Abstract
Tendon ruptures are common sports-related injuries that are often treated surgically by the use of sutures followed by immobilization. However, tendon repair by standard technique is associated with long healing time and often suboptimal repair. Methods to enhance tendon repair time as well as the quality of repair are currently unmet clinical needs. Our hypothesis is that the introduction of a unique stem cell population at the site of tendon transection would result in an improved rate and quality of repair. Achilles tendons of fifty-one Sprague-Dawley rats were transected and suture-repaired. In half of the rats, a biodegradable scaffold seeded with allogenic circulating stem cells was placed as an onlay to the defect site in addition to the suture repair. The other half was treated with suture alone to serve as the control group. Animals were randomized to a two-, four-, or six-week time group. At the time of necropsy, tendons were harvested and prepared for either biomechanical or histological analysis. Histological slides were evaluated in a blinded fashion with the use of a grading scale. By two weeks, the experimental group demonstrated a significant improvement in repair compared to controls with no failures. Average histological scores of 0.6 and 2.6 were observed for the experimental and control group respectively. The experimental group demonstrated complete bridging of the transection site with parallel collagen fiber arrangement. By four weeks, both groups showed a continuing trend of healing, with the scaffold group exceeding the histological quality of the tissue repaired with suture alone. Biomechanically, the experimental group had a decreasing cross-sectional area with time which was also associated with a significant increase in the ultimate tensile strength of the tendons, reaching 4.2MPa by six weeks. The experimental group also achieved a significantly higher elastic toughness by six weeks and saw an increase in the tensile modulus, reaching 31Mpa by six weeks. The use of circulating stem cells as an adjunct in tendon repair demonstrates superior biomechanical properties and an improved level of histological organization, when compared to the suture alone control group. These improvements were not previously observed when gene therapy, protein therapy, or current tissue engineering technologies were used.
Keywords: Orthopedic surgery, sports medicine, achilles tendon, tendon repair, circulating stem cells, tendon biome-chanics
Introduction
Tendon injuries ranging from partial to full thickness tears are some of the most common sports related injuries. These injuries can result in significant disability and dysfunction and can be devastating for both professional and amateur athletes alike. Partial tendon ruptures are often treated conservatively, while full thickness or chronic partial thickness ruptures will often require surgical intervention [1]. Current surgical treatments involve direct end-to-end repair when possible, versus the use of autografts, allografts, xenografts, synthetic polymers, or resorbable biomaterials (when large defects cannot be repaired primarily) [1].
Rehabilitation following tendon injury and repair is a long process which often lasts several weeks. During this time, immobilization and protected motion are required [2]. These restrictions put the healing tendon at risk for complications such as adhesions and a poorer quality of repair [1]. Despite the extensive remodeling that occurs, the biochemical and mechanical properties of healed tendons never match those of intact tendons. Healing of tendons in humans has been found to show remodeling for up to one year with inferior biomechanical properties when compared to normal uninjured tissue [3]. In a study of transected sheep Achilles tendons that had healed, the rupture force was only 56.7% of the normal value at 12 months [1]. This may be related to the absence of mechanical loading during the period of immobilization. Collagen that stays unstressed during the proliferative and remodeling phases remains haphazard in organization and is weaker than stressed collagen [4]. The ability to have a repaired tendon initiate early protected motion would have significant implications on the final outcome. Establishing adequate tendon healing for tendons that are capable of withstanding tension is presently a limiting factor for early motion.
Methods to enhance tendon repair time as well as the quality of repair are currently an unmet clinical need. Several strategies are currently under investigation for enhancement of tendon repairs: extracorporeal shock wave therapy, pulsed magnetic fields, gene therapy, modification of cytokines and growth factors, and tissue engineering with stem cells [1]. Mesenchymal stem cells have attracted considerable attention for their ability to undergo differentiation into a variety of specialized mesenchymal tissues including tenocytes. Several well-conducted studies have looked at the use of stem cells in the repair of tendons [5-8]. These studies have demonstrated various degrees of improvement in stem cell-mediated repairs. Biomechanical, histological, and speed of regeneration have all been investigated with positive but sometimes inconsistent results, suggesting the need for further research. The current study evaluates the use of stem cells harvested from the circulating blood in order to augment the repair of ruptured tendons. Our hypothesis is that the introduction of this unique circulating stem cell population will result in an improved rate and quality of repair than has been previously found with mesenchymal stem cell-augmented repair.
Methods
Animal model
Fifty-one adult male Sprague-Dawley Rats were acquired and acclimated in individual cages for at least one week before any surgical procedures. Each rat subsequently underwent an index procedure and was assigned to either an experimental or control group. Histomorphometric analysis of the surgical site was performed at a period of two weeks and four weeks after surgery. Biomechanical analysis of the repaired tendon was performed at two, four and six week time points. The use of animals was in accordance with protocols approved by the Institutional Animal Care and Use Committee at the North Shore-Long Island Jewish Health System/the Feinstein Institute for Medical Research.
Stem cell preparation
Circulating stem cells (CSC) were isolated using a sponge wound model. Subcutaneously-implanted polyurethane sponges (1×1×0.25cm) were surgically placed in a group of ten male Sprague-Dawley Rats under a separate experimental protocol. The induction of a local inflammatory response results in sponge infiltration by circulating stem cells. These sponges were retrieved at two weeks time. Creation of single cell suspension was achieved using collagenase, DNase, and protease digestion. Stem cells were isolated by Percoll density gradient centrifugation and subsequently cultured at 37°C, 5% CO2 in standard tissue media (M199, FBS) on Matrigel-coated flasks. These cells were loaded onto PGA nonwoven scaffolds to be used in the current study.
Surgical technique
The animals were anesthetized in accordance with IACUC protocol. The skin overlying the Achilles tendon was shaved using surgical clippers. With the use of an aseptic technique, a longitudinal skin incision was made directly over the Achilles tendon. A complete transverse laceration was made with a surgical blade through the midsubstance of the Achilles tendon to simulate tendon rupture. All transected tendons were immediately repaired with 4-0 Vicryl sutures (Ethicon) using a Mason Allen stitch. In half of the rats (n=25), a PGA nonwoven scaffold seeded with allogenic stem cells was attached as an onlay to the defect site. The other half (n=26) was treated with suture repair alone to serve as the control group. Skin closure was performed in a routine manner with use of Vicryl sutures. The animals were not immobilized post-operatively. After 2, 4, or 6 weeks post surgery, the animals were sacrificed and the tendons were harvested for histological and biomechanical analysis.
Histology and morphometry
At the time of necropsy, tendons were harvested, fixed in formalin, decalcified, and paraffin embedded. Samples were sectioned and stained with hematoxylin and eosin. Histological evaluation using a scoring system previously described was conducted on samples from the two- and four-week time points [5]. This system takes into account the orientation and degree of organization of the repaired tendon tissue. Scores range from 0 to 3 with 0 being normal tendon architecture and 3 characterized by marked changes with greater than 50% disorganization in collagen architecture [1]. All researchers were blinded to the treatment groups.
Biomechanical testing
Biomechanical testing was performed at two-, four-, and six-weeks post surgery. Upon animal sacrifice, the Achilles tendon was dissected, leaving the bone-tendon interface intact, and then frozen at -80°C. On the day of testing, the samples were thawed individually at 4°C, excess muscle was removed, and the bone was trimmed with clippers to facilitate mounting. All mechanical testing was performed on an In-stron testing frame (Model 5566) where samples were tested while submersed in a bath containing PBS. Precise displacement control was applied to each specimen, and the resulting load was measured using a 100N load cell with load accuracy of +/−0.5%. All data acquisition and device control was through a personal computer, where data was acquired at 10 Hz.
Samples were tested in uniaxial tension to determine the tensile stiffness (S) and modulus (E), and elastic toughness. Specimens were mounted in hydraulic grips between two roughened surface plates and sand paper, in order to prevent slippage of the sample during pull testing. A pre-load of 0.5N was applied to the specimens, and the length of the specimen was recorded. Tensile displacement was applied to the samples at a strain rate of 0.1 %/sec until failure. After failure, the dimensions of the fracture surface were measured with calipers to calculate the effective cross-sectional area of each sample. The tensile modulus was determined from the linear portion of the stress-strain curve. Ultimate tensile strength (UTS) was calculated from the maximum load and fractured surface area. Elastic toughness (K) was also calculated numerically using a Riemann sum method. Results were compared against a database of previously published control samples using the same suture tendon repair technique described in this study [1].
Results
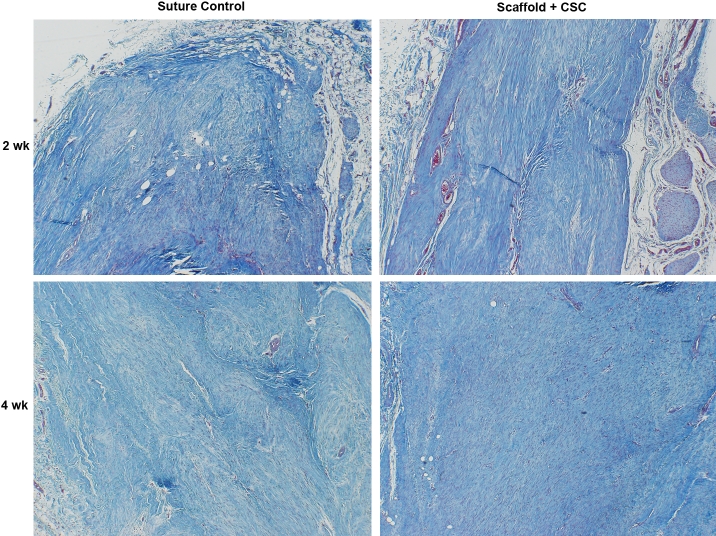
All animals enrolled in the study tolerated the surgery well with no post-operative complications. Analysis of the histological scores of the scaffold + CSC group demonstrated significant improvement in repair compared to suture-only controls. By two weeks, the scaffold + CSC group had an average histological scores of 0.6±0.4SD, which was significantly greater than the repair seen in the control only group (2.6±0.7SD; p<0.05). The scaffold repair demonstrated complete bridging of the transection site with parallel collagen fiber arrangement (Figure 1). Control repairs resulted in a repair characterized by collagen disorganization and instances of lack of bridging of tendon tissue (Figure 1). By four weeks, both groups showed a continuing trend of healing, with the scaffold group exceeding the histological quality of the tissue repaired with suture alone (Figure 1). The scaffold + CSC group had a decreasing cross-sectional area with time, (0.167±0.04 at two weeks vs. 0.134±0.03 at six weeks, p<0.05). This was also associated with a significant increase in the UTS of the tendons, reaching 4.2MPa by six weeks (Figure 2; p<0.05). An increase in the tensile modulus was seen over time (Figure 2), reaching 31Mpa by six weeks (p<0.05). The scaffold + CSC group also achieved a significantly higher elastic toughness by six weeks.
Figure 1.
Circulating stem cell augmented repair (right) vs. control (left) at 2 weeks (top) and 4 weeks (bottom) ×200 the original magnification (Mallory' trichrome Stain). Note the improved organization of the experimental groups four weeks post-operatively.
Figure 2.
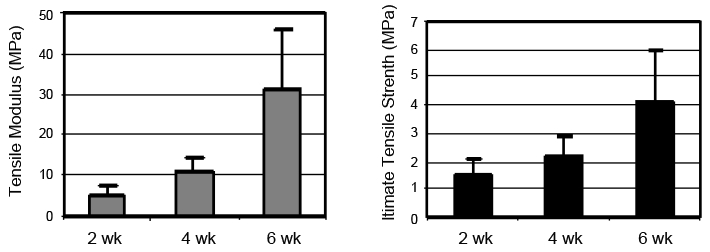
Tensile modulus (left) and UTS (right) of repair with CSCs.
Discussion
The findings from this study support our hypothesis that circulating stem cells, when used as an adjunct in tendon repair, significantly improved not only rate of repair, but contributed to a level of collagen organization not previously observed using gene therapy, protein therapy, or current tissue engineering technologies. Although the use of mesenchymal stem cells has been associated with ectopic bone and cartilage formation [1], there was no evidence of this occurrence in our samples. Of note, there was no increase in angiogenesis within the tendon tissue which, if present, could translate into reduced biomechanical strength. Increased intra-substance vascularity is often associated with healing tissues, including tendon. This improvement in tendon tissue regeneration in the absence of increased angiogenesis suggests either unique cellular qualities exhibited by our stem cells or a novel mechanism facilitating tendon repair. It is unclear as to whether the repair was primarily due to the implanted cells on scaffold, or whether the presence of the CSCs stimulated the repair process of the native tendon fibroblasts.
The addition of circulating stem cells to the site of injury also appears to have improved the strength of the repair tissue as demonstrated via biomechanical testing. The UTS, tensile modulus, and the elastic toughness were found to increase over time, reaching significantly higher values than suture only controls. This is clinically noteworthy as most tendon injuries have been shown to heal with a final strength that is markedly below pre-injury baseline strength [6]. A decrease in cross sectional area at the site of injury was also found to be associated with the addition of circulating stem cells suggesting a higher degree of remodeling. Limiting scar formation associated with a tendon injury is crucial especially in flexor tendon lacerations of the hand where repairs must not only be strong, but also capable of passing through the tendon sheath and the associated pulley system of the fingers.
The use of circulating stem cells has been successfully incorporated into other facets of medicine with few adverse reactions and minimal complications [1]. The ability to increase retrieval of these cells has been enhanced in humans by using Lenograstim (Granulocyte 34, Aventis Pharma) [9]. These autologous circulating stem cells have been successfully used to re -establish hematopoietic recovery following the treatment of acute myeloid leukemia [1, 2]. They have also seen utilization in the field of interventional cardiology in the treatment of myocardial damage following acute myocardial infarction [10].
Although making headway in the treatment of other diseases, the use of this circulating stem cell population in the treatment of common musculoskeletal injuries has been limited as is evidenced by the scarcity of literature evaluating its efficacy. In future studies, the addition of controlled mechanical loading during the repair process will be explored. This could be useful to evaluate both the histological and biomechanical consequences of early range of motion and from a practical standpoint may contribute to new post repair rehabilitation protocols in humans. Cell labeling for in vivo tracking of the cells will also be performed to determine the CSCs role in this enhanced repair process. It would also be useful in determining which area of the tendon these cells take up residence as tenocyte function has been found to vary depending on the region of origin [1]. Cell-to-collagen ratio has been evaluated in stem cell seeded construct as described by Juncosa et al. Accordingly, the use of different cell concentrations should be evaluated to examine a dosage dependent response in-vivo.
The basic cellular biology of tendon repair is still not fully understood. Although significant progress has been made, further work remains to overcome the challenges and morbidity associated with tendon injuries.
Acknowledgments
This study was funded by a grant from Embro, Inc.
References
- 1.Kvist M. Achilles tendon injuries in athletes. Sports Med. 1994;18:173. doi: 10.2165/00007256-199418030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Winter E, Weise K, Weller S, Ambacher T. Surgical repair of Achilles tendon rupture. Comparison of surgical with conservative treatment. Arch Orthop Trauma Surg. 1998;117:364. doi: 10.1007/s004020050267. [DOI] [PubMed] [Google Scholar]
- 3.Thein TB, Becker JH, Theis JC. Rehabilitation after surgery for flexor tendon injuries in the hand. Cochrane Database System Rev. 2004;4:CD003979. doi: 10.1002/14651858.CD003979.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Rosberg HE, Carlsson KS, Hojgard S, Lindgren B, Lundborg G, Dahlin LB. What determines the costs of repair and rehabilitation of flexor tendon injuries in zone II? A multiple regression analysis of data from southern Sweden. J Hand Surg [Br] 2003;28:106–112. doi: 10.1016/s0266-7681(02)00352-2. [DOI] [PubMed] [Google Scholar]
- 5.Indelicato PA. Isolated medial collateral ligament injuries in the knee. J Am Acad Orthop Surg. 1995;3:9–14. doi: 10.5435/00124635-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bruns J, Kampen J, Kahrs J, Plitz W. Achilles tendon rupture: experimental results on spontaneous repair in a sheep model. Knee Surg Sports Tramatol Arthrosc. 2000;8:364–9. doi: 10.1007/s001670000149. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto E, Hayashi K, Yamamoto N. Mechanical properties of collagen fascicles from stress-shielded patellar tendons in the rabbit. Clin Biomech. 1999;14:418–425. doi: 10.1016/s0268-0033(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Maffulli N. Tendon injury and ten-donopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 9.Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH. Tissue engineering of ligament and tendon healing. Clinical Orthopaedics and Related Research. 1999;367:312–323. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 10.Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendonhealing in a rabbit achilles tendon model. J Bone Joint Surg Am. 2007;89:74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 11.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AL. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Engineering. 1999;5:267–77. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 12.Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Butler DL. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Engineering. 2006;12:681–9. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- 13.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 14.Dines J, et al. Transactions of the Orthopedic Research Society. 2007 Paper #27. [Google Scholar]
- 15.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Porat Y, Belkin D, Belleli A, Elkayam J, Shimoni D, Porozov S, Sarel I, Ash I, Fulga V. Challenges in the development of autologous cell therapy products. BioProcessing Journal. 2007;6:46–53. [Google Scholar]
- 17.Pompilio G, Cannata A, Peccatori F, Bertolini F, Nascimbene A, Capogrossi MC, Biglioli P. Autologous peripheral blood stem cell transplantation for myocardial regeneration: a novel strategy for cell collection and surgical injection. Ann Thoracic Surg. 2004;78:1808–12. doi: 10.1016/j.athoracsur.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 18.Raos M, Nemet D, Bojanic I, Sertic D, Batinic D, Dusak V, Dubravcic K, Mazic S, Serventi-Seiwerth R, Mrsic M, Golubic-Cepulic B, Labar B. Collection and composition of autologous peripheral blood stem cells graft in patients with acute myeloid leukemia: influence on hematopoietic recovery and outcome. Coll Antropol. 2010;34:105–15. [PubMed] [Google Scholar]
- 19.Porat Y, Porozov S, Belkin D, Shimoni D, Fisher Y, Belleli A, Czeiger D, Silverman WF, Belkin M, Battler A, Fulga V, Savion N. Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial, and neural lineages. Br J Haematol. 2006;135:703–14. doi: 10.1111/j.1365-2141.2006.06344.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein MB, Pham H, Yalamanchi N, Chang J. Flexor tendon wound healing in vitro: the effect of lactate on tendon cell proliferation and collagen production. J Hand Surg Am. 2001;26:847–854. doi: 10.1053/jhsu.2001.26185. [DOI] [PubMed] [Google Scholar]