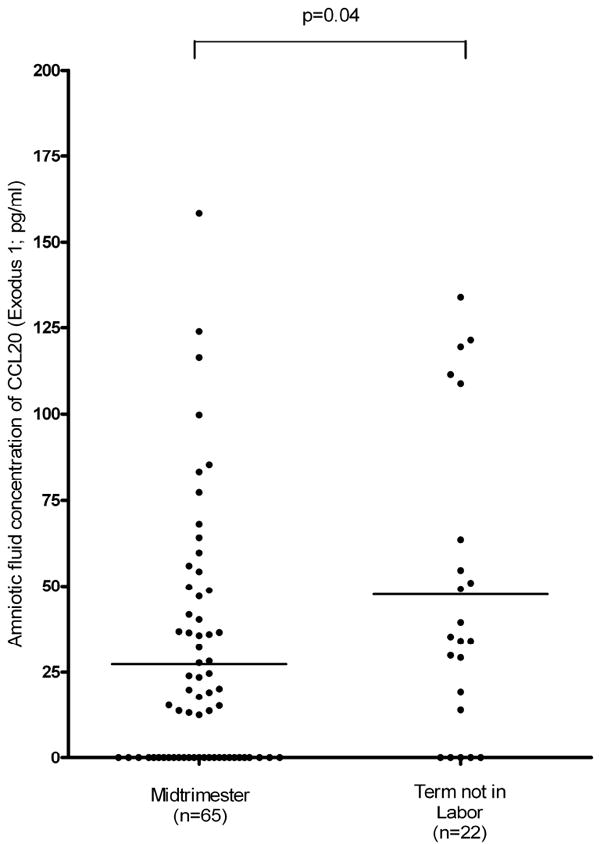
The median amniotic fluid (AF) concentrations of CCL20 in women at term not in labor (TNL) and in the mid-trimester of pregnancy. Amniotic fluid concentration of CCL20 increased significantly with advancing gestational age (TNL: 34.6 pg/ml, range: 0.0 – 134.0 vs. mid-trimester: 15.3 pg/ml, range: 0 – 158.3; p=0.04).
Spontaneous Labor at Term is Associated with Increased AF CCL20
Women at term in spontaneous labor had significantly higher median amniotic fluid concentrations of CCL20 than women at term not in labor (TIL: 76.4 pg/ml, range: 0-3933.6 vs. TNL: 34.6 pg/ml, range: 0-134; p=0.001; Figure II).
Figure II.
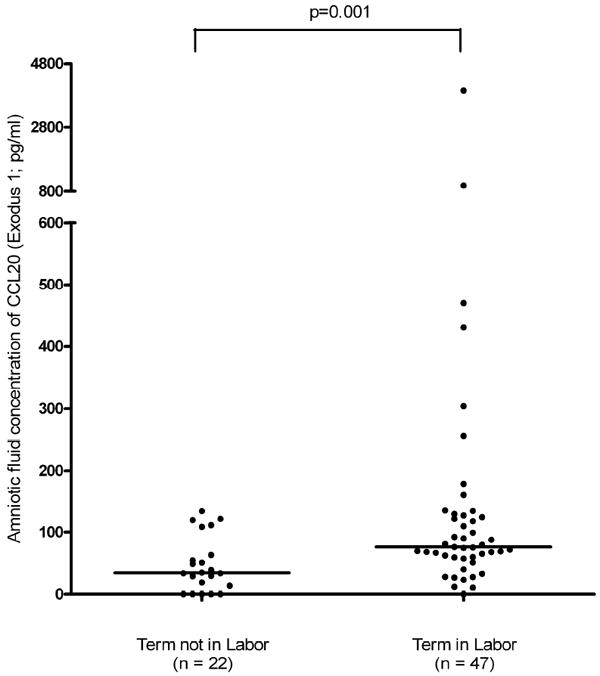
AF concentration of CCL20 in women with normal term gestations comparing women not in labor (TNL) and women in spontaneous labor (TIL). Women at term in spontaneous labor had significantly higher median amniotic fluid concentrations of CCL20 than women at term not in labor (TIL: 76.4 pg/ml, range: 0-3933.6 vs. TNL: 34.6 pg/ml, range: 0-134; p=0.001).
Spontaneous Preterm Labor without Intra-Amniotic Infection/Inflammation is Associated with Increased AF CCL20 Concentration
Patients with spontaneous PTL without IAI had significantly higher concentrations of CCL20 than patients with PTL who delivered at term (Preterm labor without IAI: 119.9 pg/ml, range: 0-15905.4 vs. Preterm labor with term delivery: 45.6 pg/ml, range: 0-293.4; p<0.001; Figure III).
Figure III.
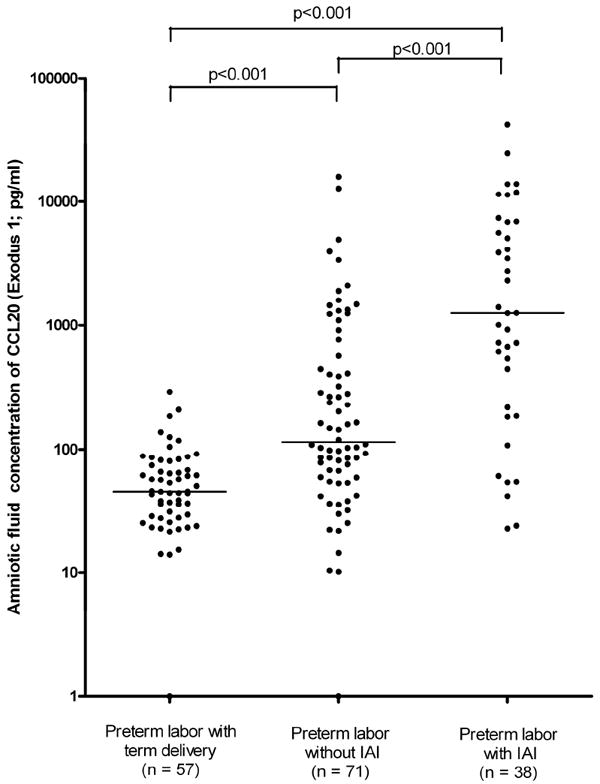
AF concentration of CCL20 in women with spontaneous preterm labor (PTL) and intact membranes. The median AF concentration of CCL20 was significantly higher in women with PTL with intra-amniotic infection/inflammation (IAI) than in those with PTL without IAI (1250.3 pg/ml, range: 22.8-42049.8 vs. 119.9 pg/ml, range: 0-15905.4, respectively; p<0.001), and than in patients with preterm labor who delivered at term (45.6 pg/ml, range: 0-293.4 respectively; p<0.001). Additionally, patients with PTL without IAI had significantly higher concentrations of CCL20 than patients with PTL who delivered at term (119.9 pg/ml, range: 0-15905.4 vs. 45.6 pg/ml, range: 0-293.4, respectively; p<0.001). Data are displayed on Log10 scale.
Intra-amniotic Infection/Inflammation is Associated with Dramatic Elevations of AF CCL20
Among patients with spontaneous PTL and intact membranes, those with IAI had significantly higher concentrations of CCL20 than those without IAI (1250.3 pg/ml, range: 22.8-42049.8 vs. 119.9 pg/ml, range: 0-15905.4, respectively; p<0.001), and than those who delivered at term (45.6 pg/ml, range: 0-293.4 respectively; p<0.001; Figure III).
DISCUSSION
Principal findings of this study
1) CCL20 is frequently detectable in amniotic fluid; 2) The median amniotic fluid concentration of CCL20 is higher at term than in the midtrimester; 3) Spontaneous labor (term and preterm) is associated with high AF concentrations of CCL20; and 4) Intra-amniotic infection/inflammation is accompanied by dramatic elevations in AF CCL20.
What is CCL20?
CCL20, also known as Exodus-1 [54] or macrophage inflammatory protein-3α (MIP-3α) [108], or liver and activation-regulated chemokine (LARC) [6,50], is a chemokine known to interact exclusively with the CC chemokine receptor CCR6 [6,50,54,108,118]. Expression of CCL20 mRNA has been demonstrated in adult tissues such as skin [116], lung [50,97,124], liver [50], stomach [97], appendix [97,108], colon [97], thymus [97,108], and lymph nodes [108]. Several disease processes are associated with CCL20 production including atopic dermatitis [92], rheumatoid arthritis [81,110], psoriasis [22,52,116], tonsillitis [21,22], inflammatory bowel disease [115], cystic fibrosis [124], and cancers of the breast and cervix [7,114]. Some biological fluids are also known to contain CCL20; specifically, cerebrospinal fluid in patients with acute pneumococcal meningitis [69] and E. coli infected urine [98]. Additionally, mRNA expression of CCL20 has been described in vaginal epithelium [19], the uterine cervix [38], placenta [97], and fetal lung and liver [108].
The CCL20 gene is located on chromosome 2q33-37 [50]. Mapping studies demonstrate that gene transcription of CCL20 is modifiable through its promoter region which contains binding sites for several transcription factors including nuclear factor-κB (NF-κB) [45,50,74,93,126]. Mounting evidence suggests that NF-κB is a critical nuclear factor involved in the regulation of CCL20 transcription [19,34,39,45,53,59,60,63,68,74,94,109,126]: 1) deletion and mutation of the NF-κB promoter region results in abrogated luciferase reporter activity for CCL20 [34,45,68,126]; 2) transcription of CCL20 is decreased in vitro by specific NF-κB inhibitors including MG-132, a protease inhibitor that blocks NF-κB gene activation [53,63], helenalin, an NF-κB specific DNA-binding inhibitor [68], and Bay 11-7085 an intracellular NF-κB pathway inhibitor [19]; 3) mutations in the subunits of the NF-κB inhibitor IkB, abrogates the activation of NF-κB and results in decreased luciferase reporter activity and decreased CCL20 protein expression [60,63,94,126]; 4) CCL20 expression, both mRNA and protein, is markedly enhanced by exposing cells in-vitro to pro-inflammatory factors including LPS [53,54,114,117] and the cytokines TNF-α [34,52-54,60,63,68,114,124], IL-1β [19,34,52,53,60,68,74,117,124], IL-1α [63,68], and IL-17 [52,68]; proteins known to act via NF-κB [5,46,68,76,88,89,119,134]; and 5) Immunohistochemical staining (Alexa Fluor 488) demonstrates translocation of the NF-κB subunit p65 from the cytoplasm to the nucleus after IL-17 stimulation [68].
Interestingly, NF-κB is produced after ligation of Toll-like receptors (TLRs) which, in turn, are involved in initiating the innate immune response [102]. This activation is followed by the production of cytokines, chemokines, and antimicrobial peptides. Together with its receptor CCR6, CCL20 participates in directing chemotaxis in the normal physiologic state and under inflammatory stress. The evidence in support of this is: 1) CCL20 expression, both mRNA and protein, is identifiable in normal tissues [19,38,50,97,108,116,124]; 2) CCL20 is chemotactic for immature dendritic cells [6,10,17,21,22,40,54,58,62,93,114,133,135], effector/memory T-cells [78,93,113,135], and memory B-lymphocytes [9,11,23,73,79] as measured by chemotaxis assays using microchemotaxis chambers; 3) calcium mobilization studies demonstrate increased cytosolic calcium concentrations indicative of cellular activation [6,21,79,93]; 4) CCL20, both mRNA and protein, is inducible by inflammatory cytokines [19,34,52-54,60,63,68,74,114,117,124]; and 5) CCL20 is expressed in both acutely [21,22] and chronically [22,52,81,92,110,116] inflamed tissues.
CCL20 and pregnancy
The study reported herein is the first report of CCL20 concentrations in amniotic fluid. The findings that the amniotic fluid concentration of CCL20 changes with advancing gestational age and in the presence of labor, both term and preterm, are novel. Only one study has reported CCL20 concentrations in relation to parturition. Laudanski et al [77]. examined maternal serum concentrations of nine chemokines, including CCL20, in three groups: women at 26-36 weeks in preterm labor who delivered preterm, (n=17) women not in labor with subsequent term delivery (n=13), and women in labor at term (n=8). No difference was found in the maternal serum concentration of CCL20 between groups; however, this study did not control for intra-amniotic infection/inflammation a process associated with preterm parturition [12,15,41,90,100,101,105,106,136]. Additionally, it is possible that the small sample size may account for the lack of difference in the serum concentration of CCL20 among these groups making a type II error possible. It remains to be determined if maternal serum concentrations of CCL20 change in the presence of intra-amniotic infection/inflammation.
Activation of innate immunity is a component of normal pregnancy [107]. Phenotypic changes occur in maternal monocytes and granulocytes in normal pregnant women [91]. The result of which is up-regulation of inflammatory mediators as evidenced by increased expression of CD11b, CD14, and CD64 as well as increased production of intracellular reactive oxygen species [91]. The finding that amniotic fluid concentration of CCL20 is increased in women at term in labor compared to women at term not in labor supports the hypothesis that spontaneous labor is an inflammatory state [43].
Intravascular inflammation is also present in preeclampsia [13,37,44,99,111]. Recently, Huang et al. [55] compared the expression of CCL20, using immunohistochemistry and ELISA, in decidual tissue obtained at the time of cesarean delivery in placental bed biopsies from normal (n=7) and preeclamptic patients (n=7) matched for gestational age. In preeclampsia, the expression of CCL20 was significantly increased.
Intra-Amniotic Infection/Inflammation and AF CCL20
The finding that intra-amniotic infection/inflammation in patients with spontaneous preterm labor is associated with highly increased median amniotic fluid CCL20 concentrations is novel and suggests that this chemokine participates in the host response to microbial invasion as well as intra-amniotic inflammation in which microorganisms cannot be detected with standard cultivation techniques. CCL20 joins several chemokines known to exist in the amniotic fluid including IL-8 [28,33,51,51,70,83,85,131], IL-18 [51,65,84,95], monocyte chemotactic protein-1 (MCP-1) [16,30,31,51,66], MCP-2 [51,64], MCP-3 [51,64], GRO-α [18], and psoriasin [96].
The origin of CCL20 in the amniotic fluid is not known and may be produced from either maternal [77] and/or fetal compartments. The fetal membranes are known to secrete cytokines, including IL-1α [33,56,75,83,85], IL-1β [28,83,85,86,138], and TNF-α [32,33,83,138], as well as the chemokine IL-8 [28,33,70,83,85,131]. Additionally, epithelial tissues appear to be the major producers of CCL20 [7,19,21,22,38,50,52,81,92,97,110,114,116,124]. Since the amnion and chorion are derived from epithelium, it is plausible that these tissues produce and release CCL20 into the amniotic fluid. Fetal tissue may also be a source of CCL20 as in vitro evidence examining fetal lung and liver demonstrate expression of CCL20 mRNA [63,108].
Interestingly, CCL20 secreted from primary cultures of human airway epithelia demonstrates a spectrum of salt-sensitive antimicrobial activity specifically to E. coli, Klebsiella pneumonia, L. monocytogenes, M. catarrhalis, and P. aeruginosa PA01 [124]; an effect that may be mediated by inducing increased bacterial membrane permeability [124]. Amniotic fluid is known to be antimicrobial [3,35,124,127,129,130], an activity that may partially be due to the presence of specific antimicrobial peptides [1,29,49,80,123,137]. Furthermore, B2-defensin, an antimicrobial peptide found in amniotic fluid, uses the CCL20 receptor: CCR6 [123,135]. It is possible that CCL20 retains its antimicrobial activity in amniotic fluid thereby accounting for the marked increase in CCL20 concentration in the setting of intra-amniotic infection.
CCL20 may also function as a chemoattractant in the reproductive organs. Dendritic cells, both mature and immature, have been identified in human decidua basalis and decidua parietalis from first trimester abortions [4,36,55,61,67], placental bed biopsies at the time of delivery [55], and cervical stroma [57,125]. Umbilical cord blood is also a source of dendritic cell populations [2,122,132]. Additionally, both mature and immature dendritic cells have been isolated in maternal serum between 20 and 35 weeks gestation [132] and at delivery [2,132]. Interestingly, the percentage of dendritic cells in maternal serum is significantly lower than non-pregnant controls [2]. This observation may represent cellular trafficking to the reproductive organs; no direct evidence exists at this time that uterine or cervical dendritic cells, mature or immature, participate in parturition. However, this is an interesting area of investigation.
Effector/memory T-cells (CD4+ and CD45RO+) are present in peripheral blood from normal pregnant patients and those with complications [13,20,82], as well as in human decidua [112,121]. Additionally, while B cells are found infrequently in the endometrium [87] and cervical stroma [8], memory B cells have not been described in human gestational tissues. Furthermore, neither immature dendritic cells, effector/memory T-cells, nor memory B-lymphocytes have been identified in amniotic fluid; the site from which CCL20 was measured in this report. Therefore, the role of CCL20 may be unrelated to previously recognized functions.
In conclusion, the amniotic fluid concentration of CCL20 increased as a function of gestational age and spontaneous labor (term and preterm), as well as intra-amniotic infection/inflammation, is associated with a significant increase in the amniotic fluid concentration of this chemokine. We propose that CCL20 is involved in the mechanisms of parturition as well as in the host response to microbial invasion and other pathologic processes.
Acknowledgments
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191:2090–6. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Aldebert D, Diallo M, Niang M, Sarr D, Cisse C, Moreau JC, et al. Differences in circulating dendritic cell subtypes in peripheral, placental and cord blood in African pregnant women 1. J Reprod Immunol. 2007;73:11–9. doi: 10.1016/j.jri.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum PC, Shulman G, Chambers NL, Simon NV, Granados JL, Fairbrother PF, et al. Studies on the growth-inhibiting property of amniotic fluids from two United States population groups. Am J Obstet Gynecol. 1980;137:579–82. doi: 10.1016/0002-9378(80)90699-7. [DOI] [PubMed] [Google Scholar]
- 4.Askelund K, Liddell HS, Zanderigo AM, Fernando NS, Khong TY, Stone PR, et al. CD83(+)dendritic cells in the decidua of women with recurrent miscarriage and normal pregnancy 1. Placenta. 2004;25:140–5. doi: 10.1016/S0143-4004(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 5.Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–44. [PubMed] [Google Scholar]
- 6.Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–8. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 7.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas 1. J Exp Med. 1999;190:1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy 1. Hum Reprod. 1997;12:586–90. doi: 10.1093/humrep/12.3.586. [DOI] [PubMed] [Google Scholar]
- 9.Brandes M, Legler DF, Spoerri B, Schaerli P, Moser B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int Immunol. 2000;12:1285–92. doi: 10.1093/intimm/12.9.1285. [DOI] [PubMed] [Google Scholar]
- 10.Carramolino L, Kremer L, Goya I, Varona R, Buesa JM, Gutierrez J, et al. Down-regulation of the beta-chemokine receptor CCR6 in dendritic cells mediated by TNF-alpha and IL-4. J Leukoc Biol. 1999;66:837–44. doi: 10.1002/jlb.66.5.837. [DOI] [PubMed] [Google Scholar]
- 11.Casamayor-Palleja M, Mondiere P, Verschelde C, Bella C, Defrance T. BCR ligation reprograms B cells for migration to the T zone and B-cell follicle sequentially. Blood. 2002;99:1913–21. doi: 10.1182/blood.v99.6.1913. [DOI] [PubMed] [Google Scholar]
- 12.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor 1. J Clin Invest. 1989;83:430–6. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol. 2002;187:889–93. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 14.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–82. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 16.Chaiworapongsa T, Romero R, Tolosa JE, Yoshimatsu J, Espinoza J, Kim YM, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med. 2002;12:159–64. doi: 10.1080/jmf.12.3.159.164. [DOI] [PubMed] [Google Scholar]
- 17.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J Exp Med. 1999;190:1755–68. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35:23–9. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 19.Cremel M, Berlier W, Hamzeh H, Cognasse F, Lawrence P, Genin C, et al. Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction 1. J Leukoc Biol. 2005;78:158–66. doi: 10.1189/jlb.0305147. [DOI] [PubMed] [Google Scholar]
- 20.Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, et al. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2007;58:39–45. doi: 10.1111/j.1600-0897.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 21.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, it-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–18. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois B, Massacrier C, Caux C. Selective attraction of naive and memory B cells by dendritic cells. J Leukoc Biol. 2001;70:633–41. [PubMed] [Google Scholar]
- 24.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87:94–8. doi: 10.1016/0029-7844(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 25.Dudley DJ, Spencer S, Edwin S, Mitchell MD. Regulation of human decidual cell macrophage inflammatory protein-1 alpha (MIP-1 alpha) production by inflammatory cytokines. Am J Reprod Immunol. 1995;34:231–5. doi: 10.1111/j.1600-0897.1995.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 26.Ebert LM, McColl SR. Up-regulation of CCR5 and CCR6 on distinct subpopulations of antigen-activated CD4+ T lymphocytes. J Immunol. 2002;168:65–72. doi: 10.4049/jimmunol.168.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–84. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 28.Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–7. doi: 10.1034/j.1600-0897.2001.d01-11.x. [DOI] [PubMed] [Google Scholar]
- 29.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 30.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–71. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 32.Fortunato SJ, Menon R, Swan KF. Expression of TNF-alpha and TNFR p55 in cultured amniochorion. Am J Reprod Immunol. 1994;32:188–93. doi: 10.1111/j.1600-0897.1994.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 33.Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol. 1996;174:1855–61. doi: 10.1016/s0002-9378(96)70221-1. [DOI] [PubMed] [Google Scholar]
- 34.Fujiie S, Hieshima K, Izawa D, Nakayama T, Fujisawa R, Ohyanagi H, et al. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB [correction of NK-kappaB] Int Immunol. 2001;13:1255–63. doi: 10.1093/intimm/13.10.1255. [DOI] [PubMed] [Google Scholar]
- 35.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970;106:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 36.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–46. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 37.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 38.Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer. 2002;97:654–9. doi: 10.1002/ijc.10084. [DOI] [PubMed] [Google Scholar]
- 39.Gobert AP, Vareille M, Glasser AL, Hindre T, de ST, Martin C. Shiga toxin produced by enterohemorrhagic Escherichia coli inhibits PI3K/NF-kappaB signaling pathway in globotriaosylceramide-3-negative human intestinal epithelial cells. J Immunol. 2007;178:8168–74. doi: 10.4049/jimmunol.178.12.8168. [DOI] [PubMed] [Google Scholar]
- 40.Godefroy S, Guironnet G, Jacquet C, Schmitt D, Staquet MJ. A combination of MIP-3alpha and TGF-beta1 is required for the attraction of human Langerhans precursor cells through a dermal-epidermal barrier. Eur J Cell Biol. 2001;80:335–40. doi: 10.1078/0171-9335-00169. [DOI] [PubMed] [Google Scholar]
- 41.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 42.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–93. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 43.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haller H, Ziegler EM, Homuth V, Drab M, Eichhorn J, Nagy Z, et al. Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension. 1997;29:291–6. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- 45.Harant H, Eldershaw SA, Lindley IJ. Human macrophage inflammatory protein-3alpha/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-alpha via a non-standard NF-kappaB site. FEBS Lett. 2001;509:439–45. doi: 10.1016/s0014-5793(01)03138-6. [DOI] [PubMed] [Google Scholar]
- 46.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–G1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 47.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–92. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 48.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–44. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 49.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27:513–8. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 50.Hieshima K, Imai T, Opdenakker G, Van DJ, Kusuda J, Tei H, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–53. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 51.Holst RM, Laurini R, Jacobsson B, Samuelsson E, Savman K, Doverhag C, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: Relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20:885–93. doi: 10.1080/14767050701752601. [DOI] [PubMed] [Google Scholar]
- 52.Homey B, eu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–32. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 53.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin. Clin Exp Immunol. 2005;142:285–91. doi: 10.1111/j.1365-2249.2005.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hromas R, Gray PW, Chantry D, Godiska R, Krathwohl M, Fife K, et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood. 1997;89:3315–22. [PubMed] [Google Scholar]
- 55.Huang S, Chen CP, Schatz F, Rahman M, Abrahams V, Lockwood C. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–36. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 56.Huleihel M, Amash A, Sapir O, Maor E, Levy S, Katz M, et al. Lipopolysaccharide induces the expression of interleukin-1alpha distinctly in different compartments of term and preterm human placentae. Eur Cytokine Netw. 2004;15:30–6. [PubMed] [Google Scholar]
- 57.Hussain LA, Kelly CG, Fellowes R, Hecht EM, Wilson J, Chapman M, et al. Expression and gene transcript of Fc receptors for IgG, HLA class II antigens and Langerhans cells in human cervico-vaginal epithelium. Clin Exp Immunol. 1992;90:530–8. doi: 10.1111/j.1365-2249.1992.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J Biol Chem. 1996;271:21514–21. doi: 10.1074/jbc.271.35.21514. [DOI] [PubMed] [Google Scholar]
- 59.Imaizumi Y, Sugita S, Yamamoto K, Imanishi D, Kohno T, Tomonaga M, et al. Human T cell leukemia virus type-I Tax activates human macrophage inflammatory protein-3 alpha/CCL20 gene transcription via the NF-kappa B pathway. Int Immunol. 2002;14:147–55. doi: 10.1093/intimm/14.2.147. [DOI] [PubMed] [Google Scholar]
- 60.Inoue Y, Tsushima H, Ando K, Sawayama Y, Sakai M, Yamasaki R, et al. Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3alpha/CCL20 is induced by inflammatory cytokines. Exp Hematol. 2006;34:19–26. doi: 10.1016/j.exphem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Ivanova E, Kyurkchiev D, Altankova I, Dimitrov J, Binakova E, Kyurkchiev S. CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol. 2005;53:199–205. doi: 10.1111/j.1600-0897.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 62.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–G719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsson B, Holst RM, Andersson B, Hagberg H. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand. 2005;84:566–71. doi: 10.1111/j.0001-6349.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 65.Jacobsson B, Holst RM, Mattsby-Baltzer I, Nikolaitchouk N, Wennerholm UB, Hagberg H. Interleukin-18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation and preterm delivery. BJOG. 2003;110:598–603. [PubMed] [Google Scholar]
- 66.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161–7. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 67.Kammerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kampgen E, et al. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–69. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 69.Kastenbauer S, Angele B, Sporer B, Pfister HW, Koedel U. Patterns of protein expression in infectious meningitis: a cerebrospinal fluid protein array analysis. J Neuroimmunol. 2005;164:134–9. doi: 10.1016/j.jneuroim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol Reprod. 1997;57:1438–44. doi: 10.1095/biolreprod57.6.1438. [DOI] [PubMed] [Google Scholar]
- 71.Keelan JA, Wang K, Chaiworapongsa T, Romero R, Mitchell MD, Sato TA, et al. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod. 2003;9:535–40. doi: 10.1093/molehr/gag068. [DOI] [PubMed] [Google Scholar]
- 72.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–9. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 73.Krzysiek R, Lefevre EA, Bernard J, Foussat A, Galanaud P, Louache F, et al. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells. Blood. 2000;96:2338–45. [PubMed] [Google Scholar]
- 74.Kwon JH, Keates S, Simeonidis S, Grall F, Libermann TA, Keates AC. ESE-1, an enterocyte-specific Ets transcription factor, regulates MIP-3alpha gene expression in Caco-2 human colonic epithelial cells. J Biol Chem. 2003;278:875–84. doi: 10.1074/jbc.M208241200. [DOI] [PubMed] [Google Scholar]
- 75.Laham N, Brennecke SP, Bendtzen K, Rice GE. Labour-associated increase in interleukin-1 alpha release in vitro by human gestational tissues. J Endocrinol. 1996;150:515–22. doi: 10.1677/joe.0.1500515. [DOI] [PubMed] [Google Scholar]
- 76.Lappas M, Yee K, Permezel M, Rice GE. Lipopolysaccharide and TNF-alpha activate the nuclear factor kappa B pathway in the human placental JEG-3 cells. Placenta. 2006;27:568–75. doi: 10.1016/j.placenta.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Laudanski P, Lemancewicz A, Pierzynski P, Akerlund M, Laudanski T. Decreased serum level of macrophage inflammatory chemokine-3beta/CCL19 in preterm labor and delivery. Eur J Obstet Gynecol Reprod Biol. 2006;124:23–6. doi: 10.1016/j.ejogrb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha 9. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 79.Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J Immunol. 2002;168:4871–80. doi: 10.4049/jimmunol.168.10.4871. [DOI] [PubMed] [Google Scholar]
- 80.Marinoni E, Di IR, Letizia C, Villaccio B, Alberini A, Cosmi EV. Amniotic fluid concentrations of adrenomedullin in preterm labor. Obstet Gynecol. 1999;93:964–7. doi: 10.1016/s0029-7844(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 81.Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, et al. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125:155–61. doi: 10.1046/j.1365-2249.2001.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthiesen L, Berg G, Ernerudh J, Hakansson L. Lymphocyte subsets and mitogen stimulation of blood lymphocytes in normal pregnancy. Am J Reprod Immunol. 1996;35:70–9. doi: 10.1111/j.1600-0897.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 83.Menon R, Ismail L, Ismail D, Merialdi M, Lombardi SJ, Fortunato SJ. Human fetal membrane expression of IL-19 and IL-20 and its differential effect on inflammatory cytokine production. J Matern Fetal Neonatal Med. 2006;19:209–14. doi: 10.1080/14767050500440986. [DOI] [PubMed] [Google Scholar]
- 84.Menon R, Lombardi SJ, Fortunato SJ. IL-18, a product of choriodecidual cells, increases during premature rupture of membranes but fails to turn on the Fas-FasL-mediated apoptosis pathway. J Assist Reprod Genet. 2001;18:276–84. doi: 10.1023/A:1016626620137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006;56:112–8. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 86.Menon R, Swan KF, Lyden TW, Rote NS, Fortunato SJ. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol. 1995;172:493–500. doi: 10.1016/0002-9378(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 87.Michimata T, Ogasawara MS, Tsuda H, Suzumori K, Aoki K, Sakai M, et al. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol. 2002;47:196–202. doi: 10.1034/j.1600-0897.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 88.Mordmuller B, Krappmann D, Esen M, Wegener E, Scheidereit C. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep. 2003;4:82–7. doi: 10.1038/sj.embor.embor710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moynagh PN, Williams DC, O’Neill LA. Interleukin-1 activates transcription factor NF kappa B in glial cells. Biochem J. 1993;294(Pt 2):343–7. doi: 10.1042/bj2940343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murtha AP, Greig PC, Jimmerson CE, Roitman-Johnson B, Allen J, Herbert WN. Maternal serum interleukin-6 concentrations in patients with preterm premature rupture of membranes and evidence of infection. Am J Obstet Gynecol. 1996;175:966–9. doi: 10.1016/s0002-9378(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 91.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 92.Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, Hieshima K, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 93.Nelson RT, Boyd J, Gladue RP, Paradis T, Thomas R, Cunningham AC, et al. Genomic organization of the CC chemokine mip-3alpha/CCL20/larc/exodus/SCYA20, showing gene structure, splice variants, and chromosome localization. Genomics. 2001;73:28–37. doi: 10.1006/geno.2001.6482. [DOI] [PubMed] [Google Scholar]
- 94.Okudaira T, Yamamoto K, Kawakami H, Uchihara JN, Tomita M, Masuda M, et al. Transactivation of CCL20 gene by Epstein-Barr virus latent membrane protein 1. Br J Haematol. 2006;132:293–302. doi: 10.1111/j.1365-2141.2005.05877.x. [DOI] [PubMed] [Google Scholar]
- 95.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–43. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 96.Porre S, Heinonen S, Mantyjarvi R, Rytkonen-Nissinen M, Perola O, Rautiainen J, et al. Psoriasin, a calcium-binding protein with chemotactic properties is present in the third trimester amniotic fluid. Mol Hum Reprod. 2005;11:87–92. doi: 10.1093/molehr/gah141. [DOI] [PubMed] [Google Scholar]
- 97.Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med. 1997;186:825–35. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Real JM, Munro P, Buisson-Touati C, Lemichez E, Boquet P, Landraud L. Specificity of immunomodulator secretion in urinary samples in response to infection by alpha-hemolysin and CNF1 bearing uropathogenic Escherichia coli. Cytokine. 2007;37:22–5. doi: 10.1016/j.cyto.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 99.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 102.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero R, Gomez R, Araneda H. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993;168:A57. [Google Scholar]
- 104.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–13. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 106.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 107.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–8. [PubMed] [Google Scholar]
- 108.Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158:1033–6. [PubMed] [Google Scholar]
- 109.Rumbo M, Sierro F, Debard N, Kraehenbuhl JP, Finke D. Lymphotoxin beta receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004;127:213–23. doi: 10.1053/j.gastro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 110.Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, Qin S, et al. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–88. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 111.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 112.Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ito A, et al. A study of CD45RO, CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol Lett. 1994;40:193–7. doi: 10.1016/0165-2478(93)00019-a. [DOI] [PubMed] [Google Scholar]
- 113.Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, et al. Chemokine receptor expressions and responsiveness of cord blood T cells. J Immunol. 2001;166:1659–66. doi: 10.4049/jimmunol.166.3.1659. [DOI] [PubMed] [Google Scholar]
- 114.Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, et al. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981–8. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 115.Scheerens H, Hessel E, de Waal-Malefyt R, Leach MW, Rennick D. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10-/- mice and Rag-2-/- mice reconstituted with CD4+CD45RBhigh T cells. Eur J Immunol. 2001;31:1465–74. doi: 10.1002/1521-4141(200105)31:5<1465::AID-IMMU1465>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 116.Schmuth M, Neyer S, Rainer C, Grassegger A, Fritsch P, Romani N, et al. Expression of the C-C chemokine MIP-3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Exp Dermatol. 2002;11:135–42. doi: 10.1034/j.1600-0625.2002.110205.x. [DOI] [PubMed] [Google Scholar]
- 117.Schutyser E, Struyf S, Menten P, Lenaerts JP, Conings R, Put W, et al. Regulated production and molecular diversity of human liver and activation-regulated chemokine/macrophage inflammatory protein-3 alpha from normal and transformed cells. J Immunol. 2000;165:4470–7. doi: 10.4049/jimmunol.165.8.4470. [DOI] [PubMed] [Google Scholar]
- 118.Schutyser E, Struyf S, Van DJ. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 119.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–73. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 120.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–97. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 121.Slukvin II, Merkulova AA, Vodyanik MA, Chernyshov VP. Differential expression of CD45RA and CD45RO molecules on human decidual and peripheral blood lymphocytes at early stage of pregnancy. Am J Reprod Immunol. 1996;35:16–22. [PubMed] [Google Scholar]
- 122.Sorg RV, Kogler G, Wernet P. Identification of cord blood dendritic cells as an immature CD11c- population. Blood. 1999;93:2302–7. [PubMed] [Google Scholar]
- 123.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–33. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 125.Stjernholm Y, Sennstrom M, Granstrom L, Ekman G, Liang Y, Johansson O. Neurochemical and cellular markers in human cervix of late pregnant, postpartal and non-pregnant women. Acta Obstet Gynecol Scand. 2000;79:528–37. [PubMed] [Google Scholar]
- 126.Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, et al. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J Immunol. 2002;168:5621–8. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
- 127.Tafari N, Ross SM, Naeye RL, Galask RP, Zaar B. Failure of bacterial growth inhibition by amniotic fluid. Am J Obstet Gynecol. 1977;128:187–9. doi: 10.1016/0002-9378(77)90685-8. [DOI] [PubMed] [Google Scholar]
- 128.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–8. [Google Scholar]
- 129.Thadepalli H, Appleman MD, Maidman JE, Arce JJ, Davidson EC., Jr Antimicrobial effect of amniotic fluid against anaerobic bacteria. Am J Obstet Gynecol. 1977;127:250–4. doi: 10.1016/0002-9378(77)90463-x. [DOI] [PubMed] [Google Scholar]
- 130.Thadepalli H, Bach VT, Davidson EC., Jr Antimicrobial effect of amniotic fluid. Obstet Gynecol. 1978;52:198–204. [PubMed] [Google Scholar]
- 131.Trautman MS, Dudley DJ, Edwin SS, Collmer D, Mitchell MD. Amnion cell biosynthesis of interleukin-8: regulation by inflammatory cytokines. J Cell Physiol. 1992;153:38–43. doi: 10.1002/jcp.1041530107. [DOI] [PubMed] [Google Scholar]
- 132.Ueda Y, Hagihara M, Okamoto A, Higuchi A, Tanabe A, Hirabayashi K, et al. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol. 2003;64:1144–51. doi: 10.1016/j.humimm.2003.08.342. [DOI] [PubMed] [Google Scholar]
- 133.Vanbervliet B, Homey B, Durand I, Massacrier C, it-Yahia S, de BO, et al. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur J Immunol. 2002;32:231–42. doi: 10.1002/1521-4141(200201)32:1<231::AID-IMMU231>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 134.Wang JH, Manning BJ, Wu QD, Blankson S, Bouchier-Hayes D, Redmond HP. Endotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanism. J Immunol. 2003;170:795–804. doi: 10.4049/jimmunol.170.2.795. [DOI] [PubMed] [Google Scholar]
- 135.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 136.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 137.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53:211–6. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 138.Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71:1296–302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]


