Abstract
Measuring hormone metabolites from excreta is a powerful method to study hormone–behavior relationships. Currently, fecal corticosterone metabolite concentrations are used to estimate individual short-term stress responses. From the free-roaming, semitame flock of greylag geese (Anser anser), as many fecal samples as possible were collected over 3 h following a challenge (social density stress) or in a control situation. This time span corresponds to the gut passage time of geese. It was asked how many samples were necessary to determine differences in excreted corticosterone immunoreactive metabolites (CORTs) between control and social density stress and which parameters (means, maxima, range) reliably showed this difference. A large variation of CORT was found between consecutive samples. Still, means, maxima, and ranges of the samples in a fecal series consistently showed the response to a stressor both within and between individuals. Three samples sufficed if the maximum value of CORT was used, whereas four or more samples were necessary to work with the mean. It was concluded that by increasing the number of fecal samples collected, the course of CORT could be measured more precisely and an individual’s acute stress response inferred more reliably.
Keywords: corticosterone–immunoreactive metabolites, sampling effort, noninvasive sampling, individual variation, short-term stress response, greylag goose, Anser anser
INTRODUCTION
The method of invasive sampling of circulating hormones from blood is well established; however, it may also have disadvantages. For example, blood samples show concentrations that occur in a very narrow time frame.1 Plasma glucocorticoids, for example, can vary with time of the day and have pulsatile secretory patterns;2–4 therefore, timing of when to sample becomes an issue. Alternatively, hormone metabolites from excretion products may show integration over a certain period.
Gut passage time and the dynamics of excretion determine the temporal resolution of the method. In geese, fecal samples are assumed to represent an integrated, proportional record of the plasma level within a frame of 2–4 h prior to defecation.5 Short-term hormonal changes in reaction to specific situations were measured from feces,6 as were long-term endocrine profiles due to seasonal variations7–9 or patterns during stress-related disorders.10 One potential disadvantage of fecal sampling is that the time course of excretion varies widely between species, ranging from a maximum of days in mammals11 to a low of hours or minutes in passerine12,13 and nonpasserine14 birds. Another disadvantage is that the sensitivity of the method can vary between different assays, and results can differ between seasons9 and weather conditions.15
Individuals differ greatly in their stress-induced glucocorticoid responses.12,16,17 These interindividual differences might show an individual’s ability to cope with potentially unfavorable environmental demands or challenging situations within the social context. The latter topic is of key interest in our study on our flock of free-ranging, semitame greylag geese (Anser anser). Generally, we are interested in answering questions pertaining to individual costs and benefits of living socially. We intend to determine individual variation in response to a variety of stressors within the intact social environment as well as stress-reducing effects of social partners.18 Because our geese are human acquainted, we opted to collect excreta, as has been done in the past.7,8,19
It was our goal to optimize the resolution of the noninvasive approach of fecal hormone collection through increasing our sampling efforts—that is, collection of as many fecal samples as possible and determining how many samples were necessary to consistently find a difference in excreted corticosterone immunoreactive metabolites (CORTs) between control and an experimental challenge. We also wanted to establish which parameter (mean or maximum) was most reliable in finding this difference. In addition, we asked whether CORT would show consistent interindividual variation.
We intended to record exact metabolite profiles of individual geese to see how CORT develops in a series of consecutive fecal samples within 3 h. Furthermore, we wanted to determine whether we could detect the short-term stress response and pinpoint if there was a peak excretion of CORT after a certain time after a challenge. We were also interested in determining how large the variation was in a fecal series collected over that time and whether there were comparable patterns within the series of one individual.
MATERIALS AND METHODS
Animals
A nonmigratory flock of greylag geese was introduced into the Upper Austrian valley of the Alm River by Konrad Lorenz in 1973,20 and individual life history data and social backgrounds of all individuals have been continuously monitored since then.21 Individuals are unrestrained and roam the valley between the Konrad Lorenz Forschungsstelle (KLF) and a lake approximately 10 km to the south, where they roost at night. Geese are supplied with pellets and grain twice daily in the meadows around the research station, with low quantities from spring to fall and with sustaining quantities during winter. The flock is subjected to natural selection, and losses to predators, the most common being red foxes (Vulpes vulpes), white-tailed eagles (Haliaeetus albicilla), and golden eagles (Aquila chrysaetos), and may account for up to 10% of the flock loss per year.22 The flock consisted of approximately 170 individuals at the time of data collection. All are marked with colored leg bands. In addition to being raised by hand, the geese readily breed in the valley, either at natural nest sites or breeding boxes provided by the KLF. All geese are habituated to the presence of humans,6 and goose-raised flock members neither show avoidance if approached to a distace of 1 m, nor do they excrete elevated levels of corticosterone-immunoreactive metabolites following such situations (Frigerio, unpublished data). This finding indicates that human presence does not cause stress and probably does not negatively affect agonistic motivation even in goose-raised geese. More detailed information of flock demography is presented elsewhere.23,24
Data Collection and Analysis
Our main research goal was to quantify costs of behavior in individual greylag geese, and we therefore continuously collected data and fecal samples for extraction of hormone metabolites—in particular, CORT and testosterone–immunoreactive metabolites. In general, data collection was such that we observed the geese starting from the morning feeding for up to 1 h. Observations were performed either on a “control day” with no experimental stressor or on a “stress day” when some sort of stressor was presented. Data presented here were collected under a “social density stress” feeding situation. During this experiment, food was spread widely (~160 m2) on a control day, whereas the same amount of food was spread over a much closer area (~40 m2, social density stress). This situation has been previously shown to induce a competitive feeding situation25 (Frigerio, unpublished data) and actually produces an increased excretion of corticosterone–immunoreactive metabolites (Frigerio and Scheiber, unpublished data).
From the beginning of the distribution of food until 3 h thereafter, we collected fecal samples for extraction of CORT. Because geese defecated up to 11 times (mean ± SE: 7.15 ± 0.212) within 3 h, we attempted to collect short series of samples of feces per individual per observation day. In geese, fecal samples represent an integrated, proportional record of the plasma level within a frame of 2–4 h prior to defecation,5,14,26 as gut passage time is not constant. To avoid the effect of diurnal variation, we collected individual fecal samples only during morning hours. Our sampling started well after the early morning peak of endogenous corticosterone.27 Because separation of urine and feces in geese is not entirely possible,35 we collected and analyzed both together. Samples were frozen at −20°C within 1 h after collection.
We assayed fecal samples with an enzyme immunoassay (EIA).8,28 Fecal samples (0.5 g) were extracted in methanol and hydrolyzed as described by Kotrschal et al.8 Recently, a new group-specific antibody was developed (Möstl et al., unpublished data) that recognizes groups of metabolites (i.e., 5b,3a,11b-diol glucocorticoid metabolites) other than those previously used, which recognized 11b,210H,20-oxo-corticosterone metabolites. To assess the resolution of the 5β,3α,11β-diol glucocorticoid metabolite assay, and to validate the procedure, we also collected fecal samples from four domestic geese (Anser domesticus) in collaboration with the Department of Veterinary Medicine, University of Vienna (Möstl et al., unpublished data). In this case, we collected every dropping over the course of 16 h, that is, from dusk to dawn, from four geese: two males and two females (range: 27–49 droppings). The recently developed assay was validated both through an experiment infusing trace amounts of radioactive-labeled hormone and an ACTH challenge experiment (Möstl et al., unpublished data). We found that the new assay is considerably more sensitive in the biological sense, resulting in higher peak values in the same sample.15 Therefore, all data presented were analyzed using the 5β,3α,11β-diol glucocorticoid metabolite assay.
Concentration limits for reliable measurements ranged from 0.15 ng/g to 250.5 ng/g. Intra- and interassay coefficients of variation were determined from homogenized pool samples. The mean intraassay coefficient of variation was 7.4%, and the interassay coefficient of variation was 13.2%.
For this study, we combined data from individuals that defecated either six (N = 18), seven (N = 11), eight (N = 6), or nine (N = 3) times in 3 h, which yielded 38 individual fecal series. To avoid pseudoreplication, each individual was used only once per situation (Ncontrol = 9; Nsocial density stress = 11). However, some were used once in control and once in stress (Ncontrol/social density stress = 9). If more than one series of a particular individual was available, we picked the longest series.
Data Analysis
Data were analyzed using the SPSS statistical package.29 Results of all tests are two-tailed, and the level of significance was set to 0.05. Whether data were normally distributed was tested with Shapiro–Wilk tests, and appropriate parametric or nonparametric tests were then applied accordingly. Fecal samples were collected from male and female geese both in control and social density stress feeding situations. We analyzed possible differences between the sexes with Student’s t-tests.
We determined whether differences existed between control and social density stress situations within the following parameters: (a) defecation intervals, (b) CORT means of all samples per series, (c) CORT maxima of the individual series, (d) CORT range of the samples in the series, and (e) coefficients of variance. Defecation intervals were defined as the elapsed time between the first and last sample divided by the length of the series, that is, the number of samples in the series. The range of the series was defined as the difference of the sample with the lowest concentrations of CORT within a series subtracted from the highest concentration of CORT within the same series. To compare the relative amount of variation of CORT within the series, we calculated the coefficient of variance, which determines the spread of the standard deviation from the mean as a percentage. Statistical comparisons were performed with parametric Student’s t-tests.
Next we compared the differences in control and social density stress feeding situations in the nine individuals for which we had data in both situations: We compared individual means, maxima, ranges, and the variance coefficients with paired Student’s t-tests.
To determine whether there was an excretion peak in CORT following a challenge after a certain time, we conducted Kruskal–Wallis ANOVAs, comparing the occurrence of maxima over ten 15-min intervals, starting from 30 min after the beginning of the feeding situation and continuing until 3 h after the feeding started.
The change of concentration between two consecutive samples over time was defined as the slope of CORT changes over time. Because data were not normally distributed, we calculated differences between the possible combinations with nonparametric Wilcoxon Signed Rank tests.
Often it is impossible to collect every dropping of an individual goose over a certain period, because, for example, defecation might occur in the water. Therefore, we decided to determine the number of samples necessary to predictably find the difference in CORT between control and social density stress, as well as which parameter is most likely to find this difference. In an attempt to mimic the collection of an incomplete series, we reduced all original six-sample series by one randomly chosen sample to obtain a five-sample series. This procedure was repeated five times. If we still found significant differences, we continued to reduce the original six-sample series by two, etc. We then calculated whether we could still find the difference in CORT between control and social density stress, using either the mean or the maximum. Because test results were equivocal when we reduced the original series by two, three, or four samples—some test results within these reduced series were significant, whereas others were not—we wanted to pinpoint the exact number of samples necessary to ensure that significant differences did not just occur by chance. We therefore repeated the reduction procedure as previously described five more times to get a total of 10 repetitions. We then used binomial tests to determine whether the difference in either means or maxima was statistically different between the four- and three-sample series or between the three- and two-sample series.
RESULTS
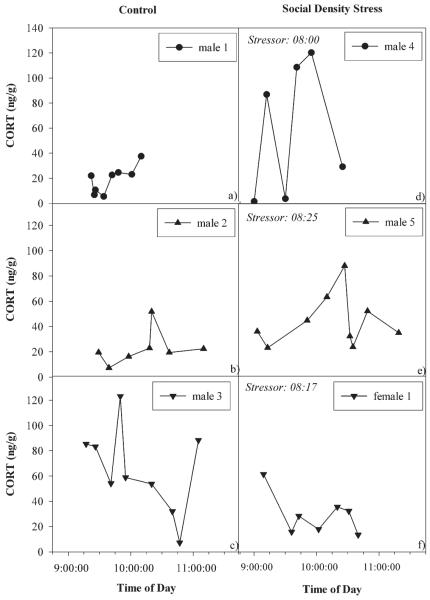
Within a series, concentrations can vary widely from one dropping to the next, both in control (Fig. 1a–1c) and experimental stress conditions (Fig. 1d–1f). Fluctuations in CORT over the course of the day are shown in Figure 2.
Figure 1.
Examples of CORT excretion profiles over time during control (a)–(c) and social density stress feeding (d)–(f) in nanograms of CORT per gram of feces. Beginning of the social density stress feeding situation is depicted in the upper right corner of panels (d)–(f).
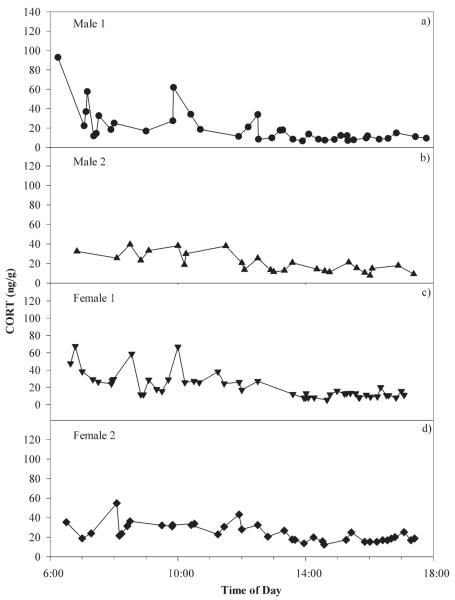
Figure 2.
CORT excretion profiles of four domestic geese over time during control feeding in nanograms of CORT per gram of feces. Series are of different lengthes: (a) filled circle, 38-sample series; (b) filled triange, 49-sample series; (c) inverted, filled triange, 27-sample series; and (d) filled diamond, 38-sample series. Individuals in (a) and (b) are male; those in (c) and (d) are female.
CORT values (expressed in nanograms per gram) were normally distributed both in control and social density stress in males and females. There was no significant difference of mean CORT between the genders either in control (females: N = 10; ; males: N = 7; ; Student’s t-test corrected for unequal variances: T = = −0.512; df = 8.827; P = .627) or during social density stress (females: N = 13; ; males: N = 8; ; Student’s t-test: T = −1.030; df = 19; P = .316). Therefore, we combined data form both genders in further analyses.
Geese defecation intervals were shorter during social density stress feedings (N = 21) than during control feedings (N = 17) (control: ; social density stress: ; Mann–Whitney U test: U = 88, P = .016), indicating enhanced systemic CORT.
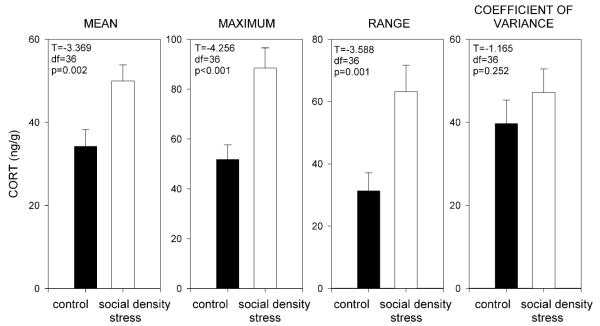
Figure 3 reveals that means of CORT concentration maxima (T = −4.256, df = 36; P < .001), series means (T = −3.369, df = 36; P = .002), and series ranges (T = −3.588, df = 36; P = .001) differed significantly between control (N = 17) and social density stress (N = 21), whereas the coefficients of variance did not (T = −1.165, df = 36; P = .252). Therefore, we did not combine data from control and social density stress. Similar results were obtained when we compared the nine individuals, for which we had data both during control and social density stress feeding situations (Table 1). Maxima and means of CORT concentration differed within individuals, and ranges of the series tended to differ, whereas the coefficients of variance did not differ between control and social density stress (Table 1).
Figure 3.
Differences in CORT (ng/g) means, maxima, ranges, and coefficients of variances. Black bars indicate control feeding situations; white bars indicate social density stress feeding situations. Standard errors are given. Note different scaling of the y-axis.
TABLE 1.
Statistical results of various parameters from CORT series of nine individual greylag geese used in control as well as social density stress (SDS)
| Mean ± S.E. control (ng/g) |
Mean ± S.E. SDS (ng/g) |
T | df | P | |
|---|---|---|---|---|---|
| Maxima | 39.56 ± 6.39 | 67.20 ± 9,30 | −2.810 | 8 | .023 |
| Means | 22.99 ± 5.02 | 40.34 ± 6.37 | −2.390 | 8 | .044 |
| Ranges | 25.13 ± 3.06 | 43.05 ± 9.20 | −1.957 | 8 | .086 |
| Coefficients of variance | 49.97 ± 6.93 | 47.15 ± 6.51 | 0.296 | 8 | .775 |
Note: Paired Student’s T-tests were applied.
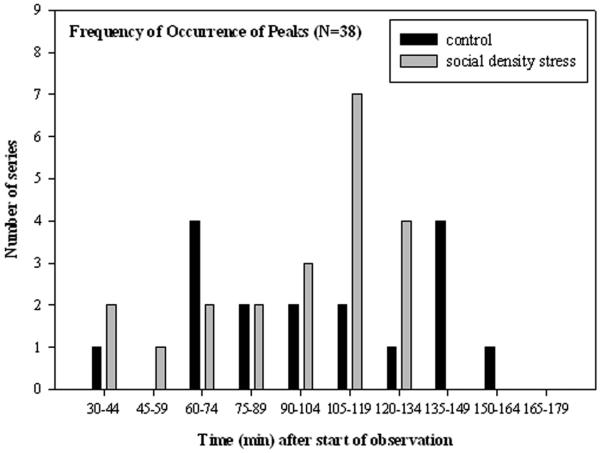
The point when peak excretion occurred did not differ between control and social density stress (Mann–Whitney U test: U = –159; P = .581). Within either control series or social density stress series, there was no apparent excretion peak at a certain point in time during the control period (Kruskal–Wallis ANOVA: H = 10.5, df = 7; P = .62). However, peak excretion occurred at approximately 120 min after a stressor was given (Kruskal–Wallis ANOVA: H = 19.064, df = 6; P = .004; Fig. 4).
Figure 4.
Histogram of the occurrences of maxima during control and social density stress feeding situations. Bins represent 14-min intervals, starting 30 min after the beginning of the feeding situation until more than 180 min after the beginning of the feeding situation have passed.
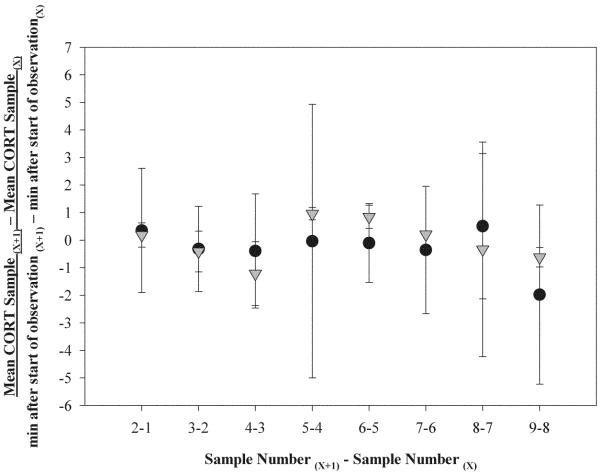
We then calculated the concentration change over time between two consecutive samples (Fig. 5). All comparisons of control and social density stress values were not significantly different from one another and fluctuated around zero (Wilcoxon Signed Rank tests: results not significant).
Figure 5.
Concentration change in two consecutive CORT samples over time during control and social density stress. Data were calculated using the formula: Mean CORT Sample(X+1) − Mean CORT sample(X)/min after start of observation(X+1) − min after start of observation(X).
To determine how many samples were necessary and which parameter to use, we focused on series with six samples, for which we had the most examples (N = 18). Data for these series were normally distributed. Means and maxima differed significantly between control and social density stress situation (means: Student’s t-test: T = −2.591, df = 16, P = .020; maxima: Student’s t-test: T = −3.673, df = 16, P = .002).
When reducing the original six-sample series randomly by one sample to obtain five-sample series, the significant differences in control and social density stress between maxima and means were retained in all but one case (Table 2). This, however, was not the case if the original six-sample series were reduced by two samples at random to four-sample series. To calculate the number of samples necessary to reveal the difference between control and stress, we repeated the reduction procedure of the original six-sample series to four, three, and two samples, for a total of 10 repeats in means and maxima (Table 2). Four- versus three-sample series were significantly different in means (significant repeats four-sample series: N = 8, three-sample series: N = 4; binomial test: P = .024; Table 3a) and maxima (significant repeats four-sample series: N = 10, three-sample series: N = 6; binomial test: P < .001; Table 3b). The comparison between the three- to two-sample series revealed a difference when comparing the maxima (significant repeats three-sample series: N = 6, two-sample series: N = 2; binomial test: P = .012; Table 3d), but not the means (significant repeats three-sample series: N = 4, two-sample series: N = 0; binomial test: P = .242; Table 3c).
TABLE 2.
Random reduction of the original 6-sample series by one to four CORT samples repeated either five (1-sample reduction) or 10 times (2- to 4-sample reductions)
| 6-Sample series reduced by |
Repeat 1/6 | Repeat 2/7 | Repeat 3/8 | Repeat 4/9 | Repeat 5/10 |
|---|---|---|---|---|---|
| Mean | |||||
1( 5 samples used) 5 samples used) |
T = −2.540 P = .022 |
T = −2.177 P = .045 |
T = −2.475 P = .025 |
T = −2.064 P = .056 |
T = −2.804 P = .013 |
2( 4 samples used) 4 samples used) |
T = −1.747 P = .124 |
T = −2.956 P = .021 |
T = −2.420 P = .046 |
T = −2.933 P = .022 |
T = −2.590 P = .036 |
|
T = −2.615 P = .035 |
T = −1.985 P = .088 |
T = −2.905 P = .023 |
T = −3.147 P = .016 |
T = −2.947 P = .022 |
|
3( 3 samples used) 3 samples used) |
T = −2.041 P = .081 |
T = −1.386 P = .208 |
T = −2.941 P = .022 |
T = −2.720 P = .030 |
T = −1.796 P = .115 |
|
T = −2.590 P = .036 |
T = −1.923 P = .096 |
T = −1.544 P = .166 |
T = −2.420 P = .046 |
T = −1.455 P = .189 |
|
4( 2 samples used) 2 samples used) |
T = −1.342 P = .221 |
T = −2.000 P = .086 |
T = −2.044 P = .080 |
T = −0.264 P = .800 |
T = −0.507 P = .335 |
|
Z = −1.122 P = .262 |
T = −0.990 P = .355 |
Z = −1.828 P = .076 |
Z = −1.958 P = .052 |
T = −1.983 P = .088 |
|
| Maximum | |||||
1( 5 samples used) 5 samples used) |
T = −4.514 P = .003 |
T = −2.664 P = .032 |
T = −4.921 P = .002 |
T = −2.475 P = .025 |
T = −4.614 P = .003 |
2( 4 samples used) 4 samples used) |
T = −2.956 P = .021 |
T = −4.095 P = .005 |
T = −3.230 P = .014 |
T = −3.668 P = .008 |
T = −2.933 P = .022 |
|
T = −3.147 P = .016 |
T = −2.047 P = .022 |
T = −2.905 P = .023 |
T = −3.987 P = .005 |
T = −2.615 P = .035 |
|
3( 3 samples used) 3 samples used) |
T = −2.041 P = .081 |
T = −2.941 P = .022 |
Z = −2.067 P = .040 |
T = −3.237 P = .014 |
Z = −1.680 P = .093 |
|
T = −4.247 P = .004 |
T = −3.685 P = .008 |
Z = −1.122 P = .262 |
T = −1.893 P = .100 |
T = −2.353 P = .050 |
|
4( 2 samples used) 2 samples used) |
Z = −2.380 P = .017 |
Z = −1.820 P = .069 |
Z = −1.260 P = .208 |
Z = −0.560 P = .575 |
T = −0.990 P = .355 |
|
Z = −1.431 P = .156 |
Z = −1.985 P = .088 |
T = −2.177 P = .045 |
T = −1.386 P = .208 |
Z = −1.828 P = .076 |
Note: Student’s T-tests or Wilcoxon Signed Rank tests were used as means or maxima of the reduced series; they were not always disributed normally. Significant differences between control and social density feeding situation appear in boldface type. Degrees of Freedom equaled seven (df = 7) in all Student’s T-tests.
TABLE 3.
2 × 2 contingency tables of a number of significant and not significant test results either in the reduced 4- vs. 3-sample series (a and b) or 3- vs. 2-sample series (c and d)
| (a) 4- and 3-sample series | (b) 4- and 3-sample series | ||||
|---|---|---|---|---|---|
| Mean | Not significant |
Significant | Maximum | Not significant |
Significant |
| 4 samples | 2 | 8 | 4 samples | 0 | 10 |
| 3 samples | 6 | 4 | 3 samples | 4 | 6 |
| (c) 3- and 2-sample series | (d) 3- and 2-sample series | ||||
|---|---|---|---|---|---|
| Mean | Not significant |
Significant | Maximum | Not significant |
Significant |
| 3 samples | 6 | 4 | 3 samples | 4 | 6 |
| 2 samples | 10 | 0 | 2 samples | 8 | 2 |
NOTE: Means are displayed in panels (a) and (c); maxima are displayed in panels (b) and (d).
DISCUSSION
We have demonstrated that, although CORT represents an integrated, proportional record of the corticosterone plasma level within a time frame of 2–4 h prior to defecation in geese,5,7,8,26 it can be used to determine differences between individuals in response to an acute stressor if a sufficient number of samples are collected. We showed that means and ranges of the samples within a series as well as the maxima are suitable parameters for determining differences in control versus challenge situations. Similar results were found in black grouse, where Baltic et al.30 found individual differences in means of excreted corticosterone immunoreactive metabolites despite large variation within the samples. In geese, the coefficient of variance, on the other hand, did not reveal a difference. This indicates that in geese, the variation around a mean in successive defecations was similar in control and social density stress feeding situations; therefore, a challenge does not change the scale of variance relative to the mean. In the case of geese, the aim to detect differences requires the collection of three samples and use of the maximum of these. One could also collect at least four samples and take the mean to determine individual differences. The more samples are used to calculate the mean, the more precise the results will be, and the greater the chance not to erroneously accept the null hypothesis (or commit a type II error). These findings apply to geese only, but might be different in other species.
Whether repeated fecal sampling is a useful alternative to collecting blood samples will depend not only on the study question and the feasibility of collection but also on several other important issues. For example, high defecation intervals in the relevant collection time are desirable. Also, the shorter the gut passage time of an animal, the more useful this method will be. It is particularly suitable for birds, in which gut passage times are shorter5,8,12,13,26,31 than in mammals.11,32 Consecutive urine33 and fecal samples may show large variations in CORT, even in relatively short collection windows—for example, less than 1 h in chimpanzees and less than 20 min in geese. This may lead to over- or underestimation of differences both within individuals and between studies. Sufficient—and more standardized—sampling effort can level out this variation.
If fecal samples are used for measuring acute stress responses, several factors must be addressed before starting data collection. It should be determined how metabolite excretion profiles of the study species in question look. For example, not all avian species exhibit the excretion patterns found in geese. Chickens (Gallus gallus domesticus) show the expected, relatively smooth bimodal CORT excretion profile, with an early urine and later fecal peak measured over the course of the day.34 The curve of the excretion profile will influence when and how many fecal samples should be collected. For example, domestic geese showed higher variation in consecutive fecal samples in the morning than in the afternoon (Fig. 2). This finding might indicate a biologically relevant difference in CORT excretion in the morning relative to the afternoon. It is also possible, however, that lower afternoon values result from geese habituating to the sampling procedure over the course of the day. In addition, greylag geese varied widely in their excretion of CORT: Whereas some series had wide variation between consecutive samples, others displayed only very little variation in CORT within a single series (e.g., Fig. 1, upper two panels). This was the case not only in control series but also in series collected during social density stress. Whether this lack of variability is biologically important, and how much variation constitutes biological relevance, needs to be determined. It should also be investigated whether this variability is a random effect between different series or if it is due to differences in individuals. If the latter were true, we would expect consistency in several series of the same individual. However, at this point we have too few series per individual to answer this question. An estimate of the number of samples necessary and which parameter to use is of uppermost importance to answer questions concerning acute stress responses. This will vary from species to species, but can vary even within the same species, depending on the type of stressor. Finally, for extraction, in general samples are weighed in at 0.5 g. Whether this is the best amount possible to even out heterogeneities within the sample itself is not known. It might be worthwhile to compare different amounts of the same fecal sample (i.e., 1 g, 2 g, etc.; Möstl, personal communication) to reduce the effect of a possible CORT concentration gradient within the sample. Ultimately, an ample fecal sampling scheme will result in more accurate assessments of physiological processes and their relationship to behavior.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the FWF Project 15766-B03, from the “Verein der Förderer,” and from the “Herzog von Cumberland Stiftung.”
We are grateful to M. Kalas, M. Kirnbauer, V. Pilorz, and T. Stern for assistance in collecting fecal samples. The corticosterone enzyme immunoassays were conducted in the laboratories of E. Möstl at the Department of Biochemistry of the Veterinary University of Vienna as well as the laboratory ofJ. Dittami at the Department of Ethology, University of Vienna, and by A. Schöbitz and A. Aschauer. C. Schlögl, C. Pribersky-Schwab, and J. Hemetsberger offered statistical advice. S. Rettenbacher and C. Yussif helped collecting the domestic goose data, and the Möstl and Palme laboratories performed the biochemical validations of the new assay. B. Weiss. D. Frigerio, and the participants of the ESF technical workshop provided discussion on the topic. T. Bugnyar, W. Goymann, S. Jenni-Eiermann, and E. Möstl provided constructive criticism of the manuscript.
REFERENCES
- 1.Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm. Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Monfort SL, Brown JL, Wildt DE. Episodic and seasonal rhythms of cortisol secretion in male Eld’s deer (Cervus eldi thamin) J. Endocrinol. 1993;138:41–49. doi: 10.1677/joe.0.1380041. [DOI] [PubMed] [Google Scholar]
- 3.Thun R, et al. Twenty-four hour secretory pattern of cortisol in the bull: evidence of episodic and circadian rhythm. Endocrinology. 1981;109:2208–2212. doi: 10.1210/endo-109-6-2208. [DOI] [PubMed] [Google Scholar]
- 4.Fulkerson WJ, Tang BY. Ultradian and circadian rhythms in the plasma concentration of cortisol in sheep. J. Endocrinol. 1979;81:135–141. doi: 10.1677/joe.0.0810135. [DOI] [PubMed] [Google Scholar]
- 5.Hirschenhauser K, et al. Endocrine and behavioral responses of male greylag geese (Anser anser) to pairbond challenges during the reproductive season. Ethology. 2000;106:63–77. [Google Scholar]
- 6.Frigerio D, Weiss B, Dittami J, Kotrschal K. Social allies modulate corticosterone excretion and increase success in agonistic interactions in juvenile hand-raised greylag geese (Anser anser) Can. J. Zool. 2003;81:1746–1754. [Google Scholar]
- 7.Hirschenhauser K, Möstl E, Kotrschal K. Seasonal patterns of sex steroids determined from feces in different social categories of greylag geese (Anser anser) Gen. Comp. Endocrinol. 1999;114:67–79. doi: 10.1006/gcen.1998.7236. [DOI] [PubMed] [Google Scholar]
- 8.Kotrschal K, Hirschenhauser K, Möstl E. The relationship between social stress and dominance is seasonal in greylag geese. Anim. Behav. 1998;55:171–176. doi: 10.1006/anbe.1997.0597. [DOI] [PubMed] [Google Scholar]
- 9.Kotrschal K, et al. Effects of physiological and social challenges in different seasons on fecal testosterone and corticosterone in male domestic geese (Anser domesticus) Acta Ethol. 2000;2:115–122. [Google Scholar]
- 10.Sgoifo A, et al. Social stress: acute and long-term effects on physiology and behavior. Physiol. Behav. 2001;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- 11.Goymann W, et al. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. Gen. Comp. Endocrinol. 1999;114:340–348. doi: 10.1006/gcen.1999.7268. [DOI] [PubMed] [Google Scholar]
- 12.Carere C, et al. Fecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Horm. Behav. 2003;43:540–548. doi: 10.1016/s0018-506x(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 13.Hiebert SM, et al. Noninvasive methods for measuring and manipulating corticosterone in hummingbirds. Gen. Comp. Endocrinol. 2000;120:235–247. doi: 10.1006/gcen.2000.7559. [DOI] [PubMed] [Google Scholar]
- 14.Hirschenhauser K, et al. Seasonal relationships between plasma and fecal testosterone in response to GnRH in domestic ganders. Gen. Comp. Endocrinol. 2000;118:262–272. doi: 10.1006/gcen.2000.7463. [DOI] [PubMed] [Google Scholar]
- 15.Frigerio D, Dittami J, Möstl E, Kotrschal K. Excreted corticosterone metabolites co-vary with ambient temperature and air pressure in male greylag geese (Anser anser) Gen. Comp. Endocrinol. 2004;137:29–36. doi: 10.1016/j.ygcen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Piazza PV, et al. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc. Natl. Acad. Sci. USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwabl H. Individual variation of the acute adrenocortical resonse to stress in the white-throated sparrow. Zoology. 1995;99:113–120. [Google Scholar]
- 18.Scheiber IB, et al. Active and passive social support in families of greylag geese (Anser anser) Behaviour. 2005 doi: 10.1163/156853905774831873. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frigerio D, Möstl E, Kotrschal K. Excreted metabolites of gonadal steroid hormones and corticosterone in greylag geese (Anser anser) from hatching to fledging. Gen. Comp. Endocrinol. 2001;124:246–255. doi: 10.1006/gcen.2001.7706. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz K. Hier bin ichwo bist Du? Ethologie der Graugans. Piper Verlag; München: 1988. [Google Scholar]
- 21.Hemetsberger J. Die Entwicklung der Grünauer Graugansschar seit 1973. In: Kotrschal K, Müller G, Winkler H, editors. Konrad Lorenz und seine verhaltensbiologischen Konzepte aus heutiger Sicht. Filander Verlag; Fürth, Germany: 2001. pp. 249–260. [Google Scholar]
- 22.Kotrschal K, Hemetsberger J, Dittami J. Vigilance in a flock of semitame greylag geese (Anser anser) in response to approaching eagles Haliaeetus albicilla and Aquila chrysaetos. Wildfowl. 1992;43:215–219. [Google Scholar]
- 23.Hemetsberger J. Populationsbiologische Aspekte der Grünauer Graugansschar (Anser anser) University of Vienna; Vienna: 2002. Ph.D. thesis. [Google Scholar]
- 24.Kotrschal K, Hemetsberger J, Weiss BM. Homosociality in greylag geese. In: Vasey P, Sommer V, editors. Homosexuality Behaviour in Animals: An Evolutionary Perspective. Cambridge University Press; Cambridge: 2005. In press. [Google Scholar]
- 25.Kotrschal K, Hemetsberger J, Dittami J. Food exploitation by a winter flock of greylag geese: behavioral dynamics, competition and social status. Behav. Ecol. Sociobiol. 1993;33:289–295. [Google Scholar]
- 26.Krawany M. Die Entwicklung einer nichtinvasiven Methode zum Nachweis von Steroidhormonmetaboliten im Kot von Gänsen. Institute of Biochemistry, Veterinary University of Vienna; Vienna, Austria: 1996. Ph.D. thesis. [Google Scholar]
- 27.Schütz K, Wallner B, Kotrschal K. Diurnal patters of steroid hormones from feces in greylag goslings (Anser anser) Adv. Ethol. 1997;32:66. [Google Scholar]
- 28.Möstl E, et al. Proc. Symp Analysis Steroids. Sopron, Hungary: 1987. Oestrogen determination in feces of mares by enzyme immunoassay on microtitre plates; pp. 219–224. [Google Scholar]
- 29.SPSS . SPSS for Windows. version 11.0.1 2001. [Google Scholar]
- 30.Baltic M, et al. A noninvasive technique to evaluate human-generated stress in black grouse. Ann. N.Y. Acad. Sci. 2005;1046:81–95. doi: 10.1196/annals.1343.008. [DOI] [PubMed] [Google Scholar]
- 31.Goymann W, Möstl E, Gwinner E. Corticosterone metabolites can be measured noninvasively in excreta of European Stonechats (Saxicola torquata rubicola) AUK. 2002;119:1167–1173. [Google Scholar]
- 32.Wasser S, Risler L, Steiner RA. Excreted steroids in primate feces over the menstrual cycle and pregnancy. Biol. Reprod. 1988;39:862–872. doi: 10.1095/biolreprod39.4.862. [DOI] [PubMed] [Google Scholar]
- 33.Anestis SF, Bribiescas RG. Rapid changes in chimpanzee (Pan troglodytes) urinary cortisol excretion. Horm. Behav. 2004;45:209–213. doi: 10.1016/j.yhbeh.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Rettenbacher S, et al. Measurement of corticosterone metabolites in chicken droppings. Br. Poult. Sci. 2004;45:704–711. doi: 10.1080/00071660400006156. [DOI] [PubMed] [Google Scholar]
- 35.Klasing KC. Potential impact of nutritional strategy on noninvasive measurements of hormones in birds. Ann. N.Y. Acad. Sci. 2005;1046:5–16. doi: 10.1196/annals.1343.003. [DOI] [PubMed] [Google Scholar]