Abstract
Elevated levels of homocysteine (Hcy) known as hyperhomocysteinemia (HHcy) are associated with arrhythmogenesis and sudden cardiac death (SCD). Hcy decreases constitutive neuronal and endothelial nitric oxide (NO), and cardiac diastolic relaxation. Hcy increases the iNOS/NO, peroxynitrite, mitochondrial NADPH oxidase, and suppresses superoxide dismutase (SOD) and redoxins. Hcy activates matrix metalloproteinase (MMP), disrupts connexin-43 and increases collagen/elastin ratio. The disruption of connexin-43 and accumulation of collagen (fibrosis) disrupt the normal pattern of cardiac conduction and attenuate NO transport from endothelium to myocyte (E-M) causing E-M uncoupling, leading to a pro-arrhythmic environment. The goal of this review is to elaborate the mechanism of Hcy-mediated iNOS/NO in E-M uncoupling and SCD. It is known that Hcy creates arrhythmogenic substrates (i.e. increase in collagen/elastin ratio and disruption in connexin-43) and exacerbates heart failure during chronic volume overload. Also, Hcy behaves as an agonist to N-methyl-D-aspartate (NMDA, an excitatory neurotransmitter) receptor-1, and blockade of NMDA-R1 reduces the increase in heart rate-evoked by NMDA-analog and reduces SCD. This review suggest that Hcy increases iNOS/NO, superoxide, metalloproteinase activity, and disrupts connexin-43, exacerbates endothelial-myocyte uncoupling and cardiac failure secondary to inducing NMDA-R1.
Keywords: Heart failure, ECM, calcium channel, tachycardia, bradycardia, arrhythmia, LVH, peri-capillary fibrosis, MMP, TIMP, integrin, connexin, contraction, relaxation, neuronal endothelial myocyte coupling, NOS, sudden cardiac death, NMDA
Introduction
SCD is a major cause of mortality (Hiromasa et al., 1988; Palakurthy et al., 1989). Approximately 65% of SCD cases occur in patients with underlying acute or chronic heart disease. The incidence of SCD increases 2- to 4-fold in the presence of coronary disease and 6- to 10-fold in the presence of structural heart disease. Ventricular tachycardia (VT) leading to ventricular fibrillation (VF) is a primary cause of cardiac arrest and SCD. One of the challenges in preventing SCD lies in identifying individuals at highest risk for SCD within a lower-risk population (Podrid et al., 2005). The identification of conventional risk factors for coronary artery disease and structural heart disease during progression to arrhythmogenesis and SCD can be very daunting. Although both ischemia as well as reperfusion induced arrhythmia, only reperfusion-induced arrhythmias were sensitive to NMDA-R1 blockade (D’Amico et al., 1999). This may suggest that arrhythmias in high cardiac output are influenced by circulating factors and are mitigated by NMDA-R1 blockade. However, increased serum Hcy has been identified as a risk factor for SCD resulting from coronary fibrous plaques (Bollani et al., 1999; Albert et al., 2002; Burke et al., 2002). Cardiac interstitial fibrosis is the result of HHcy and the combination of HHcy and hypertension (Miller et al., 2002). Both cardiac arrhythmias and neurological disorders contribute to SCD, and the blockade of NMDA-R1 mitigates SCD (Folbergrova, 1994; Huang & Su, 1999; Matsuoka et al., 2002; Simandle et al., 2005). Since the induction iNOS increases SCD (Mungrue et al., 2002), and Hcy increases iNOS, therefore, the use of iNOSKO mice to determine the contribution of inducible NO in Hcy-mediated E-M uncoupling (disruption of connexin-43 and fibrosis), and generation of arrhythmogenic substrate (Gutstein et al., 2001; Kitamua et al., 2003; Poelzing & Rosenbaum, 2004) is very important for the understanding of the mechanism of iNOS-NO-mediated cardiac dysfunction. Hcy increases mitochondrial oxidative stress and activates MMPs. The MMPs are activated in VT, VF, and SCD (Hoit et al., 2002; Xu et al., 2004; Mukherjee et al., 2006). The metalloproteinases degrade connexin-43 (Hunt et al., 2002), therefore, it is important to measure metalloproteinases and connexin alteration in Hcy-mediated E-M uncoupling in heart failure.
Homocysteine (Hcy) metabolomics
Elevated levels of Hcy known as hyperhomocysteinemia (HHcy) are a significant risk factor for cardiac arrhythmia and sudden cardiac death (SCD, Bollani et al., 1999; Burke et al., 2002). There are five ways by which homocysteine (Hcy) is accumulated in the plasma and tissues: 1) by a methionine rich protein diet; 2) by hyper de-methylation of methionine by methyl transferase (MT) during DNA/RNA methylation; 3) by hypo re-methylation of Hcy to methionine by MTHFR/vitamin b12/folate deficiency; 4) by heterozygous/homozygous mutation in cystathione β synthase (CBS) activity, b6, and transsulphuration deficiency; and 5) by renal disease and volume retention (Figure 1). Mammalian vascular cells are lacking the CBS (Finkelstein, 1990; 1998). Decrease in methionine-rich diet and treatment with vitamin b12/folate reduce the levels of plasma Hcy and ameliorate vascular dysfunction, in part, by re-methylation of Hcy to methionine, however, the mechanisms of other genetic causes of HHcy are unknown. There are three ranges of hyperhomocysteinemia: moderate (16 to 30 μM), intermediate (31–100 μM), and severe (>100 μM) (Cheng & Kaplowitz, 2004). Extracellular thiols are oxidized, and only a fraction of total plasma homocysteine (Hcy) is in the reduced form in vivo and in vitro. Hcy at doses of 0.1–1.0 mM markedly inhibits endothelial cell growth over time in vitro; in contrast, vascular smooth muscle cells respond to similar concentrations of Hcy with an increase in cyclin D1 and cyclin A mRNA expression and a resulting marked increase in cell proliferation (Tsai et al., 1994).
Figure 1.
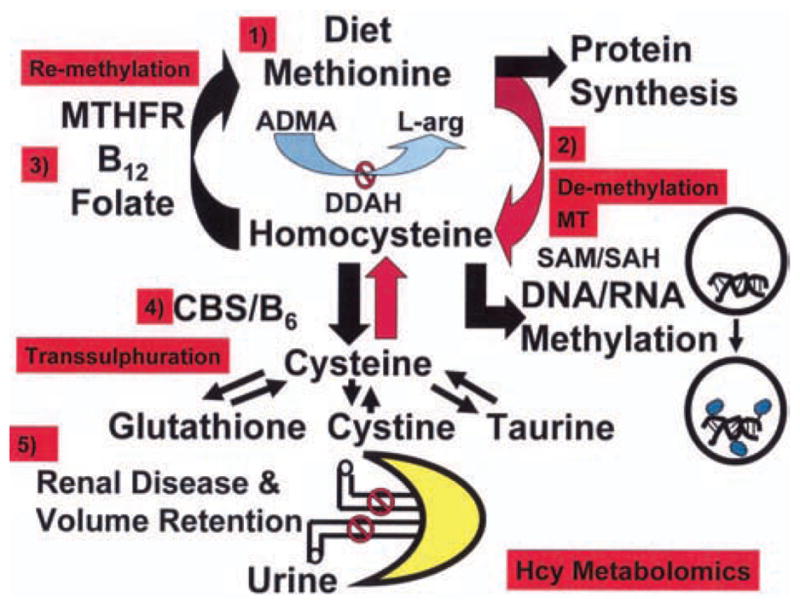
Methionine rich protein diet increases Hcy levels. The hyper de-methylation of methionine by methyl transferase (MT) and SAHH activity during DNA/RNA methylation cause HHcy. The hypo re-methylation of Hcy to methionine by MTHFR/vitamin b12/folate dependent pathways cause increase in Hcy levels. The heterozygous/homozygous in cystathione β synthase (CBS) activity, b6, and transsulphuration deficiency exacerbate HHcy. The renal disease and volume retention increase plasma Hcy levels.
Importance of endothelium in the heart
Although the volume of capillaries may account to 16%, the endothelial cell volume is probably only 2–3%, whereas red blood volume is 6% and plasma volume 7%. The importance of a cell species cannot be judged simply based on cell volume. Nonetheless, sixteen percent of the myocardial mass is capillaries, including the lumen and endothelium (Hoppeler & Kayar, 1988). The capillary endothelium is embedded in the muscle, and plays a very important role in myocardial diastolic relaxation (Roberts & Waern, 1941; Henderson et al., 1992; Smith et al., 1992; Mebazaa et al., 1995). Nitric oxide (NO) generation from the endocardial endothelium contributes to myocyte contraction, relaxation, and heart rate (Brady et al., 1994; Pinsky et al., 1997). A gradient of NO concentration (i.e. high in endocardium and low in midmyocardium) has been depicted (30) that is consistent with the notion that there is more capillary endothelium in the endocardium than in epi- or mid-myocardium (Fukuchi et al., 2001; Scarabelli et al., 2001). The importance of endocardial endothelium in cardiac contraction/relaxation is illustrated in an experiment in which the responses to CaCl2 and acetylcholine were attenuated in the endothelium-denuded myocardium (Wang & Morgan, 1992; Gattuso et al., 1999; Tyagi et al., 1999).
Endothelium-myocyte (E-M) coupling implies the E-M cell-cell connections, the thickness of the basement membrane between the E and M, and the efficiency of transport of endothelial-derived cardio-active agents to the cardiac muscle. Primarily there are three connexins in the heart, connexion-40 is in endothelium, connexion-43 and -45 are present in myocytes (Bastide et al., 1993). The disruption of connexin-43 impairs cardiac electrical impulse. The accumulation of interstitial collagen between E and M increases distance from E to M, and interferes with cardiac diastolic relaxation. In addition, the increase in distance from E to M impairs endothelial-derived NO diffusion mechanism to the cardiac muscle (Moshal et al., 2005).
Elevation of Hcy levels has been shown to increase [Ca2+]i
The treatment of spinal motorneurons with homocysteine elevated calcium, which resulted in cell death, this may contribute to SCD. Interestingly, increased levels of Hcy create myocardial conduction abnormalities and are associated with SCD (James et al., 1974; Bollani et al., 1999; Burke et al., 2002). Hcy behaves as an agonist to NMDA-R1, and NMDA induces Ca2+ and K+ currents (Robinson et al., 2005; Yang et al., 2005). Treatment of spinal motor neurons with Hcy elevated [Ca2+]i which culminated in cell death (Adalbert et al., 2002). Culturing embryonic cortical neurons and differentiated human neuroblastoma cells in folate-free medium increased Hcy, [Ca2+]i and reactive oxygen species (Ho et al., 2003). Addition of 3-deazaadenosine (DZA), an inhibitor of SAHH and Hcy formation, abrogated the formation of Hcy and the increase in ROS (Ho et al., 2003). Due to S-(1,2-dichlorovinyl)-L-Hcy, an analog of Hcy, Hcy has much more potent agonist at specific receptors, but a poor metabolic analogue, and therefore elevated [Ca2+]i nearly five fold (Vamvakas et al., 1990). Results from our laboratory showed that Hcy-mediated cardiac contractile dysfunction and increase in [Ca2+]i were amplified by subphysiological levels of angiotensin II and endothelin-1 which did not normally elicit cardiac responses (Tyagi et al., 1999; Mujumdar et al., 2000). This suggested synergism between Hcy, angiotensin II and endothelin-1, causing cardiac dysfunction.
The MMP family includes gelatinases, collagenases, and membrane type (MT-MMP) (Rosenberg, 2002). The metalloproteinase family also includes a disintegrin metalloproteinase (ADAM) (Loechel et al., 1998). These metalloproteinases are neutral proteases that act on the MVEC BM resulting in its degradation (Tyagi et al., 1996). It has been shown that association of MMP-2 with integrin results in its disengagement from ECM and promotes cell death (Frisch et al., 1994). MMPs are regulated by their interaction with tissue inhibitors of metalloproteinases (TIMPs) (Visse & Nagase, 2003). TIMPs inactivate MMPs by binding to their catalytic site. There are four TIMPs. In general, TIMP-4 inhibits MMP-2, and -7 and to a lesser extent, MMP-1, -3 and -9. TIMP-3 inhibits MMP-1, -3, -7 and -13. TIMP-1 and -2 inhibit a broad range of MMPs (Stamenkovic, 2003). Composition of extracellular matrix (ECM) in the BM is important for conductance of signals from endothelial to myocyte side. It is known that focal adhesion complex integrates incoming signals and orchestrates an intricate interrelationship between ECM, cytoskeleton and signaling cascades (Levkau et al., 2002; Spragg & Kass, 2005). Connexin-43 −/− promotes cardiac arrhythmia and SCD, in part, by inducing endothelial-myocyte uncoupling (Gutstein et al., 2001; Kitamua et al., 2003; Poelzing & Rosenbaum, 2004). In end-stage human heart failure connexin-43 is disrupted and metalloproteinases are activated (Hunt et al., 2002). We showed that most of the MMPs in the heart are latent (Tyagi et al., 1993) due to active-site Zn2+ coordination with constitutive NO in a ternary complex (MMP/NO/TIMP) in the basement-membrane-matrix of endothelium. Increased oxidative stress leads to generation of nitro-tyrosine residues in TIMP and liberates active MMP (Figure 2, Tyagi et al., 2005). This, in turn, degrades the connective matrix. Since collagen turnover is faster than other ECM components, degraded matrix is replaced by oxidized collagen (fibrosis). Two detrimental consequences of this process are: 1) degradation of ultrastructural matrix which causes disconnection (i.e. degradation of connexin-43) of the endothelium from myocytes; and 2) accumulation of oxidized collagen, which impairs the delivery of metabolites to underlying muscle, causing uncoupling. Generalized MMP activation is implicated in development of VF, SCD and CHF (Hoit et al., 2002; Xu et al., 2004; Mukherjee et al., 2006). Furthermore, increased oxidative-modification of collagen by cross-linking is associated with diastolic dysfunction in CHF (Mizushige et al., 2000; Joseph et al., 2002, 2003; Kass et al., 2004). NMDA-R1 antagonist ameliorates MMP activation (Meighan et al., 2006). TIMP-1 is induced in fibrotic myocardium (Lindsay et al., 2002) and Hcy at micro-molar range induces TIMP-1 (Torres et al., 1999). TIMP-4 is highly expressed in the heart and is decreased during cardiac failure (Tammalapalli et al., 2001). Unlike humans, rodents do not have a typical interstitial collagenase (MMP-1). Instead, they have MMP-13 equivalent to MMP-1 (Vincenti et al., 1998). MMP-2 degrades interstitial collagen as well as elastin (Senior et al., 1991; Aimes & Quigley, 1995), and under pathophysiological conditions MMP-9 at 92 kDa (gelatinase b) is induced. In addition, MMP-2 (72 kDa, gelatinase a) is present in all species. ADAM-12 is increased and connexin-43 is degraded in CHF (Hunt et al., 2002).
Figure 2.
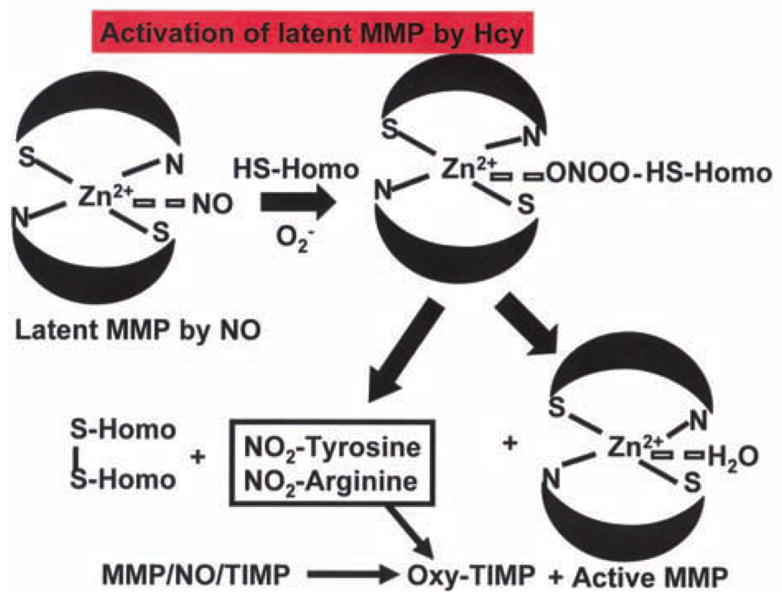
Oxidative stress and increase ROS in HHcy decrease constitutive NO in ternary MMP/NO/TIMP complex and generate RNS and nitrotyrosine. This process oxidizes the TIMP and liberates active MMP.
ECM remodeling and endothelial-myocyte uncoupling
ECM remodeling interferes with integrin-mediated cell survival signaling. This leads to formation of filamentous actin (F-actin), gaps between the cells, and disrupts endothelial-myocyte tight junctions (Tyagi, 1997; Tyagi & Hoit, 2002; Shastry & Tyagi, 2004; Lominadze et al., 2006), resulting in E-M uncoupling. In the BM of capillary endothelium, the MMPs reside in the latent ternary (MMP/NO/TIMP) complex. During HHcy TIMPs are oxidized and MMPs are activated (Figure 2, Tyagi & Hayden, 2003). MMPs are collagenases as well as elastases, therefore, because elastin turnover is relatively slower than collagen, it is replaced by oxidized collagen (Rucklidge et al., 1992; Mujumdar & Tyagi, 1999). This alters collagen/elastin ratio, disrupts connexin-43, and leads to accumulation of interstitial collagen (fibrosis) between the endothelium and myocyte. Fibrosis causes endothelial-myocyte disconnection (uncoupling) and attenuates NO diffusibility from endothelium to myocyte. The disruption of connex-in-43 and lack of constitutive NO create an environment for trigger activity.
Oxidative stress plays significant role in VT, arrhythmia, and SCD during CHF (Fukuda et al., 2005; Wolin & Gupte, 2005). Previous studies from our laboratory showed that Hcy increases oxidative stress by generating ROS and nitrotyrosine (Mujumdar et al., 2001; Tyagi et al., 2005). In tissues NADPH oxidase is a primary source of ROS generation. Most of the peroxidase activity depends on the levels of thioredoxin, the tissue level of thioredoxin is decreased as a result of an increased oxidative stress (Yamamoto et al., 2000; Shao et al., 2002; Goth & Vitai, 2003; Hui et al., 2004). In addition, the level of thioredoxin is a strong predictor of oxidative stress (Farina et al., 2001; Wong et al., 2002; Barr & Gedamu, 2003; Oh et al., 2004). Hcy increases mitochondrial oxidative stress by decreasing redoxins, SOD, and increasing NADPH oxidase (Nonaka et al., 2001; Tyagi et al., 2005). In normal myocardium, the MMPs active site is bound to NO (Tyagi & Hayden, 2003). During chronic increases in load (82 Cox et al., 2002), and oxidative stress (Hunt et al., 2002), MMPs are activated, causing a decrease in endothelial NO bioavailability (Mujundar et al., 2001). To reduce the load by dilating the heart in the absence of endothelial NO, the latent resident MMPs are activated (Tyagi & Hayden, 2003). However, persistent activation of MMP (i.e. increase in MMP/TIMP ratio) leads to degradation of connexin-43 (Hunt et al., 2002). Interestingly, matrix degradation and proteolytic shedding (activation) of NMDA receptor have been implicated by tPA, an active serine proteinase (Frey et al., 1996). Although ligand-gated glutamate binds to NMDA-R1, activates K+/Na+ channels, and increases [Ca2+]i-mediated contraction (James et al., 1974; Duchen, 2004; Robinson et al., 2005; Yang et al., 2005, 35–37, 85), the role of disruption of matrix-connection in creating pro-arrhythmic environment is unclear. Hcy activates NMDA-R1 (Chen et al., 2005; Qureshi et al., 2005) and decreases endothelial NO (Gu et al., 2002), increases iNOS-NO, ROS contents, and generates peroxynitrite (Mujumdar et al., 2001). The increase in superoxide, peroxynitrite and nitration of proteins (Mihm et al., 2001) leads to cardiac arrhythmia and failure. The blocker of NMDA-R1 inhibits MMP activation (Meighan et al., 2006) and mitigates SCD (Matsuoka et al., 2002).
Hcy, NMDA-R1, iNOS/NO, arrhythmia and SCD
Constitutively, NO released from sympathetic and parasympathetic nerves moderate neuronal-myocyte coupling and cardiac rhythm (Nihei et al., 2005), and NO released from endothelium regulates E-M coupling and cardiac diastolic relaxation (Brady et al., 1994; Pinsky et al., 1997). Interestingly, Hcy decreases both neuronal (Kim, 1999) and endothelial NO (Chen et al., 2002) but increases inducible NO through iNOS (Welch et al., 1998). The increase in iNOS induced sudden cardiomyocyte death and may lead to SCD (Mungrue et al., 2002). NMDA is a major excitatory neurotransmitter, and NMDA-R1 is present in the mammalian central nervous system (CNS, Lalo et al., 2006), in cardiomyocytes (Kraine et al., 1998; Huang & Su, 1999) and the endothelial cells (Chen et al., 2005; Qureshi et al., 2005). The relative expression of the NMDA-receptor in heart, specifically expression in cardiomyocytes versus endothelial cells and neuronal tissue (sympathetic and parasympathetic nerve endings, for example) is in the order of neuronal >cardiomyocyte >endothelial cells. The NMDA-R1 increases neuronal membrane “excitability” resulting in the excitotoxic actions of NMDA (Fridman, 1999; Jara-Prado et al., 2003). In addition, NMDA-R1 activation increases mitochondrial oxidative stress and [Ca2+]i (Duchen, 2004). The antagonist to the NMDA receptor protects against Hcy mediates oxidative toxic effects in neurons (Folbergrova, 1994), and protects against increase in heart rate by NMDA-analog (DiMicco & Monroe, 1996), suggesting that Hcy is an agonist to NMDA-receptor. In summary, activation of NMDA-R1 decreases constitutive NO, increases iNSO/NO/oxidative stress, creates arrhythmogenic condition and SCD.
Hcy, NMDA-R1, Connexin-43, collagen, elastin and arrhythmias
Paradoxically, Hcy overexpresses and nitrosylates connexin-43, which is then degraded in the mitochondria (Li et al., 2002). Hcy decreases connexin expression and impaired EDHF-mediated vasodilatation (Heil et al., 2004). Both developmental gap junction uncoupling and connexin-36 down regulation were prevented by the blockade of NMDA receptors (Arumugam et al., 2005), suggesting that the connexins are functionally linked to NMDA-R1 (Grozdanvic et al., 1998). In addition, mutations in connexin-43, elastin, and collagen genes are associated with long Q-T syndromes (Keating, 1995; Pavlovich, 1998; Gutstein et al., 2001; Kaplan et al., 2004). The overexpression of connexin-43 is associated with activation of MMP-2 and -9 (Zhang et al., 2003). Although increased Hcy levels are associated with arrhythmias and SCD, it is unclear whether Hcy mediates arrhythmia in parallel through MMP activation, collagen, elastin, and connexin degradation.
Heart rate inversely related to elastin/collagen ratio
An associative and not causative relationship between animal size and heart rate has been suggested. The higher the metabolic demand is, the higher the heart rate is. To determine whether normal heart rate depends on metabolic demand, we compared heart rate between mice and elephants (Webb et al., 1998; Breukelman et al., 2006; Gehlen et al., 2006). The data suggests that the bigger the animal is, the lower the heart rate is. The cardiac elastin/collagen ratio increases as the size of animal increases (Figure 3), suggesting cardiac function follows the structure. These data also suggest that cardiac elastin/collagen ratio may contribute to cardiac rhythm.
Figure 3.
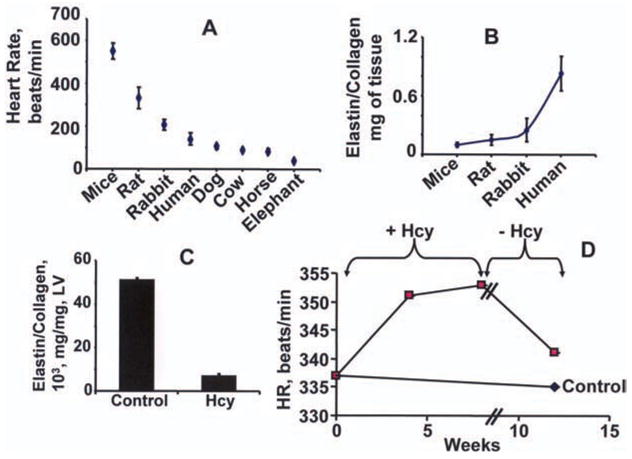
Panel A shows comparative heart rates for animals of different sizes. There was a correlation between the size and the heart rate of mice, rat, rabbit, human, and dog (Henegar et al., 2001; Cox et al., 2002; Hunt et al., 2002; Carroll & Tyagi, 2005; Moshal et al., 2005), cow, horse and elephant (Webb et al., 1998; Breukelman et al., 2006; Gehlen et al., 2006). Panels B, C and D show quantitative data. The data in panels B, C and D is from our previous reports (Tyagi et al., 1995; Sood et al., 2002). After chronic oral administration of Hcy (32 μmol/L) in drinking water for 12 weeks, the heart rate and blood pressure were measured by a PE-50 catheter in femoral artery of the rats (Sood et al., 2002).
Hcy increases heart rate
Twelve weeks of Hcy administration increases LV collagen expression and causes endocardial, peri-capillary and interstitial fibrosis (Miller et al., 2002). The elastin/collagen ratio was robustly decreased in Hcy-treated animals. The chronic administration of Hcy increases the heart rate (HR). The withdrawal of Hcy reduces the Hcy-mediated increase in HR (Figure 3). These results suggest that Hcy decreases elastin/collagen and increases HR (Tyagi et al., 1995; Sood et al., 2002). However, the inverse relationship shown for the elastin/collagen ratio (Figure 3B) and heart rate (Figure 3A) for mice, rats, rabbits and humans may be entirely unrelated. Similarly, the observations that Hcy reduces the elastin/collagen ratio (Figure 3C) and increases heart rate (Figure 3D) may be associative and not causative.
Hcy increases intracellular [Ca2+]i
Treatment of primary MVEC and SMC with Hcy induces calcium transient. Hcy increases [Ca2+]i with an EC50 of 60 nM. The concentrations of Hcy in mammals are around 5–10 μM and the process may be saturated completely under physiological baseline conditions. This may enhance the sensitivity of these cells to subphysiological levels of angiotensin II which normally do not elicit responses in these cells, suggesting a role of Hcy in cardiac calcium-mediated contraction (Mujumdar et al., 2000; Moshal et al., 2005).
Summary
There is an inverse relationship between the increase in heart rate and cardiac elastin/collagen ratio in mice, rats, rabbits and humans. The peri-capillary fibrosis attenuates endothelial ability to relax the cardiac muscle, causing diastolic dysfunction. There is direct relationship between the Hcy administration and increase in systolic blood pressure. Hcy increases intracellular Ca2+ and mitochondrial oxidative stress. Hcy decreases eNOS and increases iNOS expression, generates super oxide and peroxynitrite. Hcy activates MMP. The cardiac connexin-43 is degraded in human heart end-stage failure. The response to CaCl2 is enhanced in endocardial endothelial-denuded myocardium. Hcy damages the endocardial endothelial cells. In endocardial endothelial denuded myocardium Hcy induces contraction. The mechanism of Hcy mediated cardiac contraction and enhanced CaCl2 sensitivity in endocardial-endothelium denuded myocardium may suggest Ca2+-sensitive Hcy receptor (NMDA-R1, Chen et al., 2005; Qureshi et al., 2005) playing a significant role in Hcy mediated cardiac contractile dysfunction. Therefore, it is important to determine the role of NMDA-R1 in Hcy-mediated cardiac contraction, arrhythmia and failure (Figure 4).
Figure 4.
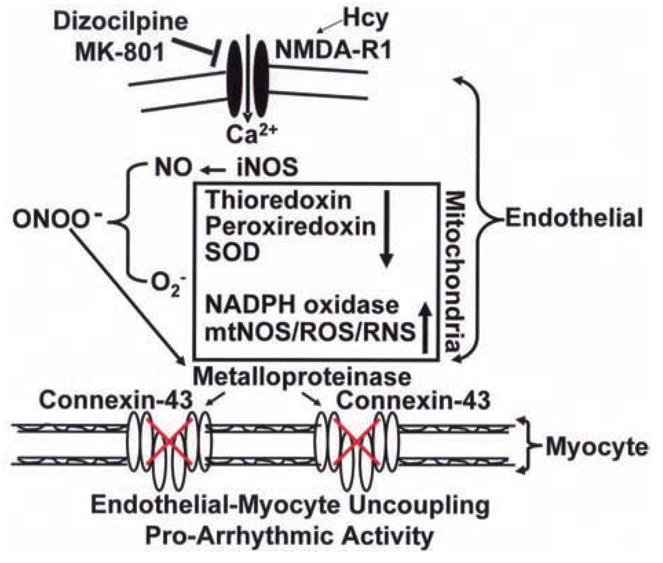
Hcy increases iNOS/NO, superoxide, metalloproteinase activity, and disrupts connexin-43, exacerbates endothelial-myocyte uncoupling and cardiac failure secondary to inducing NMDA-R1. In mitochondria Hcy decreases thioredoxin, peroxiredoxin and SOD and increases NADPH oxidase increasing ROS and RNS.
Abbreviations
- ADAM
a disintegrin and metalloproteinase
- ADMA
asymmetric dimethyl arginine
- AV
aortavenacava shunt
- L-arg
L-arginine
- BH4
tetrahydrobiopterin
- BM
basement membrane
- CBS
cystathionine beta synthatase
- CHF
chronic heart failure
- DDAH
dimethyl arginine hydrolase
- DZA
3-deazaadenosine
- ECM
extracellular matrix
- EDRF
endothelial-derived relaxing factor
- EDHF
endothelial-derived hyperpolarizing factor
- EE
endocardial endothelial
- EET
epoxy-eicosatrienoic acid
- E-M
endothelial-myocyte
- eNOS
endothelial nitric oxide synthase
- Hcy
homocysteine
- HETE
20-hydroxyeicosatetraenoic acid
- HHcy
hyperhomocysteinemia
- LV
left ventricle
- MK
MK-801
- MMP
matrix metalloproteinase
- MT-MMP
membrane type-MMP
- MTHFR
methylene tetrahydrofolate reductase
- MVEC
microvascular endothelial cells
- NADPH
nicotinamide adenosine diphosphate
- NE
norepinephrine
- NMDA-R1
N-methyl-D-aspartate receptor-1
- nNOS
neural nitric oxide synthase
- PVC
premature ventricle contraction
- Redox
reduction-oxidation
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAHH
S-adenosyl-homocysteine hydrolase
- SAM
S-adenosyl-methionine
- SOD
superoxide dismutase
- TIMP
tissue inhibitor of metalloproteinase
- t-PA
tissue plasminogen activator
- VF
ventricular fibrillation
- VT
ventricular tachycardia
- Q-RT-PCR
quantitative real time polymerase chain reaction
- WT
wild type
Footnotes
This work was supported in part by NIH grant HL-71010, and HL-74185.
References
- Adalbert R, Engelhardt JI, Siklos L. Hcy application disrupts calcium homeostasis and induces degeneration of spinal motor neurons in vivo. Acta Neuropathologica. 2002;103(5):428–36. doi: 10.1007/s00401-001-0485-5. [DOI] [PubMed] [Google Scholar]
- Aimes RT, Quigley JP. MMP-2 is an interstitial collagenase. J Biol Chem. 1995;270:5872–6. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, Hcy and plasma lipid levels as predictors of SCD. Circulation. 2002;105(22):2595–9. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8(12):1720–6. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- Barr SD, Gedamu L. Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J Biol Chem. 2003;278(12):10816–23. doi: 10.1074/jbc.M212990200. [DOI] [PubMed] [Google Scholar]
- Bastide B, Neyses L, Ganten D, Paul M, Willecke K, Traub O. Gap junction protein connexin-40 is preferentially expressed in vascular endothelial and conductive bundles of rat myocardium and is increased under hypertensive conditions. Circ Res. 1993;73:1138–49. doi: 10.1161/01.res.73.6.1138. [DOI] [PubMed] [Google Scholar]
- Bollani G, Ferrari R, Bersatti F, Ferrari M, Cattaneo M, Zighetti MI, Visioli O, Assanelli D. A hyperhomocysteinemia study in a population with a familial factor for acute MI and SCD at a young age. Cardiologia. 1999;44(1):75–81. [PubMed] [Google Scholar]
- Brady AJ, Warren JB, Poole-Wilson PA, Williams TJ, Harding SE. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1994;265:H176–82. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Breukelman S, Mulder EJ, van Oord R, Jonker H, van der Weijden BC, Taverne MA. Continuous fetal heart rate monitoring during late gestation in cattle by means of Doppler ultrasonography: reference values obtained by computer-assisted analysis. Theriogenology. 2006;65(3):486–98. doi: 10.1016/j.theriogenology.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Burke AP, Fonseca V, Kolodgie F, Zieske A, Fink L, Virmani R. Increased serum Hcy and SCD resulting from cornary atherosclerosis with fibrous plaques. Arterioscler Thromb Vasc Biol. 2002;22(11):1936–41. doi: 10.1161/01.atv.0000035405.16217.86. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Tyagi SC. Extracellular matrix remodeling in heart of homocysteinemic obese rabbit. Am J Hypertens. 2005;18(5):692–8. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Chen C, Conklin BS, Ren Z, Zhong DS. Homocysteine decreases endothelium-dependent vasorelaxation in porcine arteries. J Surg Res. 2002;102(1):22–30. doi: 10.1006/jsre.2001.6304. [DOI] [PubMed] [Google Scholar]
- Chen H, Fitzgerald R, Brown AT, et al. Identification of a Hcy receptor in the peripheral endothelial and its role in proliferation. J Vasc Surg. 2005;41(5):853–60. doi: 10.1016/j.jvs.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Cheng J, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic injury. World J Gastroenterol. 2004;10(12):1699–708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M, Sood H, Hunt M, Chandler D, Henegar J, Aru G, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol. 2002;282:H1197–206. doi: 10.1152/ajpheart.00483.2001. [DOI] [PubMed] [Google Scholar]
- D’Amico M, Di Filippo C, Rossi F. Arrhythmias induced by myocardial ischemia-reperfusion are sensitive to ionotrophic excitatory amino acid receptor antagonists. Euro J Pharmacol. 1999;366(2–3):167–74. doi: 10.1016/s0014-2999(98)00914-5. [DOI] [PubMed] [Google Scholar]
- DiMicco J, Monroe AJ. Stimulation of metabotropic glutamate receptors in the dorsomedial hypothalamus elevates heart rate in rats. Am J Physiol. 1996;270:R1115–21. doi: 10.1152/ajpregu.1996.270.5.R1115. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Role of mitochondria in health and disease. Diabetes. 2004;53:S96–102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- Farina AR, et al. Thioredoxin alters the MMP/TIMP balance and stimulates human SK-N-SH neuroblastoma cell invasion. Eur J Biochem. 2001;268(2):405–13. doi: 10.1046/j.1432-1033.2001.01892.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–37. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. The metabolism of Hcy: pathways and regulation. Eur J Pediatr. 1998;157(S–2):S40–4. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- Folbergrova J. NMDA and not non-NMDA receptor antagonists are protective against seizures induced by homocysteine in neonatal rats. Exp Neurol. 1994;130(2):344–50. doi: 10.1006/exnr.1994.1213. [DOI] [PubMed] [Google Scholar]
- Frey U, Muller M, Kuhl D. A different form of long lasting potentiation revealed in tPA mutant mice. J Neurosci. 1996;16(6):2057–63. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman O. Hyperhomocysteinemia: atherosclerosis and neurotoxicity. Acta Physiol Pharmacol Ther Latinoam. 1999;49(1):21–30. [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Watanabe J, Kumagai K, Katori Y, Baba S, Fukuda K, Yagi T, Iguchi A, Yokoyama H, Miura M, Kagaya Y, Sato S, Tabayashi K, Shirato K. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J Am Coll Cardiol. 2001;37:1436–42. doi: 10.1016/s0735-1097(01)01125-1. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ, 2nd, Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97(12):1262–9. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- Gattuso A, Mazza R, Pellegrino D, Tota B. Endocardial endothelium (EE) mediates luminal acetylcholine-nitric oxide signaling in isolated frog heart. Am J Physiol. 1999;276:H633–41. doi: 10.1152/ajpheart.1999.276.2.H633. [DOI] [PubMed] [Google Scholar]
- Gehlen H, Marnette S, Rohn K, Stadler P. Stress echocardiography in warmblood horses: comparison of dobutamine/atropine with treadmill exercise as cardiac stressors. J Vet Intern Med. 2006;20(3):562–8. doi: 10.1892/0891-6640(2006)20[562:seiwhc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Goth L, Vitai M. The effects of hydrogen peroxide promoted by homocysteine and inherited catalase deficiency on human hypocatalasemic patients. Free Radic Biol Med. 2003;35(8):882–8. doi: 10.1016/s0891-5849(03)00435-0. [DOI] [PubMed] [Google Scholar]
- Grozdanvic Z, Gossrau R. Co-localization of NOS I and NMDA receptor-1 at the neuromuscular junction in rat and mouse skeletal muscle. Cell Tissue Res. 1998;291(1):57–63. doi: 10.1007/s004410050979. [DOI] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, et al. S-nitrosylation of MMPs: signaling pathways to neuronal cell death. 2002;297:1186–90. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin-43. Circ Res. 2001;88:333–9. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SG, DeVriese AS, Kluijtmans LA, Mortier S, Den Heijer M, Blom HJ. The role of HHcy in NO and EDHF-mediated vasodilation. Cell Mol Biol. 2004;50(8):911–6. [PubMed] [Google Scholar]
- Henderson AH, Lewis MJ, Shah AM, Smith JA. Endothelium, endocardium, and cardiac contraction. Cardiovasc Res. 1992;26(4):305–8. doi: 10.1093/cvr/26.4.305. [DOI] [PubMed] [Google Scholar]
- Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–7. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- Hiromasa S, Ikeda T, Kubota K, Hattori N, Coto H, Maldonado C, Kupersmith J. Ventricular tachycardia and sudden death in myotonic dystrophy. Am Heart J. 1988;115:914–5. doi: 10.1016/0002-8703(88)90901-5. [DOI] [PubMed] [Google Scholar]
- Ho PI, et al. Folate deprivation induces neurodegeneration: roles of oxidative stressand increased Hcy. Neurobiol Dis. 2003;14(1):32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Takeishi Y, Cox MJ, Gabel M, Kirkpatrick D, Walsh RA, Tyagi SC. Remodeling of the left atrium in pacing-induced cardiomyopathy. Mol Cell Biochem. 2002;238:145–50. doi: 10.1023/a:1019988024077. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Kayar SR. Capillary and oxidative capacity of muscles. News in Physiol Sci. 1988;3:113–6. [Google Scholar]
- Huang CF, Su MJ. Positive inotropic action of NMDA receptor antagonist (+)-MK801 in rat heart. J Biomed Sci. 1999;6(6):387–98. doi: 10.1007/BF02253670. [DOI] [PubMed] [Google Scholar]
- Hui TY, et al. Mice lacking thioredoxin interacting protein provide linking cellular redox state to appropriate response to nutritional signals. J Biol Chem. 2004;279(23):24387–93. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Aru GM, Hayden MR, Moore CK, Hoit BD, Tyagi SC. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am J Physiol. 2002;283(2):L239–45. doi: 10.1152/ajplung.00001.2002. An editorial on this paper, L237–38. [DOI] [PubMed] [Google Scholar]
- James TN, Carson NAJ, Froggatt P. Coronary vessels and conduction system in homocystinuria. Circulation. 1974;XLIX:367–74. doi: 10.1161/01.cir.49.2.367. [DOI] [PubMed] [Google Scholar]
- Jara-Prado A, Ortega-Vazquez A, Martinez-Ruano L, Rios C, Santamaria A. Hcy-induced brain lipid peroxidation: effectsa of NMDA receptor blockade, antidaxidant, treatment and NOS inhibition. Neurotox Res. 2003;5(4):237–43. doi: 10.1007/BF03033381. [DOI] [PubMed] [Google Scholar]
- Joseph J, Joseph L, Shekhawat NS, Devi S, et al. Hyperhomocysteinemia leads to pathological ventricular hypertrophy in normotensive rats. Am J Physiol Heart & Circ Physiol. 2003;285:H679–86. doi: 10.1152/ajpheart.00145.2003. [DOI] [PubMed] [Google Scholar]
- Joseph J, Washington A, Joseph L, Koehler L, Fink LM, et al. Hyperhomocysteinemia leads to adverse cardiac remodeling in hypertensive rats. Am J Physiol Heart & Circulatory. 2002;283:H2567–74. doi: 10.1152/ajpheart.00475.2002. [DOI] [PubMed] [Google Scholar]
- Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1(1):3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–42. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- Keating MT. Genetic approaches to cardiovascular disease. Supravalvular aortic stenosis, Williams syndrome, and long-QT syndrome. Circulation. 1995;92(1):142–7. doi: 10.1161/01.cir.92.1.142. [DOI] [PubMed] [Google Scholar]
- Kim WK. S-nitrosation ameliorates homocysteine-induced neurotoxicity and calcium responses in primary culture of rat cortical neurons. Neurosci Lett. 1999;265(2):99–102. doi: 10.1016/s0304-3940(99)00212-8. [DOI] [PubMed] [Google Scholar]
- Kitamua H, Yoshida A, Ohnishi Y, Okajima K, et al. Correlation of connexin-43 expression and late ventricular potentials in nonischemic dialated cardiomyopathy. Circ J. 2003;67(12):1017–21. doi: 10.1253/circj.67.1017. [DOI] [PubMed] [Google Scholar]
- Kraine D, Bai G, Okamoto S, Carlos M, et al. Synergistic activation of the NMDA receptor subunit 1 promoter by myocyte enhancer factor 3C and sp1. J Biol Chem. 1998;273(40):26218–24. doi: 10.1074/jbc.273.40.26218. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26(10):2673–83. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkau B, Kenagy RD, Karsan A, Weitkamp B, Clowes AW, Ross R, Raines EW. Activation of metalloproteinases and their association with integrins: apoptotic pathway in human endothelial cells. Cell Death Differ. 2002;9:1360–7. doi: 10.1038/sj.cdd.4401106. [DOI] [PubMed] [Google Scholar]
- Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS. Paradoxical pver expression and translocation of connexin 43 in Hcy-treated endothelial cells. Am J Physiol. 2002;282:H2124–33. doi: 10.1152/ajpheart.01028.2001. [DOI] [PubMed] [Google Scholar]
- Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of LV diastolic dysfunction and fibrosis in hypertension. Hypertension. 2002;40:136–41. doi: 10.1161/01.hyp.0000024573.17293.23. [DOI] [PubMed] [Google Scholar]
- Loechel F, Gilpin BJ, Engvall E, Albrechtsen R, Wewer UM. Human ADAM 12 (meltrin alpha) is an active metalloprotease. J Biol Chem. 1998;3;273(27):16993–7. doi: 10.1074/jbc.273.27.16993. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart & Circulatory Physiol. 2006;290:H1206–13. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka N, Kodama H, Arakawa H, Yamaguchi I. NMDA receptor blockade by dizocilpine prevents stress-induced sudden death in cardiomyopathic hamsters. Brain Res. 2002;944 (1–2):200–4. doi: 10.1016/s0006-8993(02)02885-8. [DOI] [PubMed] [Google Scholar]
- Mebazaa A, Wetzel R, Cherian M, Abraham M. Comparison between endothelial and great vessel endothelial cells: morphology, growth, and prostaglandin release. Am J Physiol. 1995;268:H250–9. doi: 10.1152/ajpheart.1995.268.1.H250. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, et al. Effects of ECM-degrading proteases MMP-3 and -9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96(5):1227–41. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104(2):174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hyperten. 2002;15:157–63. doi: 10.1016/s0895-7061(01)02286-5. [DOI] [PubMed] [Google Scholar]
- Mizushige K, Yao L, Noma T, et al. Alteration in LV diastolic filling and accumulation of mycardial collagen at insulin-resistant prediabetic stage of a type 2 diabetic rat model. Circulation. 2000;101:899–907. doi: 10.1161/01.cir.101.8.899. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Tyagi N, Henderson B, Ovechkin AV, Tyagi SC. Protease activated receptor and endothelial-myocyte uncoupling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2005;288(6):H2770–7. doi: 10.1152/ajpheart.01146.2004. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem. 2001;82(3):491–500. doi: 10.1002/jcb.1175. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Hayden MR, Tyagi SC. Homocysteine induces calcium secondary messenger in vascular smooth muscle cells. J Cell Physiol. 2000;183:28–36. doi: 10.1002/(SICI)1097-4652(200004)183:1<28::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Tyagi SC. Temporal Regulation of ECM Components in Transition from Compensatory Hypertrophy to Decompensatory Heart Failure. J Hypertension. 1999;17:261–70. doi: 10.1097/00004872-199917020-00011. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Herron AR, Lowry AS, Stroud RE, Stroud MR, Wharton JM, Ikonomidis JS, Crumbley AJ, 3rd, Spinale FG, Gold MR. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol. 2006;97(4):532–7. doi: 10.1016/j.amjcard.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Gros R, You X, Pirani A, Azad A, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109(6):735–43. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei M, Lee JK, Honjo H, Yasui K, et al. Decreased vagal control over heart rate in rats with right-sided congestive heart failure: downregulation of nNOS. Circ J. 2005;69(4):493–9. doi: 10.1253/circj.69.493. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Tsujino T, Watari Y, Emoto N, Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation. 2001;104(10):1165–70. doi: 10.1161/hc3601.093976. [DOI] [PubMed] [Google Scholar]
- Oh JH, et al. Thioredoxin sectreted unpon UV A irradiation modulates MMP-2 and TIMP in human dermal fibroblasts. Arch Biochem Biophys. 2004;423(1):218–26. doi: 10.1016/j.abb.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Palakurthy P, McGraw P, Maldonado C. Electrocardiographic changes are associated with elevated blood pressure rather than a direct effect of raised intracranial pressure. In: Hoff JT, Betz AL, editors. Intracranial Pressure. 7. Berlin, Hildeberg, New York, Tokyo: Springer-Verlag; 1989. pp. 442–4. [Google Scholar]
- Pavlovich ER. Connective tissue components of conducting and working myocardium of the sinoatrial region of the heart in patients of various ages with idiopathic long QT syndrome (quantitative evaluation) Morfologiia. 1998;114(5):45–50. Russian. [PubMed] [Google Scholar]
- Pinsky DJ, Patton S, Mesaros S, Brovkovych V, Kubaszewski E, Grunfeld S, Malinski T. Mechanical Transduction of NO synthesis in the beating heart. Circ Res. 1997;81:372–9. doi: 10.1161/01.res.81.3.372. [DOI] [PubMed] [Google Scholar]
- Podrid PJ, Myerburg RJ. Epidemiology and stratification of risk for sudden cardiac death. Clin Cardiol. 2005;28(11 Suppl 1):I3–11. doi: 10.1002/clc.4960281303. [DOI] [PubMed] [Google Scholar]
- Poelzing S, Rosenbaum DS. Altered connexin-43 expression produces arrhythmia substrate in heart failure. Am J Physiol. 2004;287(4):H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- Qureshi I, Chen H, Brown AT, et al. Hcy-induced vascular dysregulation is mediated by the NMDA receptor. Vasc Med. 2005;10(3):215–23. doi: 10.1191/1358863x05vm626oa. [DOI] [PubMed] [Google Scholar]
- Roberts JT, Wearn JT. Quantitative changes in the capillary-muscle relationship in human hearts during normal growth and hypertrophy. Am Heart J. 1941;16:617–33. [Google Scholar]
- Robinson AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005;280(47):39316–23. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix Metalloproteinases in Neuroinflammation. Glia. 2002;39:279–91. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rucklidge GJ, Milne G, McGaw BA, Milne E, Robins SP. Turnover rates of different collagen types measured by isotope ratio mass spectrometery. Biochim Biophys Acta. 1992;11:1156–7. doi: 10.1016/0304-4165(92)90095-c. [DOI] [PubMed] [Google Scholar]
- Scarabelli T, Stephanou A, Rayment N, Pasini E, Comini L, Curello S, Ferrari R, Knight R, Latchman D. Apoptosis of endothelial cells precedes myocytes cell apoptosis in ischemia/reperfusion injury. Circulation. 2001;104:253–256. doi: 10.1161/01.cir.104.3.253. [DOI] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Eliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991;266:7870–5. [PubMed] [Google Scholar]
- Shao LE, et al. Thioredoxin-related regulation of NO/NOS activities. Ann N Y Acda Sci. 2002;962:140–50. doi: 10.1111/j.1749-6632.2002.tb04064.x. [DOI] [PubMed] [Google Scholar]
- Shastry S, Tyagi SC. Homocysteine induces metalloproteinase and shedding of beta-1 integrin in microvessel endothelial cells. J Cell Biochem. 2004;93:207–13. doi: 10.1002/jcb.20137. [DOI] [PubMed] [Google Scholar]
- Simandle SA, Kerr BA, Lacza Z, Eckman DM, Busija DW, Bari F. Piglet pial arterioles respond to N-methyl-d-aspartate in vivo but not in vitro. Microvasc Res. 2005;70(1–2):76–83. doi: 10.1016/j.mvr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Smith JA, Shah AM, Fort S, Lewis MJ. The influence of endocardial endothelium on myocardial contraction. Trends in Pharmacological Sci. 1992;13:113–6. doi: 10.1016/0165-6147(92)90040-d. [DOI] [PubMed] [Google Scholar]
- Sood HS, Cox MJ, Tyagi SC. Generation of Nitrotyrosine Precedes the Activation of Matrix Metalloproteinase in Left Ventricle of Hyperhomocystenemia Rats. Antioxidant & Redox Signaling. 2002;4(5):799–804. doi: 10.1089/152308602760598954. [DOI] [PubMed] [Google Scholar]
- Spragg DD, Kass DA. Controlling the gap: myocytes, matrix and mechanics. Circ Res. 2005;96:485–7. doi: 10.1161/01.RES.0000160607.30286.54. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodeling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Torres L, Garcia-Trevijano ER, Rodriguez JA, et al. Induction of TIMP-1 expression in rat hepatic stellate cells: a new role for Hcy in liver fibrosis. Biochim Biophys Acta. 1999;1455(1):12–22. doi: 10.1016/s0925-4439(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci USA. 1994;91:6369–73. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummalapalli CM, Heath BJ, Tyagi SC. Tissue inhibitor of metalloproteinase-4 instigates apopotosis in transformed cardiac fibroblasts. J Cell Biochem. 2001;80:512–21. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol Cell Biochem. 1997;168:1–12. doi: 10.1023/a:1006850903242. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Hayden MR. Role of nitric oxide in matrix remodeling in diabetes and heart failure. Heart Failure Reviews. 2003;8:23–8. doi: 10.1023/a:1022138803293. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Hoit BD. Metalloproteinase in myocardial adapatation and maladaptation. J Cardiovasc Pharmaol & Therapeutics. 2002;7(4):241–6. doi: 10.1177/107424840200700407. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Kumar SG, Alla SR, Reddy HK, Voelker DJ, Janicki JS. Extracellular matrix regulation of metalloproteinase and antiproteinase in human heart fibroblast cells. J Cell Physiol. 1996;167(1):137–47. doi: 10.1002/(SICI)1097-4652(199604)167:1<137::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Meyer L, Schmaltz RA, Reddy HK, Voelker DJ. Proteinases and Restenosis in Human Coronary Artery: Extracellular Matrix Production Exceeds the Expression of Proteolytic Activity. Atherosclerosis. 1995;116:43–57. doi: 10.1016/0021-9150(95)05520-7. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Ratajska A, Weber KT. Myocardial matrix metalloproteinases: localization and activation. Mol Cell Biochem. 1993;126:49–59. doi: 10.1007/BF01772207. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Rodriguez W, Patel AM, Roberts AM, Falcone JC, Passmore JC, Fleming JT, Joshua IG. Hyperhomocysteinemic Diabetic Cardiomyopathy: Oxidative stress, Remodeling, and Endothelial-Myocyte Uncoupling. J Cardiovasc Pharmacol Therapeutics. 2005;10(1):1–10. doi: 10.1177/107424840501000101. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Moshal KS, Ovechkin AV, Tyagi SC. Mechanisms of Homocysteine-Induced Oxidative stress. Am J Physiol, Heart & Circulatory Physiol. 2005;289(6):H2649–56. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Smiley LM, Mujumdar VS. Homocyst(e)ine impairs endocardial endothelial function. Canad J Physiol & Pharmacology. 1999;77:950–7. [PubMed] [Google Scholar]
- Vamvakas S, et al. Perurbations of intracellular calcium distribution in kidney cells by nephrotoxic haloalkenyl cysteine S-conjugates. Molecular Pharmacology. 1990;38(4):455–61. [PubMed] [Google Scholar]
- Vincenti VP, Coon CI, Mengshol JA, Yocum S, Mitchell P, Brinckerhoff CE. Cloning of the gene for interstitial collagenase-3 (MMP-13) from rabbit synovial fibroblasts: differential expression with collagenase-1 (MMP-1) Biochem J. 1998;331:341–6. doi: 10.1042/bj3310341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases:structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wang J, Morgan JP. EE modulates myofilament Ca2+ responsiveness in aequorin-loaded ferret myocardium. Cir Res. 1992;70:754–60. doi: 10.1161/01.res.70.4.754. [DOI] [PubMed] [Google Scholar]
- Webb PM, Andrews RD, Costa DP, Le Boeuf BJ. Heart rate and oxygen consumption of northern elephant seals during diving in the laboratory. Physiol Zool. 1998;71(1):116–25. doi: 10.1086/515894. [DOI] [PubMed] [Google Scholar]
- Welch GN, Upchurch GR, Jr, Farivar RS, Pigazzi A, Vu K, Brecher P, Keaney JF, Jr, Loscalzo J. Homocysteine-induced nitric oxide production in vascular smooth-muscle cells by NF-kappa B-dependent transcriptional activation of Nos2. Proc Assoc Am Physicians. 1998;110(1):22–31. [PubMed] [Google Scholar]
- Wolin MS, Gupte SA. Novel roles for nox oxidases in cardiac arrhythmia and oxidized glutathione export in endothelial function. Circ Res. 2005;97(7):612–4. doi: 10.1161/01.RES.0000186804.96482.78. [DOI] [PubMed] [Google Scholar]
- Wong CM, Zhou Y, Ng RW, Kung HF, Jin DY. Cooperation of yeast peroxiredoxins Tsa1p and Tsa2p in the cellular defense against oxidative and nitrosative stress. J Biol Chem. 2002;277(7):5385–94. doi: 10.1074/jbc.M106846200. [DOI] [PubMed] [Google Scholar]
- Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;27;109(3):363–8. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Hara H, Adachi T. Effects of homocysteine on the binding of extracellular-superoxide dismutase to the endothelial cell surface. FEBS Lett. 2000;486(2):159–62. doi: 10.1016/s0014-5793(00)02260-2. [DOI] [PubMed] [Google Scholar]
- Yang A, Wang XQ, Sun CS, Wei L, Yu SP. Inhibitory effects of clofilium on membrane currents associated with Ca channels, NMDA receptor channels and Na+, K+-ATPase in cortical neurons. Pharmacology. 2005;73(3):162–8. doi: 10.1159/000083072. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nwagwu C, Le M, et al. Increased invasive capacity of connexin-43 overexpressing malignant glioma cells. J Neurosurg. 2003;99:1039–46. doi: 10.3171/jns.2003.99.6.1039. [DOI] [PubMed] [Google Scholar]


