Abstract
Objective: The study aimed to examine the effect of exogenous OT administration on the inflammation and atherosclerosis in adult male rats and its possible mechanisms. Thirty adult male rats equally divided into three groups. Control group fed regular diet; group II fed control diet supplemented with L-methionine for 10 weeks. Group III received L-methionine and oxytocin treatment for 10 weeks. RT-PCR analysis showed that OT administration increased oxytocin receptor mRNA (2 fold, P, 0.05). Blood samples were evaluated for total homocysteine, interlukin-6 (IL-6), monocyte chemoatrratant protein-1 (MCP-1) and C-reactive protein (CRP) by ELIZA, lipid profile, nitric oxide (NO), malondialdehyde (MDA) and reduced glutathione (GSH) were determined. Specimens from aorta were processed for immunohistochemical staining for Aorta nuclear factor _B (NF-κB) p65 protein. Result showed that OT administration to group III decreased the plasma levels IL-6, MCP-1 and CRP levels which were elevated in group II. Moreover, there was decrease of the oxidative stress of group III in terms of increased plasma levels of NO and GSH and decreased plasma levels of MDA in blood. In addition, rats of group II showed histological abnormalities manifested by thickening and ulceration of the aortic wall. Marked increased expression of NF-κB in aorta of in group II was detected. However, OT administration restores the histological structure of the aorta and decreased the expression of NF-κB in aorta of group III similar to the control group. Conclusion: OT has anti inflammatory pathway in atherosclerosis as it decelerates atherosclerosis by decreasing the proinflammatory responses through many mechanisms, mainly the up regulation of its receptors.
Keywords: oxytocin, atherosclerosis, inflammation, methionine
Introduction
The neurohypophysial peptide oxytocin (OT) is involved in peripheral female reproductive functions, including uterine contraction, lactation and the inflammation stimulated process. It has also been proposed that OT is a cardiovascular hormone that plays an important role in normal homeostatic mechanisms [1]. OT and its receptors are synthesized and released in the heart and vasculature of rats and human [2], and these tissues also express oxytocin receptors (OTR) [3].
Homocystiene (Hyc) is a nonprotein thiol containing amino acid, which is produced in the cell as an intermediary in methionine metabolism [4].
Several risk factors including hypercholes-terolemia, diabetes and tobacco use have been implicated in the onset of atherosclerotic lesions [5] In addition to these established risk factors, epidemiological studies have indicated that elevated plasma levels of Hey may be an independent risk factor for atherosclerosis and thrombosis [6].
Many studies have correlated associated high plasma or serum concentration of homocysteine with an increased risk of atherosclerosis [7].
Inflammation plays a key role in atherosclerosis (AS) [8]. Mild Hyperhomocystinemia (HHcy) by feeding diets rich in methionine or deficient in folate contributes to the early stages of the development of AS by increasing the expression of vascular inflammatory molecules (such as MCP-1 and ICAM-1) and decreasing the availability of nitric oxide (NO) [9].
Animal studies investigating the effects of peripheral OT administration in models of inflammatory diseases have provided evidence for the existence of potentially cardioprotective pathways involving OT. For example, peripheral OT administration has been shown to improve wound healing [10].
So, considering possible actions of OT on the inflammation stimulated process, it was hypothesized that treatment with OT may attenuate the proinflammatory process that leads to acceleration of atherosclerosis in a rat model.
The first aim of the current study is to examine the potential anti-inflammatory effects of OT involved in the pathophysiology of atherosclerosis. The second aim is to discover the possible mechanisms by which OT can act as anti-inflammatory.
Materials and methods
This study included 30 adult male rats weighing 150-200 gm, were provided from by the Institutional Animal Care and Faculty of Medicine, University of Assiut. They were maintained for a week for acclimatization under conditions of controlled temperature (24-26 C°), humidity (55-60%) and 12 hrs light- dark cycle. They were feed on a standard diet with free access to water. All experimental procedures were carried in accordance with research protocols established by the Animal Care House of Faculty of Medicine, Assiut University, Egypt.
The rats were randomly divided into:
Group I: included 10 adult male rats which was fed normal diet.
Group II: included 10 adult male rats, which fed the control diet supplemented with L-methionine 10 gm/kg [9] and Food intake was measured daily for 10 weeks.
Group III: included 10 adult male rats, which received L- methionine for the same dose as group II and oxytocin in a dose 1 mg/kg/day intraperotineal [11] for 10 weeks.
Physiologic measurements
Systolic blood pressure (SBP) was recorded by the tail-cuff device (NARCO, Biosystem, Inc., Huston, Texas) after animals have been warmed for 30 min. in a metabolic chamber maintained at approximately 30°C. All measurements were made at the same time of the day. Mean SBP values obtained from three consecutive measurements were recorded as the pressure values for a given rat at each point.
At the end of the experiment the non-fasting blood samples blood samples (5 ml) from retro-orbital vein were collected in chilled EDTA-containing microtubes and centrifuged immediately and the plasma were stored at - 20 C° until analysis. Then, the rats were killed by decapitation. The thoracic aorta was rapidly removed and dissected from the connective tissues and kept frozen in liquid nitrogen. Tissues were stored in -80C° until processed.
Other Specimens from aorta were fixed in 10% netrual-buffered formalin. Sections of 5um were stained with hematoxylin and eosin (H&E) then were examined with light microscopy for histopathology.
Plasma levels of total cholesterol (C) were determined by colorimeteric method according to the method of Allain [12], and triglyceride levels (TG) according to the method of Wahlefeld [13]. Determination of HDL-cholesterol (HDL-C) were done by a microplate enzymatic assay [14] using reagents from Synermed International Inc. LDL-cholesterol (LDL-C) was calculated using Friedewald's formula [15] (LDL-C = [total cholesterol- (HDL-cholesterol- triglyceride/5)]. Determination of NO was done by evaluation of its oxidant products, nitrates and nitrites by using Griess reaction [16]. Plasma levels of malondialdehyde (MDA) were determined as thiobarbituric acid reactivity. The product of reaction between malonic dialdhyde and thiobarbituric acid was measured as described by Oh-kawa [17]. Determination of reduced glutathione (GSH) levels were determined by HPLC methods according to the Jayatilleke and Shaw [18]. Plasma total Hcy levels were determined by enzyme-linked immunosorbent assay kit (ELIZA) technique kit according to Frantzen et al [19].
Assays for interlukin -6 (IL-6) levels were performed with an ELIZA kit (Endogen, USA) according to the manufacturer's instructions. The assay selectively recognizes IL-6 with a limit of detection of <1pg/ml. Plasma monocytes chemoattractant protein-1 (MCP-1) concentrations were analyzed using a Rat MCP-1 detector kit (Pierce-Endogen). The analysis was conducted according to the manual provided with the kit. Absorbance was detected at 450 nm, using ELISA reader (Bio-TEK Instruments) [20]. Determination of plasma levels of C-reactive protein (CRP) were performed with an ELIZA Kit (Helica Biosystems, Inc. Fullerton, CA.). Absorbance was detected at 450 nm.
Immunohistochemistry examination: Aorta nuclear factor _B (NF-κB) p65 protein
Tissue sections (4-μm thick) of formalin-fixed, paraffin-embedded specimens were deparaffinized, rehydrated in graded alcohol, and transferred to PBS (phosphate buffered saline, PH 7.6). The slides were rinsed twice with PBS, then endogenous peroxidase was blocked by the use of 3% hydrogen peroxide in for 5 min. antigen retrieval was done using microwave at 700W for 20 min in citrate buffer. After cooling the slides were washed three times with PBS. The slides were incubated for (overnight) at 4 C with prediluted primary antibody. The slides were then rinsed three times with PBS and incubated for 10 min. at room temperature with the biotinylated goat antipolyvalent. The slides were rinsed with PBS for three times and incubated for 5 min. with steptavidin peroxidase at room temperature. The sections were then washed three times with PBS, and diaminibenzidine was applied for 5 min at room temperature. The slides then rinsed in D.W., counterstained with Mayer's hematoxylin, dehydrated, and then mounted. Negative control were obtained by ommiting the primary antibody. A distinct brown cytoplasmic staining in lipid laden histocytes was scored positive for NF-κB. Photographs were taken by use of a light microscopy at a magnification of ×400.
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the rat aorta using the Blood Mini Kit (Qiagen, Hilden, Germany) Following the manufacturer's protocol. The RNA aliquots were stored at -80° C until use. After treatment with deoxyribonuclease I to eliminate possible DNA contamination, the reverse transcriptase reaction was carried out by the Qiagen one step RT- PCR kits by mixing 5-10 mg total RNA previously denatured at 70°C for 10 min in the presence of 10 ul 5 x Qiagen One Step RT-PCR Buffer, 2 ul deoxy-NTPs, 1 ml 40 U/ml RNas inhibitor, and 2 ul Qiagen One Step RT-PCR Enzyme. Then mix with template RNA (2ug/ reaction) using specific primers for OT receptors 5-GTCAATGCGCCCAAGGAAG-3 OTR antisense 5'-GATGCAAACCAATAGACACC-3, The thermal cycler conditions (Cycler, Bio- Rad Laboratories, Inc., Hercules, USA) of PCR were (denaturation 0.5-1 min at 94°C, anneling for 0.5-1 min at 55 °C, and extension at 1 min at 72° C, The number of cycles was 45 cycles, 370 bp). The PCR products thus obtained were separated by electrophoresis on 1.8 % agarose gel, visualized by ethidium bromide staining under ultraviolet light, and analyzed by scanning densitometry. β-actin was used as a control for the amount of amplified cDNA chosen over at least one intron). The specific primers of β-actin is 5 -CCTCTATGCCAACACAGTGC-3, Antisense 5 -CATCGTACTCCTGCTTGCTG-3.
The results are presented relative to the expression of the control OTR gene. Band intensities of RT-PCR products were quantified using Biometre Image software.
Statistical analysis
All values were expressed as mean ± SE for all parameters. The data were analyzed by using Prism computer program (Graph Pad version 3.0, Software, Inc., San Diego, CA, USA). For comparison of statistical significance between groups Student Newman Keuls t-test for unpaired data was used. ONE WAY ANOVA test followed by least significance differences LSD were used for multiple comparisons. Levels of significance (P) was considered as follows: P>0.05, not significant. P≤0.05, significant and P≤0.01, highly significant [21].
Results
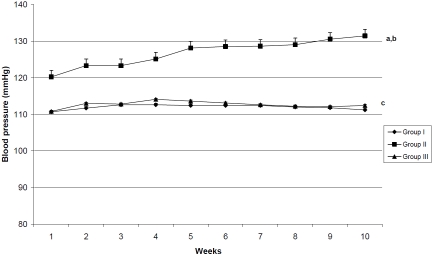
The mean systolic blood pressure values of group II were significantly higher (P>0.001) than those of groups I & III through the weeks of the study. In addition, the BP values in adult rats treated with OT were nearly similar to those of control group (Figure 1).
Figure 1.
Systolic blood pressure (SBP) mmHg in all studied groups through the weeks of the study. The mean SBP values of group II were significantly higher than those of group I & II. No significant differences were detected between the mean of SBP values of group I & III. a: p<0.001 as compared to group I. b: p<0.001 as compared to group II. c: non significant as compared to group I.
The mean plasma levels of total cholesterol of group I, II and III were (102.1±0.28, 102.5±0.38 and 102.3±0.34 mg/dl respectively). The mean plasma levels of triglyceride levels of group I, II and III were (79.75±0.19, 79.16±0.25 and 79.97±0.2mg/dl respectively). The mean plasma concentrations of HDL-C the studied groups were (27.66 ±0.13, 27.57±0.14 and 27.63±0.11 respectively). The mean plasma levels of LDL-C of the studied groups were (90.51±0.26, 90.57± 0.26 and 90.48± 0.32 respectively).
Statistical analysis of lipid profile using one way ANOVA test showed no significant difference between the all studied groups.
Significant high plasma Hcy levels were recorded in group II compared to those of group I and II (p>0.001) There was no significant differences between group I and III (Table 1).
Table 1.
Levels of plasma total Homocysteine (umol/l), MDA (ng/ml), CRP (ng/ml) and MCP-1(ug/ml) in all studied groups
| Groups | Group I | Group II | Group III |
|---|---|---|---|
| Parameters | |||
| Hcy (umol/L) | 10.76±0.17 | 18.36±0.3a,b | 10.9±0.17c |
| MDA (ng/ml) | 6.6±0.09 | 8.19± 0.09a,b | 6.6 ± 0.08c |
| GSH (mg/ml) | 39.46±0.33d | 26.74±0.25 | 39.38±0.3 |
| CRP (ng/ml) | 20.26 ±0.33 | 37.94±0.5a,b | 20.37±0.36c |
| MCP-1(ug/ml) | 16.7±0.31 | 42.77±0.27a,b | 16.84±0.18c |
| IL-6 (pg/ml) | 282.4 ±1.98 | 454.1±1.78a,b | 283.2±1.93c |
Data are expressed as mean ± S.E.M.
p<0.001 as compared to group I.
p<0.001 as compared to group III.
non significant as compared to group I.
p<0.001 as compared to group II
The mean plasma levels of CRP in group II were significantly higher than those of group I and III (37.94±0.5 versus 20.26±0.33 and 20.37±0.36, p<0.001 respectively), but there was no significant differences of these levels between group I and III.
The mean plasma levels of NO of group II were significantly lowered than those of group I and III (17.83±0.49 versus 34.47±0.71 and 32.4±0.95, P>0.001, respectively), however there was no significant differences between those of group I and III (Figure 2). The plasma levels of MDA in group II were significantly higher than those of group I and III (8.19±0.09 versus 6.56±0.09 and 6.6±0.08 respectively). No significance differences were detected between these levels of group I and III. The plasma levels of GSH of group II were significantly decreased than those of group I and III (26.74±0.25 versus 39.64±0.33 and 39.38±0.3 respectively) (Table 1). These levels of group I did not differ from those of groups II & III.
Figure 2.
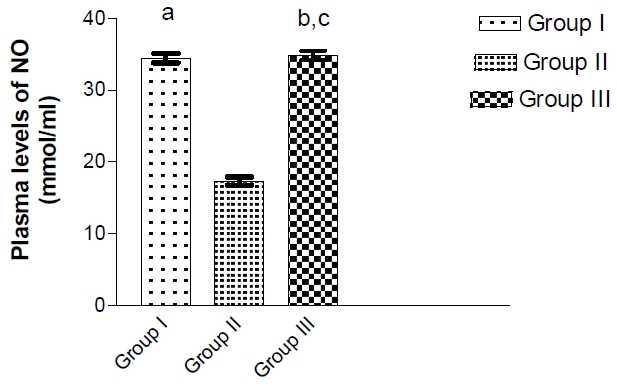
Plasma levels of nitric oxide (mmlo/ml) in all studied groups. a: p >0.001 as compared to group II. b: p >0.001 as compared to group II. c: non significant as compared to group I.
Table 1 showed that the plasma levels of MCP-1 in group II were significantly higher than those of group I and III (42.77±0.27 versus 16.7±0.31 and 16.84±0.18, p<0.001 respectively). No significance differences were detected between these levels of group I and III. The mean plasma levels of IL-6 in group II were significantly higher than those of group I and III (454.1±1.78 versus 282.4±1.98 and 283.2±1.93, p<0.001 respectively), but the mean plasma levels of IL-6 of group I did not differ significantly from those of group III (Table 1).
OTR expression
RT-PCR was used to investigate the effect of oxytocin administration to adult male rats on OTR transcripts in the rat aorta. As shown in Figure 3A, external OT treatment to adult male rats of group III augmented aortic OTR mRNA significantly (P<0.001) than those of group I and II. No significant differences between aortic OTR mRNA in group I and II (Figure 3B): Specific bands of predicted length and increasing exponentially (until 45 cycles at 370bp) were obtained with OTR specific primers in group III which in not indicated in group I and II.
Figure 3.
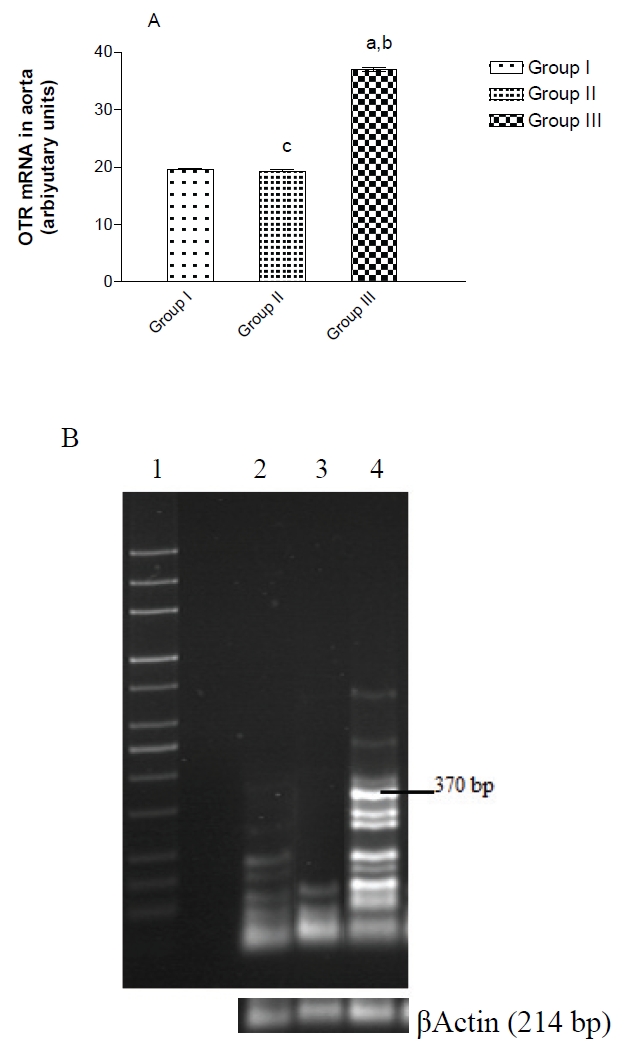
OT treatment enhances OTR mRNA in aorta of group III, a: p<0.001 as compared to group I, b: p<0.001 as compared to group II, c: non significant as compared to group I. (B) PhosphorImager analysis of RT-PCR amplification using specific oligonucleotide primers for OTR mRNA in the aorta of control, adult male rats supplemented with methionine and adult male rats treated with oxytocin. Total RNA isolated from aorta was used for RT-PCR reactions as described in Materials and methods. Lane 1 from the left is the 1-kb ladder control; lane 2: group I, lanes 2: group II, and 3: group III correspond to the primers specific for OTR at 370bp. The results are verified to β Actin mRNAPCR product used as internal standard.
Histopathological results
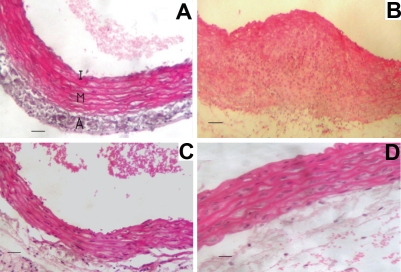
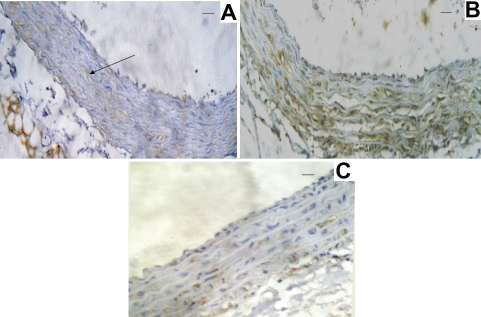
Group I: The wall of the aorta in the rats showed normal architecture with normal 3 layers a) Tunica intima with characteristic endothelial layer and delicate sub endothelial connective tissue), Tunica media that is formed of several layers of smooth muscle fibers intermingled with large amount of elastic fibers, c) Tunica adventia is formed of loose connective tissues rich in elastic fibers (Figure 4A). Immunohistochemical method for detection of NF-κB p65 demonstrates minimal reaction (Figure 5A).
Figure 4.
Photograph of transverse sections of the aorta. (A) Transverse section of the aorta of group I showing normal histological structures of intima (I), media (M) and adventitia (A). (B) Transverse section of the aorta of group II showing loss of normal architecture with infiltration by histocytes. (C) Transverse section of the aorta of group II showing ulceration of the endothelium. (D) Transverse section of the aorta of group III showing histological structures nearly similar to those of group I.
Figure 5.
Immunohistochemical staining of sections from rat aortas. (A):Normal expression of NF-κB in the group I (arrow). (B): Marked activation of NF-κB by methionine supplementation to adult male rats in group II. (C): Decreased activation of NF-κB toward normal by treatment of the male rat by oxytocin in group III.
Group II: The wall of the aorta of group II (Figure 4B) shows thickening of the wall, loss of the normal histological architecture and marked irregularity in the endothelial layer, infiltration of the media with multiple histocytes, endothelial ulceration (Figure 4C). The detection of NF-κB p65 in the aorta isolated from this group demonstrates highly positive reaction in the endothelial cell (Figure 5B).
Group III: The thickness of the walls of the aortas and histological structure of this group similar to those of the control group (Figure 4D). The detection of NF-κB p65 demonstrates in the aorta isolated from this group demonstrates a weak positivity slightly higher than the control group (Figure 5C).
Discussion
The present study was designed to examine the effect of peripheral OT administration on the proinflammatory reaction that leads to atherosclerosis in adult rat model.
In group II where methionine was supplemented in diet to the adult male rats, the plasma levels of total homocystiene were significantly increased than those of the other studied groups which was explained by Hirche et al. [22] who reported that methionine was metabolized into homocystiene. In addition, methionine and its intermediate product can inhibit homocystiene methyltransferase and thereby block the metabolism of Hcy.
HHcy causes oxidant stress by effects on cellular respiration [23]. This finding supports the results of the present study as the plasma levels of NO and GSH were significantly lower and levels of MDA of group II were increased significantly than those of group I and III. However, OT administration to group III increased the plasma levels of NO and GSH and decreased the plasma levels of MDA to normal. The present results demonstrate that oxytocin administration prevented both lipid peroxidation and GSH depletion thereby supports the maintenance of cellular integrity [24]. It appears that the protective effect of OT involves the maintenance of antioxidant capacity in protecting the tissues against oxidative stress. In addition, it was previously demonstrated that oxytocin leads to the release of NO [25].
Oxidative stress may contribute to the deleterious effects of Hcy. Homocysteine induces endothelial cell injury through an oxidant-mediated mechanism [26]. Oxidative stress can stimulate the activation of NF-κB, which plays a pivotal role in the regulation of many genes involved in the inflammatory response. Various genes whose products are putatively involved in the atherosclerotic process are regulated by NF- κB, such as MCP-1 and so forth [27] and this explain the pathological results of the present results as there was significant increase of the expression of NF-κB in the aorta of the rats of group II and significant increased levels of CRP & MCP-1 of this group than those of group I & III. This indicates that the oxidative stress-NF-κB pathway promotes the expression of MCP-1. MCP-1 is a potent chemoattractant protein that stimulates the migration of monocytes into the intima of the arterial walls in the inflammatory response. One of the important early features of AS is monocyte infiltration into the injured arterial wall, followed by differentiation into macrophages. These macrophages then take up large amounts of lipids and become foam cells [28].
The decrease in neutrophil infilteration in response to oxytocin treatment of group III could be mediated by nitric oxide (NO) as it was previously demonstrated that oxytocin leads to the release of NO, which then inhibits the adhesion and aggravation of neutrophil leukocytes [25]. That demonstrates that the pro- inflammatory responses due to methionine supplementation are alleviated by OT treatment through the mechanisms that involve an inhibitory action on tissue neutrophil infiltration, thereby inhibiting the release of ROS and inactivating inflammatory cytokines.
The present findings revealed that OT treatment activated the OT system in adult male rats as there were enhanced OTR mRNA and increased expressions of OTR in the aorta of rats of group III. This document that OTRs are abundant in aortic endothelial cells and this support that these OT and its receptors probably have a role in the damping of the early changes of atherosclerosis and OT system in the aorta may acts locally via an autocrine/ paracrine mechanism in the vascular endothelium [29]. Peripheral administration of OT to the male rats of group III increased the OTR mRNA and the expression of OTR which indicates that OT administration has a direct mechanism through up regulation of its receptors suggesting that OTR may be one factor through which OT mediates its anti-inflammatory effects on the cardiovascular system. These results were supported by Jankowski et al [30], who studied the anti-inflammatory effect of oxytocin in rat myocardial infarction, they found that myocardial infarction significantly reduced OTR mRNA in infarcts compared to the left ventricle of sham-operated rats and OT significantly increased OTR expression in the infarcts. They concluded that cardiac OTR, initially downregulated in the infarcted heart, was subsequently activated in response to OT infusion.
The present results revealed that the plasma levels of (C, TG, HDL, VLDL) did not changed through the studied groups which indicates that the observed anti-inflammatory and anti-atherogenic effects of external OT are not due to changes in lipids. These results are supported by Zhou et al [31] who reported that dietary supplementation with methionine and homocysteine for short term and long term promotes early atherosclerosis but not plaque rupture and they found that plasma levels of total cholesterol were similar in all groups, but TG was mildly reduced in the short-term, high-Met group and HDL-C did not change in the short term group.
Group II exhibited a noticeable inflammatory reaction in term of significant elevated plasma levels of CRP and thickened media intima layer of aorta. In accordance of this finding, Ross [32] indicated that inflammation has a pivotal role in the development of atherosclerosis. Moreover a previous study suggested that measurement of the inflammatory marker CRP, may provide a useful method of assessing risk of cardiovascular disease in apparently healthy persons particularly when lipid levels are normal [33].
The finding that plasma CRP levels were significantly lower in OT treated animals suggests a mechanism whereby visceral adipose tissue IL-6 secretion could be influencing levels of low grade inflammation systemically. This low-grade systemic inflammation is thought to directly impact lesion development through activation of macrophages and endothelial cells [25].
The external administration of physiologically relevant doses of OT to rats to group III lowered BP significantly than the BP values of group II, this was explained by that the OT system can elicit parasympathetic stimulation via acetylcholine release that would act on muscarinic cholinergic receptors to increase intracellular Ca concentrations within vascular cells [34].
The present study revealed that OT treatment to the rats of group III decreased significantly the expression of NF-κB, which leads to decreased the plasma levels of the pro-inflammatory cytokine IL-6, MCP-1 and CRP toward normal levels through decrease their production.
The fact that OT was able to slow the initial development of these lesions in a region of high lesion prevalence suggests that it may be working through mechanisms important during lesion initiation [35].
Conclusion
OT has anti-inflammatory pathway in atherosclerosis. OT modulates the pathophysiological mechanisms thought to be involved in the exacerbation of atherosclerosis in animals as it is capable of dampening the pro-inflammatory cytokine release. This could prevent the endothelial dysfunction characteristic of the early stages of atherosclerosis in adult male rate through upregulation of its receptors in the aorta.
The relationship between homocystiene and OTR in atherosclerosis must be investigated.
References
- 1.Chorianpoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, Frey N. FGF - inducible 14-Kda protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010;105:301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc Natl Acad Sci U S A. 2000;97:6207–6211. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutkowska J, Jankowsi M, Mukaddam-Daher S, McCann SM. Oxytocin is a cardiovascular hormone. Proc Natl Acad Sci U S A. 2000;33:625–633. doi: 10.1590/s0100-879x2000000600003. [DOI] [PubMed] [Google Scholar]
- 4.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Breslow JL. Cardiovascular disease burden increases, NIH funding decreases. Nat Med. 1997;3:600–1. doi: 10.1038/nm0697-600. [DOI] [PubMed] [Google Scholar]
- 6.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–9. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Day I, Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis. 2001;154:277–283. doi: 10.1016/s0021-9150(00)00475-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Ma J, Xia M, Zhu H, Ling W. Mild hyperhomocysteinemia induced by feeding rats diets rich in methionine or deficient in folate promotes early atherosclerotic inflammatory processes. J Nutr. 2004;134:825–830. doi: 10.1093/jn/134.4.825. [DOI] [PubMed] [Google Scholar]
- 10.Petersson M, Lundeberg T, Sohlstrom A, Wilberg U, Uvnas-Moberg U. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:701–704. doi: 10.1007/pl00005227. [DOI] [PubMed] [Google Scholar]
- 11.Biyikli NK, Tugtepe H, Şener G, Velioğlu-Öğünc A, Çetinel S, Midillioğlu Ş, Gedik N, Yeğen BC. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptide. 2006;27:2249–2257. doi: 10.1016/j.peptides.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Allian CC, Pooom LS, Chan SG, Richmond W, Fu PC. Quantitative enzymatic colorimetric determination of total HDL, cholesterol in serum or plasma. Clinc Chem. 1974;20:470–475. [Google Scholar]
- 13.Wahlefeld AW. Methods of enzymatic analysis. Bergmeyer. Vol. 5. New York: H.U. Academic press; 1974. Ouantitative enzymatic colorimetric determination of triglycerides in serum or plasma; pp. 1831–1835. [Google Scholar]
- 14.Finely PR. Enzymatic determination of HDL-C. Clin Chem. 1978;24:931–934. [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of concentrations of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activity cytokines and evidences of independent production. Immuno. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Jayatilleke E, Shaw S. A high-performance liquid chromatographic assay for reduced and oxidized glutathione in biological samples. Anal Biochem. 1993;214:452–57. doi: 10.1006/abio.1993.1522. [DOI] [PubMed] [Google Scholar]
- 19.Frantzen F, Faren AL. An enzyme conversion immunoassay for determinating total homocystiene in plasma and serum. Clinc Chem. 1998;44:311. [PubMed] [Google Scholar]
- 20.Brady M, Bhatia M, Christmas S, Boyd MT, Neoptolemos JP, Slavin J. Expression of the chemokines MCP-1/JE and cytokine induced neutrophil chemoattractant in early acute pancreatitis. Pancreas. 2002;25:260–269. doi: 10.1097/00006676-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Knapp GR, Miller MC. Clinical Epidemiology and Biostatistics 1st Eddition. Baltimoe, Maryland: Williams and Wilkins; 1992. Tests of statistical significance: Regression and Correlation; pp. 255–274. [Google Scholar]
- 22.Hirche FA, Schroder A, Knoth B, Stangl GI, Eder K. Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br J Nutr. 2006;95:879–888. doi: 10.1079/bjn20061729. [DOI] [PubMed] [Google Scholar]
- 23.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 24.Loscalzo J. The oxidant stress of hyperhomocysteinemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegan BC. Oxytocin ameliorates oxidative colonic inflammation by neutrophil-dependent mechanism. Peptide. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Moosmann B, Behl C. Secretory peptide hormones are biochemical antioxidants: structure-activity relationship. Mol Pharmacol. 2002;61:260–8. doi: 10.1124/mol.61.2.260. [DOI] [PubMed] [Google Scholar]
- 27.Hagar HH. Folic acid and vitamin B12 supplementation attenuates isoprenaline-induced myocardial infarction in experimental hyperhomocysteinemic rats. Pharmacol Res. 2002;46:213–219. doi: 10.1016/s1043-6618(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 28.Bowie A, O'Neill LA. Oxidative stress and nuclear factor kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Gutkowska J, Marcinkiewicz M, Rachelska G, Jankowski M. Genistein supplementation stimulates the oxytocin system in the aorta of ovariectomized rats. Cardiovasc Res. 2003;57:186–194. doi: 10.1016/s0008-6363(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 30.Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, Wang Y, Stoyanova E, Cloutier G, Blaise G, Gutkowska J. Antiinflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol. 2010;105:205–218. doi: 10.1007/s00395-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler. Thromb Vasc Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis - an inflammatory disease. New Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Eng J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 34.Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated Apo E-/- Mice. Psychosom Med. 2010;72:376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Woo CWH, Sung FL, Siow YL, Karmin O. Increased monocyte adhesion to aortic endothelialium in rats with hyperhomocysteinemia role of chermokine and adhesion molecules. Arterioscler. Thromb Vasc Biol. 2002;22:1777–1783. doi: 10.1161/01.atv.0000035404.18281.37. [DOI] [PubMed] [Google Scholar]