Abstract
Background
γ-Aminobutyric acid (GABA) is a known inhibitory neurotransmitter in the mammalian central nervous system, and homocysteine (Hcy) behaves as an antagonist for GABAA receptor. Although the properties and functions of GABAA receptors are well studied in mouse neural tissue, its presence and significance in non-neural tissue remains obscure. The aim of the present study was to examine the expression of GABAA receptor and its subunits in non-neural tissue.
Methods
The mice were analyzed. The presence of GABAA receptor and its subunits was evaluated using Western blot and reverse transcription polymerase chain reaction.
Results
We report that GABAA receptor protein is abundant in the renal medulla, cortex, heart, left ventricle, aorta and pancreas. Low levels of GABAA receptor protein were detected in the atria of the heart, right ventricle, lung and stomach. The mRNA protein expression of GABAA receptor subunit shows that α1, β1, β3 and γ1 subunits are present only in brain. The mRNA protein expression levels of GABAA receptor α2, α6, β2 and γ3 subunits were highly expressed in brain compared to other tested tissue, while GABAA receptor γ2 subunit was expressed only in brain and kidney. Treatment of microvascular endothelial cells with Hcy decreased GABAA receptor protein level, which was restored to its baseline level in the presence of GABAA receptor agonist, muscimol. The distribution of GABAA and GABAB receptors in wild type mice was determined and tissue-specific expression patterns were found showing that several receptor subtypes were also expressed in the central nervous system.
Conclusions
Hcy, a GABAA agonist, was found to decrease GABAA expression levels. These data enlarge knowledge on distribution of GABA receptors and give novel ideas of the effects of Hcy on different organs.
Keywords: brain, γ-aminobutyric acid receptor A (GABAA), heart, homocysteine, kidney, lung, mouse, muscimol, pancreas
Introduction
Homocysteine (Hcy) is a thiol-containing amino acid derived from the metabolic demethylation of dietary methionine. Elevated levels of Hcy, hyperhomocysteinemia (HHcy) is associated with vascular dementia and Alzheimer’s diseases. In animals, HHcy causes seizures; moreover, Hcy transduces intracellular nuclear receptors (1). It binds to γ-aminobutyric acid (GABA) receptor, and therefore competes with muscimol (agonist of GABAA receptor) and baclofen (agonist of GABAB receptor) (2, 3).
GABA, an endogenous amino acid, is present in vertebrate peripheral (4–6) and central nervous system (7, 8). This amino acid is a major functionally inhibitory neurotransmitter in the nervous system (9). It also mediates various functional responses in non-neuronal tissue (8, 10–13). The formation of GABA occurs by the decarboxylation of glutamate, catalyzed by glutamate decarboxylase (GAD) enzyme. Biochemical studies have demonstrated the significant presence of GAD enzyme in the nervous system, as well as in endocrine organs (8, 10).
GABA receptors are divided into two distinct groups: GABAA and GABAB (14). These receptors differ by their affinity for specific agonist and antagonist profiles and separate modes of action (9, 15). GABAA receptor is a ligand-gated chloride channel that exists in a hetero-pentameric structure from a number of subunits (16, 17). In rodents, GABAA receptors comprise seven different classes of subunits: α1–α6, β1–β3, γ1–γ3, ρ1–ρ3, ε, θ, δ (18–21). GABAA receptors are composed of α, β and γ subunits (22). Another receptor, GABAB, is a G-protein coupled receptor. The activation of GABAB causes an inhibition of adenylate cyclase activity, a decrease in calcium and an increase in K+ conductance in neuronal membranes (23–25). GABAB receptor is activated by baclofen (2), while its inhibition is mediated by indirect gating of either potassium or chloride channels (25). GABAA receptors play a significant role in general anesthesia, feeding, cardiovascular regulation, anxiolytic effects and anticonvulsive activity (3). However, the existence of GABAA receptor outside the central nervous system is not well studied. Activation of GABAA receptors protect against many types of seizures and convulsions in animals and humans. To date, this receptor is a target for a number of drugs with significant clinical importance.
Recent studies on rats showed that the receptor-channel complex may exist outside the central nervous system (26, 27). These studies have indicated that agonists of the GABA receptor bind to various tissues outside the central nervous system, including heart, kidney and pancreas (19, 28, 29). However, no GABAA receptors were identified in mouse non-neural tissue. As mouse is a commonly used experimental animal, and GABAA receptor may be involved in many functional aspects of a microcirculation and vascular remodeling, the objective of the present study was to identify and characterize GABAA receptor in various organs of mouse.
Materials and methods
Materials
DL-Hcy, muscimol, β-mercaptoethanol, proteinase inhibitors [phenylmethylsulfonyl fluoride (PMSF), aprotinin, leupeptin, and pepstatin], tribromoethanol, fibronectin, agarose, collagenase type B (0.2%), antibodies against GABAA receptor and β-actin were purchased from Sigma (St. Louis, MO, USA), specific antibodies against GABAA receptor subunits were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), while DMEM/Ham’s F-12 50:50 mix (DMEM/F12-50/50) was purchased from Cell Grow Media-tech, Inc. (Herndon, VA, USA). Fetal bovine serum was purchased from HyClone (Logan, UT, USA). Trypsin (0.25%)/EDTA, penicillin, streptomycin, L-glutamate, heparin and trizol were obtained from Invitrogen-Gibco (Carlsbad, CA, USA). Complete MCDB131 medium was purchased from VEC Technology, Inc. (Rensselaer, NY, USA). Bio-Rad protein assay reagents were purchased from Bio-Rad (Hercules, CA, USA).
Animals
Wild type C57BL/6J, 10–12-week-old male mice were purchased from Jackson Laboratories (Bar Harbor, MN, USA). Animal protocols were performed in accordance with the National Institutes of Health guidelines and the IACUC protocol specifications at the University of Louisville for animal safety. All of the animals were fed rodent chow diet and kept in cages in a temperature- and humidity-controlled room with a 12-h light/dark cycle.
Tissue collection
Mice were anesthetized with 0.02 mL/g body weight tribromoethanol (intraperitoneally). The brain, heart, lung, kidney, aorta, stomach and pancreas were excised, and tissues were rapidly washed twice in phosphate buffered saline (PBS). The tissue samples were stored at −20°C until further use.
Preparation of samples
The tissue samples were homogenized in 300 μL of ice-cooled lysis buffer (pH 7.4) containing Tris-HCl (20 mM), EDTA (1 mM), NaCl (50 mM), dithiothreitol (1 mM), β-mercaptoethanol (10 mM) and proteinase inhibitors pepstatin (100 μM), aprotinin (10 μM), leupeptin (100 μg/mL) and PMSF (1 mM), which were added prior to use. Cells were lysed in ice-cold-modified RIPA lysis buffer (pH 7.4) containing: Tris-HCl (50 mM), NP-40 (1%), Na-deoxycholate ( 0.25%), NaCl (150 mM), EDTA (1 mM), PMSF (1 mM) aprotinin, leupeptin, pepstatin (1 μg/mL each), Na3VO4 (1 mM) and NaF (1 mM). The clear supernatant of tissue and cell samples was collected, and protein concentration in the samples was determined by Bradford’s method (29) using bovine serum albumin as a standard.
Endothelial cell culture
Mouse brain microvascular endothelial cells (MBMEC) were purchased from ATCC. Cells were grown in modified DMEM (ATCC) with 10% fetal bovine serum. Mouse aortic endothelial cells (MAEC) were a generous gift from Dr. Kathleen Bove (Stratton VA Medical Center, Albany, NY, USA). The cells were maintained in complete DMEM/F12-50/50 mix and supplemented with 10% fetal bovine serum (30). Mouse cardiac endothelial cells (MCMEC), which were isolated as described previously (31), were grown in fibronectin-coated wells in complete MCDB131 medium with 10% fetal bovine serum.
Mouse lung endothelial (MLMEC) were isolated from lungs of wild type mice using a modification of a method described earlier (31). Briefly, both lungs from two mice were removed aseptically, rinsed in Hank’s balanced salt solution (HBSS; Invitrogen-Gibco) to remove excess blood, and minced into ~2 mm square pieces. The lung pieces were digested in 10 mL of collagenase type B for 25 min at 37°C with occasional agitation. Further digestion was carried out with 1 mL of 0.25% trypsin/EDTA for 5–10 min at 37°C. The cellular digest was filtered through sterile 40-μm nylon mesh and then centrifuged at 120×g for 5 min. After the supernatant was removed, the cell pellet was washed twice in DMEM containing 20% fetal bovine serum. MLMEC were resuspended in growth media and were grown in humidified conditions at 5% CO2 at 37°C, as described previously (32). All cells were grown at 37°C with 5% CO2, 95% air until they became 70%–80% confluence. All experiments were performed between passages 5 and 8.
Western blot analysis
Expression of GABAA receptor and its subunits was determined by Western blot analysis. Protein samples were mixed with 1:1 v/v ratio with 2× sample loading buffer, boiled at 100°C for 5 min, cooled and then centrifuged at 10,000×g for 5 min. Equal amounts of protein (50 μg) for each group were resolved by 4%–15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then electrophoretically transferred to a polyvinylidene fluoride membrane (BioRad, Hercules, CA, USA). Transferred proteins were blocked with 5% non-fat dry milk in TBS-T (pH 7.4) containing Tris-HCl (50 mM), NaCl (150 mM), Tween-20 (0.1%) for 1 h at room temperature. The blots were then incubated with respective primary antibodies in blocking solution, according to the manufacturer’s recommendations. After incubation, the blots were washed with TBS-T three times for 10 min each. The blots were incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h. After washing four times (each wash for 10 min) ECL Plus substrate (Amersham Biosciences, Pittsburgh, PA, USA) was applied to the blots for 5 min. To confirm an equal loading of the samples, each blot was probed with mouse anti-rabbit antibody directed against β-actin. The protein bands were visualized by exposing the blots to an X-ray film, as described previously (32). Image analysis of the protein bands was performed using UMAX PowerLook II (Tawin, Republic of China). The protein expression intensity was assessed by integrated optical density (IOD). To account for possible differences in total protein load, the results of the measurements were presented as a ratio of IOD of GABAA receptor protein in a tissue to the IOD of the respective β-actin.
RNA preparation and semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
Expression of GABAA receptor subunits was carried out by assessing an expression of their respective mRNAs. The extraction of total RNA from brain, heart, kidney and lung was isolated, frozen and pulverized under liquid nitrogen, followed by homogenization in trizol reagent (Invitrogen-Gibco), as described earlier (33). Briefly, the concentration of total RNA was quantified by measuring the absorbance at 260 nm. Samples with a peak area ratio of 28S-to-18S rRNA >2.0 were used. RNA (2 μg) was reverse transcribed (RT) using oligo dT primers with a total reaction volume of 20 μL. The RT program was 25°C for 10 min, 42°C for 50 min, and then 70°C for 15 min. PCR was performed using 2 μL of each RT product (cDNA), with a total reaction volume of 20 μL. The PCR thermal cycle was carried out at 94°C for 2 min, and then 35 cycles were carried out at 94°C for 30 s, at 55°C for 30 s, and finally at 72°C for 60 s. This was followed by a final extension for 5 min at 72°C. Oligonucleotide primers specified in Table 1 were synthesized, according to the published sequences (34). Each sample (20 μL) of PCR product together with negative controls were subjected to electrophoresis on 1% agarose gel and stained with ethidium bromide.
Table 1.
Sequences of oligonucleotide primers used for PCR amplification.
| Target | Length, bp | Primer sequence |
|---|---|---|
| GABAA-α1 | 231 | 5′-CTACAGCAACCAGCTATACCC-3′ 5′-GCTCTCTGTTTAAATACGTGG-3′ |
| GABAA-α2 | 282 | 5′-AAGGCTCCGTCATGATACAG-3′ 5′-ACTAACCCCTAATACAGGC-3′ |
| GABAA-α3 | 418 | 5′-GACTTGCTTGGTCATGTTGTTGGG-3′ 5′-CAGAGGCCCTGGAGATGAAGAAGA-3′ |
| GABAA-α6 | 650 | 5′-CTTCCCTATGGATGGACACG-3′ 5′-GTTGACAGCTGCGAATTCAA-3′ |
| GABAA-β1 | 540 | 5′-ATGATGCATCTGCAGCCA-3′ 5′-TGGAGTTCACGTCAGTCA-3′ |
| GABAA-β2 | 402 | 5′-GCATGTATGTCTGCAGGA-3′ 5′-CTGACACCTACTTCCTGA-3′ |
| GABAA-β3 | 224 | 5′-AGCCAAGGCCAAGAATGATCG-3′ 5′-TGCTTCTGTCTCCCATGTACC-3′ |
| GABAA-γ1 | 191 | 5′-TTTCTTACGTGACAGCAATGG-3′ 5′-CATGGGAATGAGAGTGGATCC-3′ |
| GABAA-γ2 | 351 | 5′-GCAATGGATCTCTTTGTA-3′ 5′-GTCCATTTTGGCAATGCG-3′ |
| GABAA-γ3 | 251 | 5′-TGTCGAAAGCCAACCATCAGG-3′ 5′-GACTTGCACTCCTCATAGCAG-3′ |
Statistical analysis
Data were expressed as mean±SEM, n=4 animals from each group. The arbitrary densitometry unit was represented as percentage relative to control. The data were analyzed by Student’s t-test for comparison of the results between the Hcy-treated and non-treated groups. A p-value −0.05 was considered statistically significant.
Results
The results of the present study show that expression of GABAA receptor was greater in mouse brain than in other tested tissue, such as pancreas, lung and stomach (Figure 1). In kidney, GABAA receptor was found to be expressed more in the medulla as compared to the cortex region of the kidney (Figure 2). GABAA receptor protein was present in the whole heart, with greater expression in the left ventricle and aorta and with a less expression in the atria and right ventricle (Figure 3).
Figure 1.
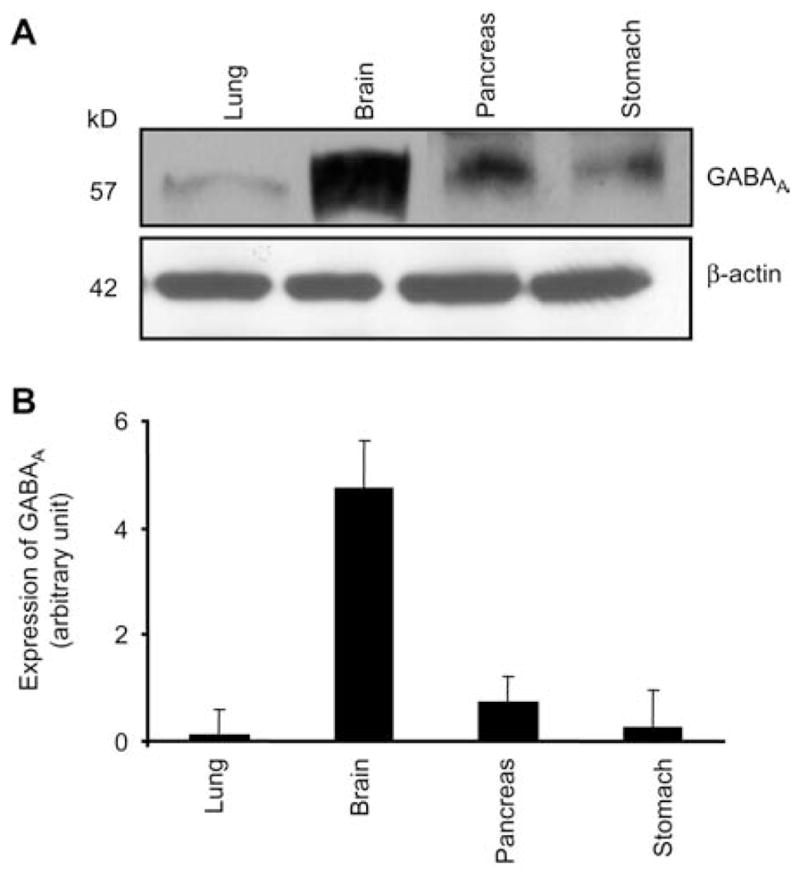
Expression of the GABAA receptor in brain (B), lung (L), pancreas (P) and stomach (S).
Immunoblot analysis (A) and corresponding densitometry analyses (B). Values are mean±SEM, n=4 animals from each group. Corresponding β-actin bands are shown.
Figure 2.
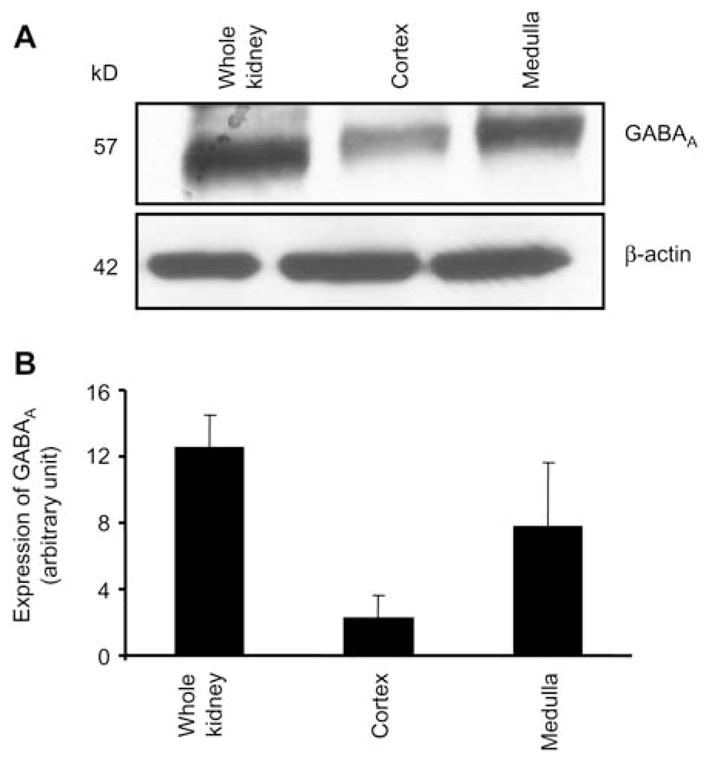
Expression of the GABAA receptor in renal system. Whole kidney, medulla and cortex.
Western blot analysis of the GABAA receptor (top) and β-actin (bottom) in renal system (A) and corresponding densitometry analyses (B). Values are mean±SEM, n=4 animals from each group.
Figure 3.
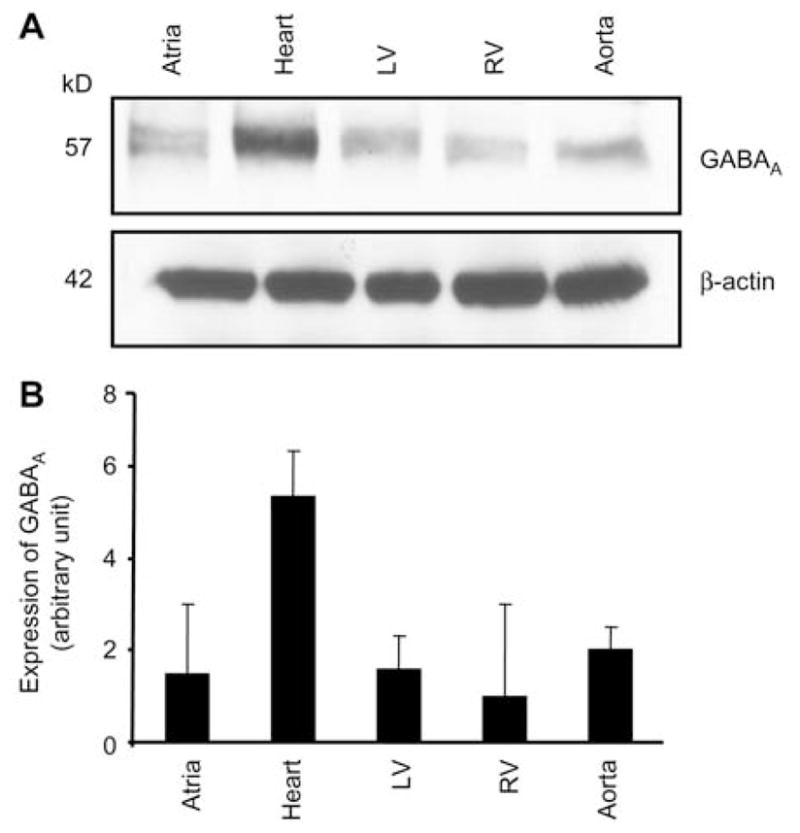
Expression of the GABAA receptor in mouse cardiovascular system.
Heart, atrium, left ventricle (LV), right ventricle (RV) and aorta. Western blot analysis of the GABAA receptor (top) and β-actin (bottom) (A) and corresponding densitometry analyses (B). Values are mean±SEM, n=4 animals from each group.
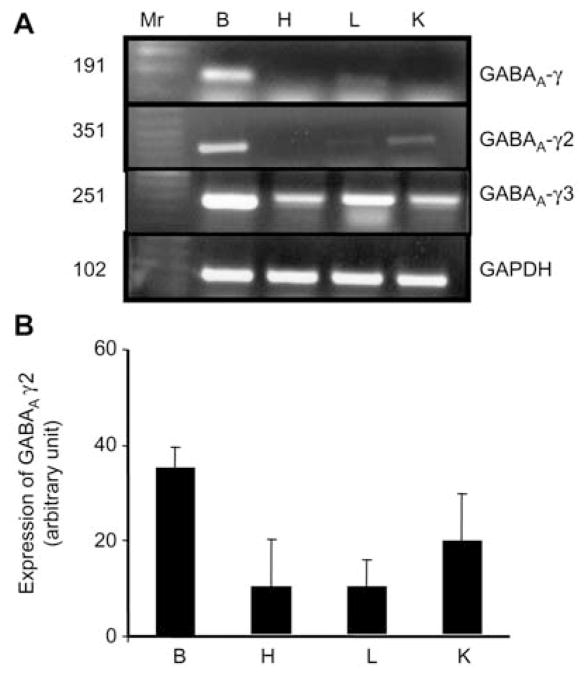
In brain, GABAA-α2 and -α6 subunit mRNAs expression levels were significantly greater than α1 subunit mRNA (Figure 4A, B). GABAA-α1 subunit mRNA was only expressed in brain and was not found in any other tested tissues. The mRNA expression level of α2 subunit was similar in heart, lung and whole kidney. The mRNA expression level of α6 subunit was greater in whole kidney as compared to heart or lung (Figure 4A, B).
Figure 4.
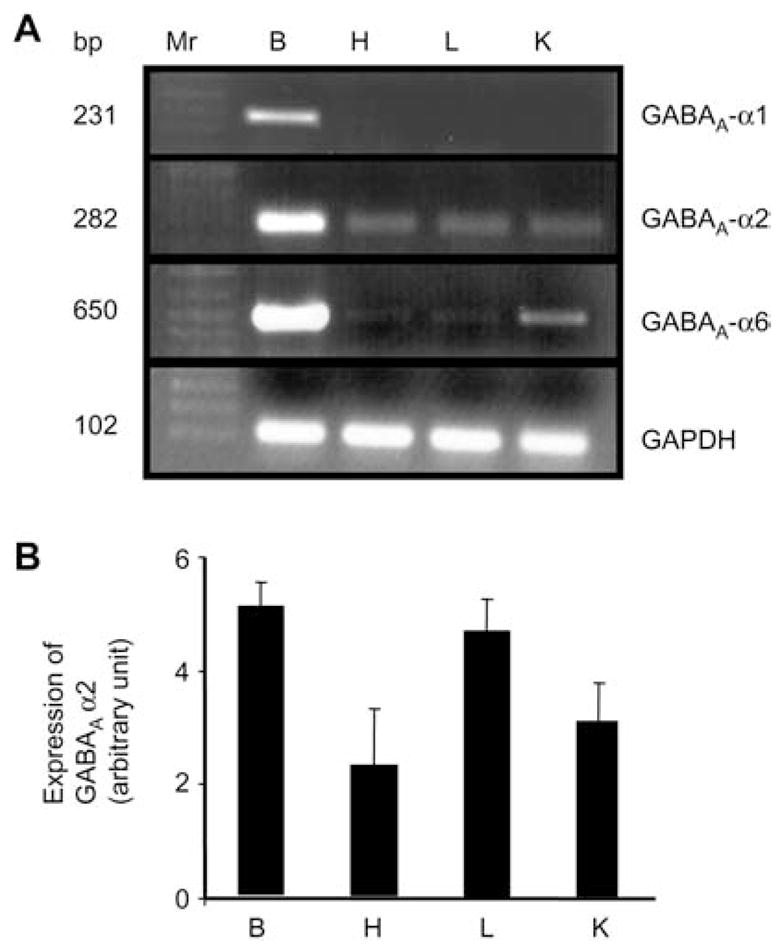
The mRNA expression of GABAA receptor α sub-units in brain (B), heart (H), lung (L) and whole kidney (K).
(A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of GABAA-α subunits. Ethidium bromide-stained 1.0% agarose gels containing cDNA amplified from RT RNA using primers for GABAA subunits (α1–6). A 100-bp DNA ladder (MW) was included at the left of the gel (Lane 1). cDNA bands of the predicted size were obtained for all the above subunits. n=4 animals from each group. (B) Corresponding densitometry analyses (bottom). Values are mean±SEM, n=4 animals from each group.
The mRNA GABAA receptor β1 and β3 subunit mRNAs were only expressed in brain and were not found in other tested tissue (Figure 5A, B). Although β2 subunit mRNA of a GABAA receptor was found in brain, lung, heart and whole kidney, its expression was greater in brain and lung compared to that in other tested tissue (Figure 5A, B).
Figure 5.
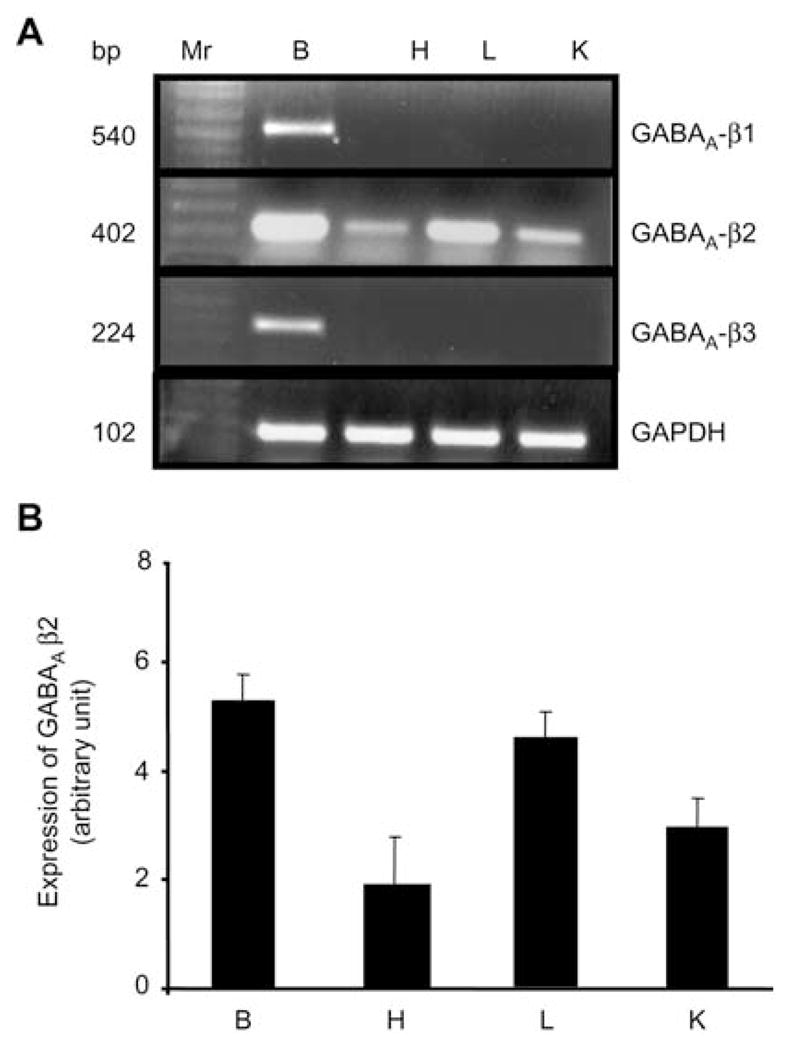
The mRNA expression of GABAA-β subunits in brain (B), heart (H), lung (L) and whole kidney (K).
(A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of GABAA-β subunits. Ethidium bromide-stained 1.0% agarose gels containing cDNA amplified from RT RNA using primers for GABAA-β1–3. A 100-bp DNA ladder (MW) was included at the left of the gel (Lane 1). cDNA bands of the predicted size were obtained for all the above subunits. n=4 animals from each group. (B) Corresponding densitometry analyses (bottom). Values are mean±SEM, n=4 animals from each group.
The γ1 subunit mRNA was not detectable in heart, lung or whole kidney (Figure 6A). It was expressed only in brain (Figure 6A). However, the γ2 subunit mRNA was expressed in all the tested tissues except heart (Figure 6B). We found an ample expression of γ3 subunit mRNA in brain and lung compared to that in heart or kidney (Figure 6B). An ubiquitous gene GAPDH was present in all the tested tissues.
Figure 6.
The mRNA expression of GABAA-γ subunits in brain (B), heart (H), lung (L) and whole kidney (K).
(A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of GABAA-γ subunits. Ethidium bromide-stained 1.0% agarose gels containing cDNA amplified from RT RNA using primers for GABAA-γ1–3. A 100-bp DNA ladder (MW) was included at the left of the gel (Lane 1). cDNA bands of the predicted size were obtained for all the above subunits. n=4 animals from each group. (B) Corresponding densitometry analyses (bottom). Values are mean±SEM, n=4 animals from each group.
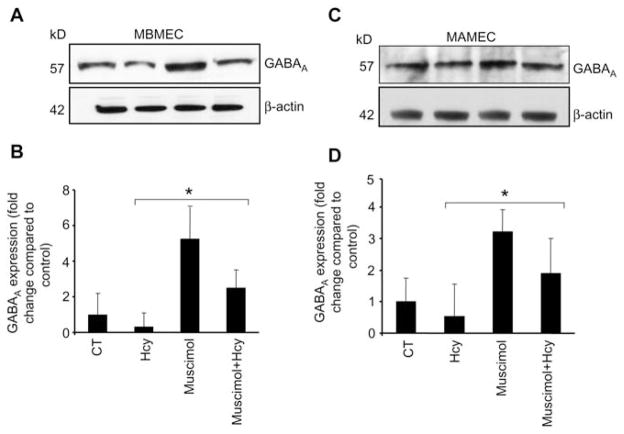
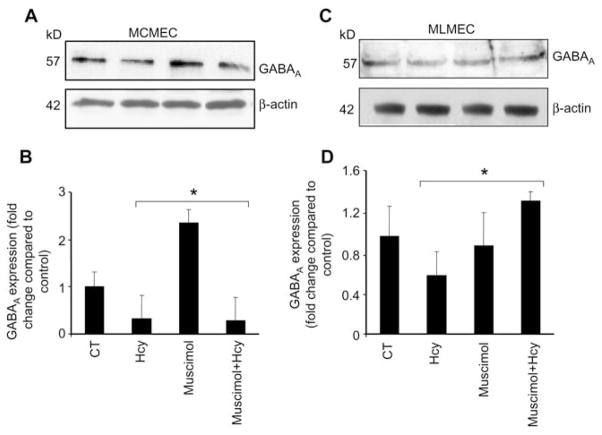
The functional studies on ECs show that Hcy diminishes and muscimol enhances GABAA receptor expression in all the EC lines: brain (Figure 7A, B), aorta (Figure 7C, D), cardiac (Figure 8A, B) and lung (Figure 8C, D). Although, muscimol did not apparently restore Hcy abrogated expression of GABAA receptor in MLMEC cell line (Figure 8C, D). This may explain the differences in lung vs. cardiac ECs.
Figure 7.
Western blot analysis showing muscimol-induced expression of GABAA receptor in cells.
Cells (5–7 passages) were plated onto cell culture dishes, grown in complete media and allowed to become 80% confluent. Cells were serum deprived. (A) Mouse brain microvascular endothelial cells (MBMEC) and (C) mouse aorta microvascular endothelial cells (MAMEC) were cultured alone (CT), with 50 μM Hcy, 50 μM muscimol or 50 μM Hcy+50 μM muscimol. After 18 h, equal amounts of cellular protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted using GABAA receptor antibody. Corresponding β-actin bands are shown. Accompanying densitometry is shown in (B) and (D), respectively (n=4). *p−0.05 vs. control.
Figure 8.
Western blot analysis showing muscimol-induced expression of GABAA receptor in cells.
Cells (5–7 passages) were plated onto cell culture dishes, grown in complete media and allowed to become 80% confluent. Cells were serum deprived. (A) Mouse cardiac microvascular endothelial cells (MCMEC) and (C) mouse lung microvascular endothelial cells (MLMEC) were cultured alone (CT), with 50 μM Hcy, 50 μM muscimol or 50 μM Hcy+50 μM muscimol. After 18 h, equal amounts of cellular protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted using GABAA receptor antibody. Corresponding β-actin bands are shown. Accompanying densitometry is shown in (B) and (D), respectively (n=4). *p−0.05 vs. control.
Discussion
Recent studies have indicated that muscimol, a specific agonist of the GABAA receptor, binds to various tissues outside the central nervous system. This includes renal system and endocrine organs, such as ovaries, adrenal glands and pancreas in rat or guinea pig (5, 8, 10, 13, 35–37). These data suggest that the GABA receptor and its subunits may exist in non-neural tissues (21). However, as of today no direct evidence is available showing that GABAA receptors are expressed in any other tissue of mouse other than the brain. The results of the present study coincide with studies of others (19, 20, 38) showing that GABAA receptor and its subunits α1, α2, α6, β1, β2, β3, γ1, γ2 and γ3 are expressed in the mouse brain. However, we found that α1, α2, α6, β1, β2, β3, γ1, γ2 and γ3 subunits of the GABAA receptor are also present in various non-neural tissues. These GABAA receptor subunits were most abundant in the mouse brain and kidney.
Sarang et al. showed that mRNA of GABAA receptor β2 and β3 subunits is expressed only in rat, rabbit and human kidney (21). However, their studies did not explore the protein and mRNA expression of other subunits in other non-neural tissue of these species, as well as in mice. The GABAA receptor β3 subunit was found primarily in the cortex and medulla of rat (21), and the β2, β3 subunits are transiently expressed in the rat proximal tubule (38, 39). Auto-radiography with use of the GABAA receptor agonist muscimol demonstrated specific binding to convoluted proximal tubules of the rat renal cortex (40). However, α1 subunit of the GABAA receptor was identified in the thick ascending limb of the loop of Henle, but not in the proximal tubule or in glomeruli in rats (41). In the present study, we found that GABAA receptor and its subunits (α2, α6, β2, γ2, γ3) are present in mouse renal system and other non-neural tissues, such as lung and heart. Although β2 and γ2 subunits were expressed in kidney, their expression was not as significant as α6, β2 and γ3 subunits. Interestingly, GABAA receptor protein expression was greater in medulla as compared to renal cortex (Figure 2).
Recently, GABAA receptor ε and π subunits were identified in heart and uterus of guinea pig (19, 20). Autoradiographic studies on the (←←3H) GABA binding sites showed presence of GABAA receptor in rat atria (39, 42). It has been suggested that GABAA receptors are to be found on the blood vessel membranes (36).
The present study showed for the first time that GABAA receptor is expressed in the adult mouse heart. It is present in the atria, left ventricle and descending aorta. The less expression of GABAA receptor was found in the right ventricle. GABAA receptor α2, α6, β2 and γ3 subunits were also present in adult mouse heart, whereas α1, β1, β3, γ1 and γ2 were not found in the heart.
Tanaka identified GABAA receptor in the lungs of guinea pig (8). Our data show that some GABAA protein was visible in mouse lung. Moreover, we found GABAA receptor α2, α6, β2, γ2 and γ3 subunits in the lung. These results confirm an existence of GABAA receptor in the adult mouse lung.
In our previous studies, we showed for the first time that mouse brain MECs contain GABAA receptor, which was stifled by Hcy (42, 43). In the present study, muscimol restored Hcy-suppressed GABAA receptor expression in mouse brain, aortic and cardiac ECs. This suggests that muscimol may ameliorate the toxic effect of Hcy on EC, such as an impairment of normal cellular function, which leads to apoptosis, lipid peroxidation, enhanced oxidative stress and impaired platelet aggregation (32, 44–47). Furthermore, our finding that GABAA receptor is expressed in aortic and cardiac MECs suggests that it is present in the endothelium lining of the vasculature of brain, lung, heart and aorta. Thus, GABAA receptor may have a significant functional role in non-neuronal tissue.
In summary, our data demonstrate that GABA receptor and its subunits are expressed in various non-neuronal tissues in mice, including lung, heart, kidney, stomach and pancreas. Our functional studies show that Hcy inhibits, while muscimol restores, expression of GABAA receptor in EC. These findings suggest that GABAA receptor may play a greater role in various physiological or pathological conditions, particularly during HHcy, than it was thought before. A specific gene knockout strategy in mice may accelerate the recognition of the role of GABAA receptor subtypes as drug targets in neuropathy, cardiopathy and renalpathy.
Acknowledgments
This study was supported in part by NIH grants (HL-71010, HL-88012 and NS-51568 to S.C.T.), American Heart Association (AHA), National SDG (grant 3 0235317N) and NIH (HL-80394 to D.L.) and the AHA post-doctoral training grant (award 3 0625579B to K.S.M.).
References
- 1.Hunt MJ, Tyagi SC. Peroxisome proliferators compete and ameliorate Hcy-mediated endocardial endothelial cell activation. Am J Physiol Cell Physiol. 2002;283:C1073–9. doi: 10.1152/ajpcell.00152.2002. [DOI] [PubMed] [Google Scholar]
- 2.Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989;10:401–7. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- 3.Martin IL, Dunn SM. GABA receptors. Tocris Review. Tocris Cookson Ltd; 2002. [Accessed 2002]. www.biotrend.com/download/gabarev.pdf. [Google Scholar]
- 4.Awapara J, Landua AJ, Fuerst R, Seale B. Free gamma-aminobutyric acid in brain. J Biol Chem. 1950;87:35–9. [PubMed] [Google Scholar]
- 5.Roberts E, Frankel S. Gamma-aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- 6.Erdo SL, editor. GABA outside the CNS. Heidelberg: Springer-Verlag; 1992. [Google Scholar]
- 7.Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine. 1998;8:213–23. doi: 10.1385/ENDO:8:3:213. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka C. Gamma-aminobutyric acid in peripheral tissue. Life Sci. 1989;30:2221–35. doi: 10.1016/0024-3205(85)90013-x. [DOI] [PubMed] [Google Scholar]
- 9.DeFeudis FV. GABA and cardiovascular regulation. Rev Clin Basic Pharmacol. 1985;5:141–58. [PubMed] [Google Scholar]
- 10.Erdo SL. Peripheral GABAergic mechanism. Trends Pharmacol Sci. 1985;6:205–7. [Google Scholar]
- 11.Erdo SL, Bowery NG, editors. GABAergic mechanism in mammalian periphery. New York: Raven Press; 1986. [Google Scholar]
- 12.Erdö SL, Kiss B. Presence of GABA, glutamate decarboxylase, and GABA transaminase in peripheral tissues: a collection of quantitative data. In: Erdö SL, Bowery NG, editors. GABAergic mechanisms in the mammalian periphery. New York: Raven Press; 1986. pp. 5–17. [Google Scholar]
- 13.Erdo SL, Wolff JR. Gamma-aminobutyric acid outside the brain. J Neurochem. 1990;54:363–72. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman RW, Hey JA, Rizzo CA, Bolser DC. GABAB receptors in the lung. Trends Pharmacol Sci. 1993;14:26–9. doi: 10.1016/0165-6147(93)90110-6. [DOI] [PubMed] [Google Scholar]
- 15.Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983;78:191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombination GABA-A receptor. J Neurosci. 1996;16:5415–24. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz RD. The GABAA receptor-gated ion channel: biochemical and pharmacological studies of structure and function. Biochem Pharmacol. 1988;37:3369–75. doi: 10.1016/0006-2952(88)90684-3. [DOI] [PubMed] [Google Scholar]
- 18.Burt DR, Kamatchi GL. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–23. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- 19.Hedblom E, Kirkness EF. A novel class of GABA-A receptor subunit in tissues of the reproductive system. J Biol Chem. 1997;272:15346–50. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 20.Whiting PJ, McAllister D, Vassilatis GD, Bonnert TP, Heavens RP, Smith DW, et al. Neuronally restricted RNA splicing regulates the expression of a novel GABA-A receptor subunit conferring atypical functional properties. J Neurosci. 1997;17:5027–37. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarang SS, Plotkin MD, Gullans SR, Cummings BS, Grant DF, Schnellmann RG. Identification of the gamma-aminobutyric acid receptor beta(2) and beta(3) subunits in rat, rabbit, and human kidneys. J Am Soc Nephrol. 2001;12:1107–13. doi: 10.1681/ASN.V1261107. [DOI] [PubMed] [Google Scholar]
- 22.Barnard EA, Skolnick P, Olsen RW. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 23.Bowery NG, Bettler B, Froestl W. Mammalian gamma-aminobutyric acid (B) receptors: structure and function. Pharmacol Rev. 2002;54:247–64. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 24.Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290:149–52. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto RR. GABA receptors: are cellular differences reflected in function? Brain Res Brain Res Rev. 1989;14:203–25. doi: 10.1016/0165-0173(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Liu G. A novel method to determine the localization of high- and low-affinity GABA transporters to the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res Brain Res Protoc. 1999;4:288–94. doi: 10.1016/s1385-299x(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HY, McPherson BC, Liu H, Maman TS, Rock P, Yao Z. H2O2 opens mitochondrial K-ATP channels and inhibits GABA receptors via PKC-epsilon in cardiomyocytes. Am J Physiol. 2002;282:H1395–403. doi: 10.1152/ajpheart.00683.2001. [DOI] [PubMed] [Google Scholar]
- 28.Gilon P, Tappaz M, Remacle C. Localization of GAD-like immunoreactivity in the pancreas and stomach of the rat and mouse. Histochemistry. 1991;96:355–65. doi: 10.1007/BF00271357. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Sen U, Moshal KS, Tyagi N, Kartha GK, Tyagi SC. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J Cell Physiol. 2006;209:767–74. doi: 10.1002/jcp.20752. [DOI] [PubMed] [Google Scholar]
- 31.Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1206–13. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi N, Ovechkin AV, Lominadze D, Moshal KS, Tyagi SC. Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J Cell Biochem. 2006;98:1150–62. doi: 10.1002/jcb.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ovechkin AV, Tyagi N, Rodriguez WE, Hayden MR, Moshal KS, Tyagi SC. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J Appl Physiol. 2005;99:2398–405. doi: 10.1152/japplphysiol.00442.2005. [DOI] [PubMed] [Google Scholar]
- 34.Geigerseder C, Doepner RF, Thalhammer A, Krieger A, Mayerhofer A. Stimulation of TM3 Leydig cell proliferation via GABA(A) receptors: a new role for testicular GABA. Reprod Biol Endocrinol. 2004;24:2–13. doi: 10.1186/1477-7827-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baum T, Becker FT. Hypotensive and postural effects of the gamma-aminobutyric acid agonist muscimol and of clonidine. J Cardiovasc Pharmacol. 1982;4:165–9. doi: 10.1097/00005344-198203000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Ong J, Kerr DI. GABA-receptors in peripheral tissues. Life Sci. 1990;46:1489–501. doi: 10.1016/0024-3205(90)90421-m. [DOI] [PubMed] [Google Scholar]
- 37.Bailey SJ, Ravier MA, Rutter GA. Glucose-dependent regulation of γ-aminobutyric acid (GABAA) receptor expression in mouse pancreatic islet α-cells. Diabetes. 2007;56:320–7. doi: 10.2337/db06-0712. [DOI] [PubMed] [Google Scholar]
- 38.Arpad P, Endre D, Joachim RW, Peter P, Erdo SL. GABA-immunoreactive structures in rat kidney. J Histochem Cytochem. 1992;40:675–80. doi: 10.1177/40.5.1573248. [DOI] [PubMed] [Google Scholar]
- 39.Amenta F, Cavallotti C, Iacopino L, Erdo SL. Autoradiographic localization of the GABA receptor agonist muscimol within rat kidney. Pharmacology. 1998;36:390–5. doi: 10.1159/000138327. [DOI] [PubMed] [Google Scholar]
- 40.Unger T, Becker H, Dietz R, Ganten D, Lang RE, Rettig R, et al. Antihypertensive effect of the GABA receptor agonist muscimol in spontaneously hypertensive rats. Role of the sympathoadrenal axis. Circ Res. 1984;54:30–7. doi: 10.1161/01.res.54.1.30. [DOI] [PubMed] [Google Scholar]
- 41.Moloney DT, Pressley T, DuBose T, Mehta P. Murine renal medulla expresses an γ-aminobutyric acid (GABAA) α1 Cl− channel that differs in cDNA sequences from the central nervous system(CNS) channel [abstract] J Am Soc Nephrol. 1993;4:875–7. [Google Scholar]
- 42.Shastry S, Moning L, Tyagi N, Steed M, Tyagi SC. GABA receptors and nitric oxide ameliorate constrictive collagen remodeling in hyperhomocysteinemia. J Cell Physiol. 2005;205:422–7. doi: 10.1002/jcp.20416. [DOI] [PubMed] [Google Scholar]
- 43.Shastry S, Tyagi N, Moshal KS, Lominadze D, Hayden MR, Tyagi SC. GABA receptors ameliorate Hcy-mediated integrin shedding and constrictive collagen remodeling in microvascular endothelial cells. Cell Biochem Biophys. 2006;45:157–65. doi: 10.1385/CBB:45:2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carsten S, Kenneth W. Death receptor induced apoptosis. A new mechanism of homocysteine-mediated endothelial cell cytotoxicity. Hypertension. 2004;43:1168. doi: 10.1161/01.HYP.0000127811.48554.12. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Slungaard A, Vercellotti GM, Iadecola C. Superoxide-dependent cerebrovascular effects of homocysteine. Am J Physiol. 1998;274:R1704–11. doi: 10.1152/ajpregu.1998.274.6.R1704. [DOI] [PubMed] [Google Scholar]
- 46.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–56. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 47.Tyagi SC, Lominadze D, Roberts AM. Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem Biophys. 2005;43:37–44. doi: 10.1385/CBB:43:1:037. [DOI] [PubMed] [Google Scholar]