Abstract
Mycobacterium tuberculosis (MTB) can persist within the human host for years without causing disease, in a syndrome known as latent tuberculosis. The mechanisms by which M. tuberculosis establishes a latent metabolic state is unknown, but it is hypothesized that reduced oxygen tension may trigger the bacillus to enter a state of latency. Therefore, we are studying anaerobic culture of M. tuberculosis (H37RV) as a model of latency. For the first time, the sequential adaptation of latent bacilli (every 90 days for 48 months) viewed under Atomic Force Microscopy (AFM). Two types of adaptation were observed and are described here. First, cells are undergoing temporary adaptation (from 1 to 18 months of latency) that includes; thickening of cell wall (20.5±1.8 nm versus 15.2±1.8 nm, P<0.05), formation of ovoid cells by “folding phenomena”(65-70%), size reduction (0.8±0.1 μm versus 2.5±0.5 μm), and budding type of cell division (20-25%).A second feature include changes that accompany development of specialized cells i.e., production of spore like cells (0.5±0.2 μm) and their progeny (filterable non -acid fast forms; 150 to 300 μm in size). Although, these cells were not real spore because they fail to form a heat resistant colony forming units, after incubation for 35-40 min at 65°C. The filterable non-acid fast forms of bacilli are metabolically active and increased their number by symmetrical type of cell-division. Therefore, survival strategies that developed by M. tuberculosis under oxygen limited condition are linked to its shape, size and conspicuous loss of acid fastness.
Keywords: Latent TB bacilli, oxygen depletion, atomic force microscopy
Introduction
Mycobacterium tuberculosis (MTB), the causative agent of human tuberculosis (TB), is responsible for more deaths in the world than any other infectious agent. This organism is estimated to infect about one- third of the world population [1]. The extraordinarily high numbers of morbidity and mortality are due to the ability of the pathogen to evade an effective immune response and to persist within the host by passing into a latent or persistent state [2]. Such latent TB infections are difficult to diagnose and treat; moreover in adults they can reactivate at any time and are often accompanied by serve destruction of lung tissue [3]. In the last two decades, significant efforts have been invested to develop several in vitro models of Mycobacterium dormancy. However, there is no direct experimental evidence to suggest that how persisting M. tuberculosis cells survive in such a state within lesions. The most widely accepted in vitro model for the transition of M. tuberculosis to an apparently dormant state is Wayne's model [4, 5]. In this model, upon gradual depletion of oxygen from culture, the bacillus exits the cell cycle and develops into a defined dormant form that is adapted to hypoxic and maintains viability for extended periods of time [6]. Growth under such conditions leads to two non-replicating, persistent (NRP) states: a mi-croaerophilic (NRP1) and a later anaerobic state (NRP2) [6]. Wayne model was amongthe best in vitro model of latency, but his hypothesis did not solve the problem on how bacilli may survive the stress generated by activated macrophages, a low PH and a high concentration of radical oxygen and nitrogen intermediates [3, 7]. Further studies have proposed the existence of acid fast, but transiently “non-culturable” bacteria in closed pulmonary lesions [8, 9]. It is perhaps surprising that most available data studied the bacillus from 1 to 12 months of latency, beyond this time the information was limited [3, 5, 10, 11, 12]. In an effort to understand how tubercle bacilli survive in hypoxic environment, we cultured M. tuberculosis under anaerobic conditions. Thereafter, every 90 days for 48 months latent cells were examined by Atomic Force Microscopy (AFM). To our knowledge this is the first sequential study that demonstrates morphological adaptation and life -cycle of TB bacilli under prolong latency periods.
Materials and methods
Strains of M. tuberculosis
All experiments were conducted with M. tuberculosis, H37RV (gifted by van Soolingen D, National Reference TB Laboratory, The Netherlands). Small aliquots of seed stocks were maintained at -70°C and sub-cultured once in liquid medium before inoculation to an experimental culture.
Cultivation in liquid medium
All liquid culture experiments were conducted in Dubos Tween-albumin broth prepared according to the manufacturer's instructions from Dubos broth base (Difco Laboratories Ltd., West Molesey, UK), and Dubos medium albumin (Difco), at a final PH 6.6±0.2. The medium was aseptically dispensed in a appropriate amounts to sterile tubes. Thereafter, strains of H37RV were sub-cultured in this medium until they reached an early exponential growth phase OD600=0.05 (5 × 106 cfu/ml). To produce dormant bacilli, pre-culture were diluted to OD600=0.005 (5 × 105 cfu/ml) and then transferred into series of screw-cap test tubes (20mm by 125mm) with a total volume of 25.5ml [5, 13]. Magnetic stirrers were added; the cultures were sealed by tightly screwing down solid caps with latex liners and stirred gently at 100-120 rpm on magnetic stirring platforms at 37°C. After 20-25 days under this oxygen-limited condition the bacilli were in their dormant state.
Estimation of oxygen consumption
Oxygen depletion was monitored via decolorization of the redox indicator methylene blue (1.5μg/ml) in control tubes. The blue dye fades and finally disappears as oxygen is depleted [5, 6]. Therefore, reduction and decolorization of this dye served as a visual indication of hypoxia. Overall, 10 parallel sets of sealed cultures (17 tubes in each sets) were included in this study. Each 90 days one culture tube from sequential series was taken for further investigations. Growth and survival were measured with spectrophotometer (OD600), and by cell counting [5, 6]. Total cell numbers were counted microscopically with a hemocytometer slide to determine the number of cells in a known volume of medium.
Atomic force microscopy (AFM)
AFM images were recorded in contact mode using an optical lever microscope equipped with a liquid cell (Nanoscope IV Multimode AFM; Veeco Metrology Group LLC, Santa Barbara, CA). To image Mycobacteria, the bacteria were immobilized by mechanical trapping onto Isopore Polycarbonate membrane (Millipore), with pore size similar to the cell size. After filtering a concentrated cell suspension, the filter was gently rinsed with deionized water, carefully cut (1×1 cm), attached to a steel sample puck (Veeco Metrology Group LLC) using a small piece of adhesive tape and the mounted sample was transferred into the AFM liquid cell. Both height and deflection images were recorded, using oxide-sharpened microfabricated Si3N4 cantilevers (Microlevers; Veeco Metrology LLC) with spring constant of 0.01 Nm-1 [14].
Reading methodology
Overall for AFM 15-20, steel sample pack was observed. The expected and observed frequencies of cell shape and cell size in different tube cultures were compared and analyzed by Fisher Exact test. AP value < 0.05 was considered statistically significant. The data presented here are the result of average observation.
Results and discussion
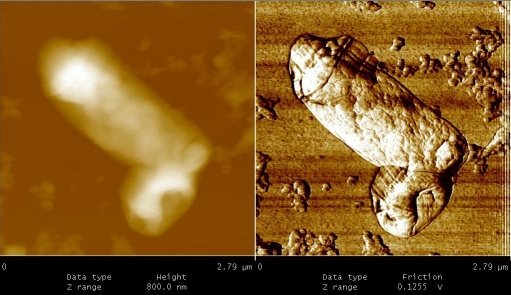
Under AFM, the sequential adaptation in anaerobic culture of M. tuberculosis was documented. In the first three months of oxygen reduction, M. tuberculosis developed a thickened cell wall. In these isolates average thickness of cell wall was 21.5±1.8 nm compared to 15.2±1.3 nm in the aerobically grown M. tuberculosis (P <0.05) (Figures 1 and 2). The thickened wall described in this report may act as a protective coat and may offer protection against hostile environments by restricting the exchange of metabolic activity. Similar changes was reported in anaerobic cultures of M. tuberculosis by other investigators [15, 16]. In addition to cell wall thickening, the latent bacilli (10-15%) reduced their size (0.8±0.1 nm versus 2.5±0.5 nm) (P <0.05) and terminates their symmetrical type of cell-division. Although, sometimes budding was seen (20-25%), in initial phase of dormancy (Figure 3). Recently, we found a co relation between susceptibility patterns and the type of cell division [14, 17]. Therefore, the latent forms of the bacterium might be resistant to anti-tuberculosis drugs [18].
Figure 1.
It shows M. tuberculosis at exponential phase.
Figure 2.
M. tuberculosis under anaerobic cultures developed a dense and homogenous layer that covers the bacilli. The thickness of cell wall reaches to 20.5±1.8 nm.
Figure 3.
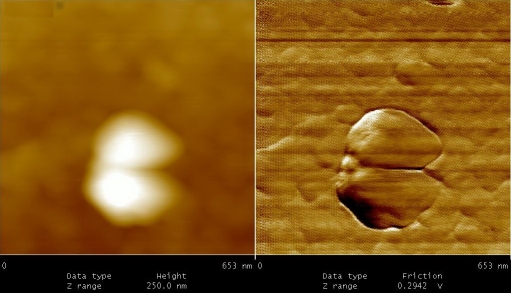
It shows budding type of cell division in M. tuberculosis. This type of division was present till 3 months post latency. Thereafter, no divisions were observed.
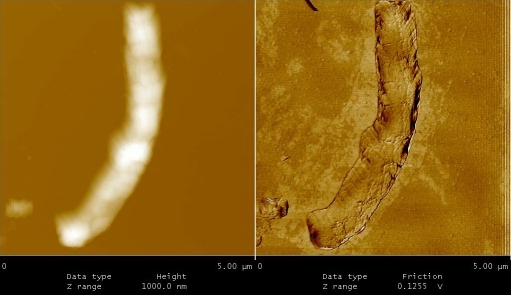
After 3 months of anaerobic conditions “folding phenomena” was observed in latent cells. As shown in Figure 4, rod shape bacteria folded from centre in such way that the two ends of bacillus (i.e. head and tail) approached each other. These cells appeared as an irregular round or oval in shape. Folding phenomena was initially observed in low number of bacilli (10-15%), but their frequencies were progressively increased. In fact, after 9 to 10 months of anaerobic culture majority of M. tuberculosis (65-70%) appeared round or oval in shape. At this stage, only small proportion of cells has their original shape (10-15%). The average size for isolates was 0.9±1 μm. The shape variations in culture of M. tuberculosis under limited conditions such oxygen depletion, exposure to nitric oxide or nutritional deficiency have been reported by many investigators, but their production remained poorly understood[10, 11, 12]. Here, we suggested two different mechanisms for ovoid or round cells production; folding phenomena (65-70%) and budding type of cell division (20-25%). These cells (1 to 18 months of latency) resuscitate by inoculation into fresh media (7H9-ADC-glycerol broth).
Figure 4.
From 4 to 10 months of dormancy” Folding phenomena” observed in latent TB bacilli.
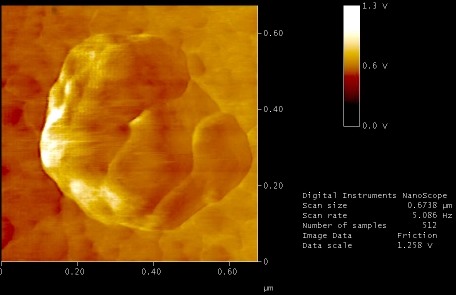
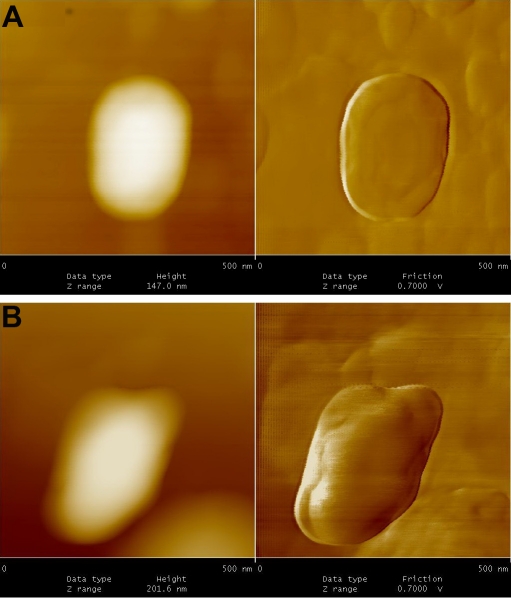
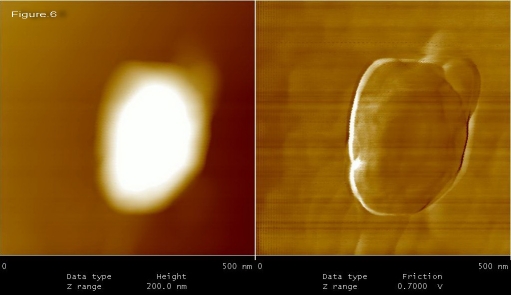
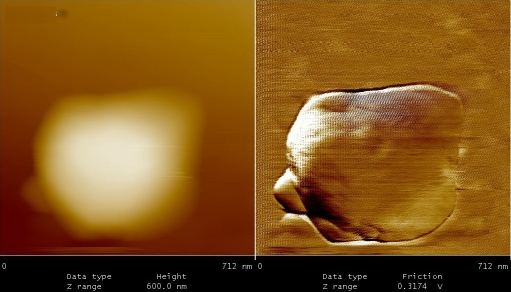
Figures 5 to 9, shows the changes in representative anaerobic culture of M. tuberculosis from 18 to 48 months of latency; alteration in bacilli accompany development of specialized cells i.e., production of spore like cells and their progeny. Spore like cells (0.7 to 0.5 μm in size) was seen from 18 to 22 months of latency (Figure 5a and 5b). The hypothesis of spore production in Mycobacterium was first proposed by Robert Koch in 1882, but his idea was refuted by further investigators [19, 20]. Recent molecular development, identified sigma factor in M. tuberculosis which is similar to SigF sporulation sigma factor from Streptomyces coelicolor [21]. This led to the suggestion that M. tuberculosis may enter a spore -like state during persistent infection [21]. In the same regards, few researchers showed spore like formation in old culture of Mycobacterium species [22]. In our study, spore like cells could not withstand the temp above 65°C and they fail to form a heat resistant colony forming units. Furthermore, these cells were metabolically active (Figure 6), hence, they are not real spore [23]. As it shown in Figure 6, spore like cells ruptured and produced a smaller progeny (Figure 6). These filterable forms (150 to 300 μm in size) are non-acid fast (Figures 7 and 8) and increased their number by symmetrical type of division (Figure 9). Culture of these cells did not resuscitated by inoculation into fresh media (7H9-ADC-glycerol broth). Although, inoculation of these cells (1×105/ml) into peritoneal cavity of mice induced active tuberculosis after 12 weeks (results not shown). During the last decades, non acid fast, cell wall -defective variants forms of tubercle bacilli was subjected of heated controversies [3, 9, 24, 25]. While few investigators isolated them from clinical specimens, others refuse to accept their presence. Even till date it is not clear why homogenized pulmonary lesions of latent TB patients with negative smear microscopy, could induce active tuberculosis in animal models [25, 26]. In the present study, AFM could reveal the first picture of nonacid fast, cell wall defective forms (Figures 7 and 8). These forms were metabolically active and they could recover their original biological properties when transferred into favorable environment (mice model). Thereby, the efforts to eradicate tuberculosis must not solely rely on attacking the actively growing tubercle bacilli but must also include bacilli in the latent stages. In conclusions, two type of alterations was observed in latent TB bacilli; temporary changes in which cell remain acid fast, but developed differences in shape, size and thickness of cell. A second alteration includes development of specialized cells with conspicuous loss of cell wall. This remarkable resistance to adverse conditions along with reversibility is of fundamental importance for the bacteriology, pathology and treatment of tuberculosis. Last but not the least, the sequential adaptation that we demonstrated here may help to improve our understanding on life cycle of latent bacilli and may help scientists to identify targets for novel therapies.
Figure 5.
A.Spore- like cells at 18 months of dormancy. These cells could not withstand the temp at 65 °C, therefore, they are not real spore. B. Spore -like cells at 24 months of dormancy
Figure 9.
Nonacid fast cells increased their number by symmetrical type of cell -division.
Figure 6.
Spore-like cells ruptured and produced a smaller progeny.
Figure 7.
Non acid fast cell wall defective forms of TB bacilli; at 36 months of dormancy.
Figure 8.
These Nonacid-fast cells induced active tuberculosis in mice model.
Acknowledgments
The authors wish to thank the staff of the Microbiology Unit at the Republican Research and practical Centre for Epidemiology and Microbiology (Minsk, Belarus).
Support statement
This study was sponsored by Ministry of Health, Medical Education, Deputy of Research (2009-2011) and National Research Institute of TB & Lung Diseases (NRITLD), Tehran, Iran.
Statement of interest
The authors have no conflicts of interest.
References
- 1.World Health Organization. Geneva: World Health Organi zation; 2001. Global tuberculosis control. WHO report 2001 (WHO/CDS/TB/2001.287) [Google Scholar]
- 2.Ulrichs T, Kaufmann SHE. Mycobacterial resistance and immunity. Front Biosci. 2002;7:458–469. doi: 10.2741/A788. [DOI] [PubMed] [Google Scholar]
- 3.Cardona PJ, Ruiz-Manzano J. On the nature of M. tuberculosis -latent bacilli. Eur Respir J. 2004;24:1044–1051. doi: 10.1183/09031936.04.00072604. [DOI] [PubMed] [Google Scholar]
- 4.Wayne LG. Dormancy of M. tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 5.Wayne LG. Synchronized replication of M. tuberculosis. Infect Immun. 1977;17:528–530. doi: 10.1128/iai.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayne LG. Dynamics of submerged growth of M. tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1979;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich T, Kaufmann SHE. Cell-mediated immune response. In: Rom WN, Garay SM, editors. Tuberculosis. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 251–262. [Google Scholar]
- 8.Meylan PRA, Richman DD, Konbluth RS. Reduced intracellular growth of mycobacteria in human macrophages cultivated at physiologic oxygen pressure. Am Rev Respir Dis. 1992;145:947–953. doi: 10.1164/ajrccm/145.4_Pt_1.947. [DOI] [PubMed] [Google Scholar]
- 9.Khomenko AG. The variability of M. tuberculosis in patients with cavitary pulmonary tuberculosis in the course of chemotherapy. Tubercle. 1987;68:243–253. doi: 10.1016/0041-3879(87)90064-x. [DOI] [PubMed] [Google Scholar]
- 10.Shleeva MO, Kudykina YK, Vostroknutova GN, Suzina NE, Mulyukin AL, Kaprelyants AS. Dormant ovoid cells of M. tuberculosis are formed in response to gradual external acidification. Tuberculosis. 2011;91:146. doi: 10.1016/j.tube.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 11.McCune RM, Tompsett R. Fate of M. tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug susceptibile tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp Med. 1965;104:737–760. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manabe YC, Bishai WR. Latent M. tuberculosis -persistence, patience and winning by waiting. Nat Med. 2000;16:327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 13.Qei BM, Dick T. Bactericidal activity of nitro furans against growing and dormant M. bovis BCG. J Antimicrob Chemother. 2000;46:917–919. doi: 10.1093/jac/46.6.917. [DOI] [PubMed] [Google Scholar]
- 14.Farnia P, Masjedi MR, Farnia P, Merza MA, Tabarsi P, Zhavnerko GK, Ibrahim TA, Kuan HO, Ghanavei J, Farnia P, Ranjbar R, Poleschuyk NN, Titov LP, Owlia P, Kazampour M, Setare M, Sheikolslami M, Migliori GB, Velayati AA. Growth and cell -division in extensive (XDR) and extremely drug resistany (XXDR) tuberculosis strains: transmission and atomic force observation. Int J Clin Exp Med. 2010;3:320–326. [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: Cell wall thickening and localization of the 16-kilodalton α-Crystallin Homology. J. Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahl J. Electron microscopy analysis of M. tuberculosis cell division. FEMS Microbiol Lett. 2004;240:15–20. doi: 10.1016/j.femsle.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Velayati AA, Farnia P, Merza MA, Zhavnerko GK, Tabarsi P, Titov LP, Ghanavei J, Farnia P, Setare M, Poleschuyk NN, Owlia P, Sheikolslami M, Ranjbar R, Masjedi MR. New insight into extremely drug-resistant tuberculosis: using atomic force microscopy. Eur Respir J. 2010;36:1490–3. doi: 10.1183/09031936.00064510. [DOI] [PubMed] [Google Scholar]
- 18.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of M. tuberculosis. Antimicrob Agents Chemother. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch R. Die Aetiologie der Tuberculose. 1882;66:19–222. [PubMed] [Google Scholar]
- 20.Much H. Über die granulare, nach Ziehl nicht daesellbane farbbare from des tuberkulosevirus. Beitrage Klinisches Tuberkulose. 1907;63:8–85. [Google Scholar]
- 21.De Maio J, Zhang Y, Ko C, Zhang DB, Bishai WR. A stationary phase, stress response sigma factor from M. tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh L, Larsson P, Singh B, Pettersson BM, Islam NM, Sarkar SN. Sporulation in mycobacteria. Proc Natl Acad Sci USA. 2009;106:10781–10786. doi: 10.1073/pnas.0904104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tragg BA, Driks A, Stragier P, Bitter W, Broussard G, Hatfull G, Chu F, Adams KN, Ramakrishnan L, Losick R. Do Mycobacteria produce endospore? Proc Natl Acad Sci USA. 2010;107:878–881. doi: 10.1073/pnas.0911299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opie EL, Anderson JD. Tubercle bacilli in latent tuberculosis lesions and in lung tissue without tuberculosis lesions. Arch Pathol Lab Med. 1927;4:1–21. [Google Scholar]
- 25.Nyka W. Studies on the effect of starvation on Mycobacteria. Infect Immun. 1974;9:843–850. doi: 10.1128/iai.9.5.843-850.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in M tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]