Abstract
The isolation of viable extremely halophilic archaea from 250-million-year-old rock salt suggests the possibility of their long-term survival under desiccation. Since halite has been found on Mars and in meteorites, haloarchaeal survival of martian surface conditions is being explored. Halococcus dombrowskii H4 DSM 14522T was exposed to UV doses over a wavelength range of 200–400 nm to simulate martian UV flux. Cells embedded in a thin layer of laboratory-grown halite were found to accumulate preferentially within fluid inclusions. Survival was assessed by staining with the LIVE/DEAD kit dyes, determining colony-forming units, and using growth tests. Halite-embedded cells showed no loss of viability after exposure to about 21 kJ/m2, and they resumed growth in liquid medium with lag phases of 12 days or more after exposure up to 148 kJ/m2. The estimated D37 (dose of 37 % survival) for Hcc. dombrowskii was ≥ 400 kJ/m2. However, exposure of cells to UV flux while in liquid culture reduced D37 by 2 orders of magnitude (to about 1 kJ/m2); similar results were obtained with Halobacterium salinarum NRC-1 and Haloarcula japonica. The absorption of incoming light of shorter wavelength by color centers resulting from defects in the halite crystal structure likely contributed to these results. Under natural conditions, haloarchaeal cells become embedded in salt upon evaporation; therefore, dispersal of potential microscopic life within small crystals, perhaps in dust, on the surface of Mars could resist damage by UV radiation.
Keywords: Halococcus dombrowskii, Simulated martian UV radiation, LIVE/DEAD staining, Halite fluid inclusions, UV transmittance and reflectance, Desiccation
Introduction
Several extremely halophilic archaea (haloarchaea) have been isolated from alpine rock salt deposits of Permian and Triassic age, and some of them were described as novel species (Denner et al., 1994; Stan-Lotter et al., 1999, 2002; Gruber et al., 2004; Fendrihan et al., 2006). In addition, amplification of 16S rRNA genes, via the polymerase chain reaction, indicated evidence for the presence of a large haloarchaeal community in the salt deposits (Radax et al., 2001). These findings suggest the possibility of microbial long-term survival under conditions of desiccation, though it is enigmatic as to which mechanisms might be used, since haloarchaea are not known to produce resting stages such as spores (Grant et al., 1998; McGenity et al., 2000). The apparent longevity of the haloarchaeal isolates in dry salty environments is of interest for the search for extraterrestrial life. Halite has been detected in meteorites, some of which stem from Mars (Gooding, 1992; Zolensky et al., 1999; Treiman et al., 2000). Data from the martian rovers Spirit and Opportuity (Rieder et al., 2004; Squyres et al., 2006) suggest that some deposits on Mars formed from concentrated salt water. If Mars and Earth had a similar geological past (Nisbet and Sleep, 2001, Schidlowski, 2002), then there is the possibility that life occurred on Mars as well and that microbial life, or remnants of it, could still be present on Mars.
Since Mars lacks an ozone layer, strong UV radiation is inferred on the martian surface (Rontó et al., 2003; Patel et al., 2004). It is believed that, before the accumulation of oxygen and ozone, the early Earth environment experienced an influx of short-wavelength UV of high intensity (Bérces et al., 2006; Westall et al., 2006). Early life on Earth, therefore, was likely resistant to UV, and it follows that potential microbiota on Mars may have possessed, or may possess, resistance to this type of radiation as well, which can produce extensive DNA damage (Cockell, 1998; Cadet et al., 2005). Previous experiments of microbial exposure to the space environment and its radiation were carried out mainly with Bacillus spores (Horneck, 1993; Horneck et al., 2001). More recently, lichens and cyanobacteria were exposed to space conditions by Sancho et al. (2007) and Cockell et al. (2007). To date, one exposure of an extremely halophilic archaeon, Haloarcula sp., to space conditions was performed in the Biopan experiments by Mancinelli et al. (1998), who demonstrated considerable survival of cells after 14 days in space.
Haloarchaea, including Halobacterium salinarum strain NRC-1, whose total genome sequence is known (Ng et al., 2000), have been investigated for the activity of DNA repair systems after exposure to UV radiation (McCready, 1996; Baliga et al., 2004; Zhou et al., 2007); therefore, some data on their resistance to UV are available. However, these studies are generally performed with cells in a liquid salt-containing medium during exposure to UV radiation. The presence of liquid saline pools on Mars is not likely, but solid halite has been discovered in martian meteorites (Gooding, 1992; Treiman et al., 2000). Therefore, the haloarchaeal survival capacity, while enclosed in salt crystals, is of interest for astrobiological studies. The experiments performed here with Halococcus dombrowskii are part of the ground investigations of the ADAPT project (Principal Investigator P. Rettberg; for more information see http://www.nasa.gov/mission_pages/station/science/experiments/Expose.html#overview), which was selected for exposure on the International Space Station (ISS). The exposure facility was developed by ESA and is described elsewhere, along with details of trays and the localization on the ISS (Baglioni et al., 2007).
While haloarchaeal strains obtained from rock salt that is believed to be millions of years of age should be eminently suitable model organisms for exposure studies, there are also some drawbacks. In particular, the isolates grow rather slowly, and some of them possess generation times on the order of weeks or even months (Gruber et al., 2004; Fendrihan, unpublished results), which complicates the determination of various growth parameters following potentially lethal treatment of cells, especially when only a few samples with small volumes are available.
In this study, we chose to develop and test methods for the evaluation of haloarchaeal survival in small desiccated samples. The Mars-UV simulator lamp for examining the effects on organisms and on different complex organic substances at the same UV flux intensity as on the martian surface, developed by Kolb et al. (2005), was used for the experiments with cells of Halococcus dombrowskii and two noncoccoid haloarchaea (Halobacterium salinarum, Haloarcula japonica). A comparison of the spectral intensity output of the lamp between 200 and 400 nm with the theoretical flux on Mars was published by Kolb et al. (2005). For the quantitative determination of surviving haloarchaea, viable cells were counted after staining with the so-called LIVE/DEAD BacLight kit; the procedure has been described recently (Leuko et al., 2004, Stan-Lotter et al., 2006). For some experiments, fluorescence microscopy was combined with growth studies and exposure in liquid culture and on semi-solid medium; the effects of these experimental setups had not been compared before. The results of the study described here indicate the potential for UV-resistant microscopic life in small crystals, which could be present in martian dust.
Materials and Methods
Growth of halophilic microorganisms
Strains Halococcus dombrowskii H4 DSM 14522T and Haloarcula japonica DSM 6131T were grown in M2 culture medium (Tomlinson and Hochstein, 1976) that contained (g × L−1): casamino acids (Hycase, Sigma), 5.0; yeast extract (Difco), 5.0; Tris (ICN Biochemicals), 12.1; NaCl, 200.0; MgCl2 × 6H2O, 20.0; KCl, 2.0; CaCl2 × 2H2O, 0.2; in side arm flasks at 37°C with shaking at 150 rpm in an incubator (Innova 4230). The optical density (OD) of cultures was measured at 600 nm with a Novaspec II photometer (Pharmacia). When the cultures reached an OD of 0.8 to 1.0, which corresponded to cell densities of 2.5–4 × 108 per ml, cells were harvested by centrifugation at 5000 × g for 5 minutes, and the pellets were resuspended in Tris-NaCl buffer, which consisted of 4 M NaCl (Sigma) and 100 mM Tris. The pH was adjusted to 7.4 with dilute HCl. Halobacterium salinarum NRC-1 ATCC-700922 was purchased from LGC Teddington, UK, grown as described previously (Gruber et al., 2004) and prepared as described for the haloarchaea mentioned before.
Exposure of cells to UV irradiation
Forty microliters and 28 microliters, respectively, of the cell suspensions were placed on quartz discs of 11 or 7 mm diameter (Aachener Quarzglas Technologie, Aachen, Germany). Discs either contained approximately 7 × 105 or 4.8 × 105 haloarchaeal cells. The discs were inserted in the wells of 24-well plates (Becton Dickinson, USA) and dried overnight in a sterile working bench (Holten LaminAir 2010, Denmark) under laminar flow at ambient temperature. This led to the formation of flat halite crystals with embedded haloarchaeal cells. For each exposure time, three quartz discs of each sample were used (placed in plastic petri dishes of 55 mm diameter) and exposed to the Mars-UV simulator lamp at the Austrian Academy of Sciences (Kolb et al., 2005) for times ranging from 1 to 3600 s. The spot of the lamp had a 50 mm diameter at a distance of 300 mm. The intensity of the irradiation of the lamp was in the range of 39.15–42 W/m2, as measured with a spectroradiometer (Typ UL 754, Optronic Laboratories Ltd., Orlando, USA); the data were acquired and edited with OL 754 version nr. 1.16 software. For some experiments, the Mars-UV simulator lamp was transported to the laboratories at the University of Salzburg or the Hungarian Academy of Sciences in Budapest. Exposures of cells in the liquid phase were performed following plating of 50 μl each of a culture, which was diluted to 2–4 × 103 or 2–4 × 104 cells per ml, respectively, on agar plates of 55 mm diameter; the same distance as above was used during exposures. In addition, liquid cultures of the same dilutions were exposed directly by placing 1.4 ml into an empty petri dish of 55 mm diameter, creating a layer of 0.6 mm thickness, similar to the protocol by Baliga et al. (2004). Some exposure experiments were performed in a chamber equipped with a UV lamp and a SOL2000 lamp (Dr. Hönle AG, Germany) that produced a spectral range from 200 to 800 nm; this equipment was provided by the DLR (Deutsches Zentrum für Luft-und Raumfahrt), Köln, Germany,
Determination of exposure effects
For removal of cells subsequent to exposure, 250 μl of a modified Tris-NaCl buffer was added, which contained only 3 M NaCl, to each quartz disc. Fifty microliters of the suspension was used for LIVE/DEAD kit staining and fluorescence microscopy to assess viability of cells (see Leuko et al., 2004; Stan-Lotter et al., 2006). Stained cells were counted manually on photographs taken with a digital camera (type DFC300FX, Leica Microsystems) from at least 5 prints per experiment, which contained between 80 and 300 cells each. Aliquots of 50 μl from each experiment with different exposure times were inoculated into 4.95 ml of liquid medium (M2) in test tubes and incubated at 37°C in an incubator (Innova 4080) with shaking at 150 rpm. The OD of cultures was measured daily at 600 nm. All analyses also included non-irradiated control samples. Colony-forming units were determined following plating of aliquots of exposed and resuspended cells or, in the case of liquid cultures, of exposed cell suspensions, on agar plates of 90 mm diameter containing solidified M2 medium, unless noted otherwise. Incubation at 37°C to 40°C was done for several days (with Har. japonica and Hbt. salinarum) or several weeks (with Hcc. dombrowskii).
Determination of UV absorption and reflectance of halite
The spectrophotometric measurements of a quartz disc with (uninoculated) halite and of Hcc. dombrowskii in halite were taken in the spectral range between 200 and 800 nm to detect the samples’ regions of transmittance (quartz disc with halite) or reflectance (Hcc. dombrowskii in halite), or both. The illumination of the samples was accomplished with a 75 W ozone-free Xenon arc lamp (Oriel 6251) as a white light source (color temperature 5500 K), equipped with a F/1.0 condenser (Oriel 68806) to obtain a collimated light beam illuminating the sample. The light transmitted or reflected by selected areas of the sample was focused by a quartz lens (f = 140 mm) into the entrance slit of a grating spectrometer (Jobin Yvon Triax 190), diffracted by a 300 lines/mm grating, and then detected by a UV-sensitive Peltier-cooled CCD camera (Jobin Yvon) at the exit side of the spectrometer. To obtain curves for transmittance and reflectance, respectively, two spectra were taken for each measurement: the first one with the sample to be studied, the second one with a reference sample obtained under the same acquisition conditions. Division of the sample spectrum by the reference spectrum yields the transmittance or reflectance curve and eliminates the need for correcting the instrumental dependences related to the spectral efficiency of the grating and the spectral sensitivity of the CCD (Galsterer et al., 1999; Langanger et al., 2000). The quartz disc with halite was placed in the light beam such that, with use of a suitable aperture of an iris diaphragm, a diameter equivalent to the halite-covered area on the disc was achieved. An unused quartz disc was used as reference for the transmittance data. The sample containing cells of Hcc. dombrowskii in halite was fixed on an opaque black holder (nonreflecting background) with the help of an opaque black mask with a 3 × 3 mm hole in it. A white, diffuse reflecting standard (Spectralon SrS-99-010, reflectance greater than 95% from 250 to 2500 nm, greater than 99% from 400 to 1500 nm) was used as the reference for the opaque black holder.
Results
Determination of survival following UV exposure
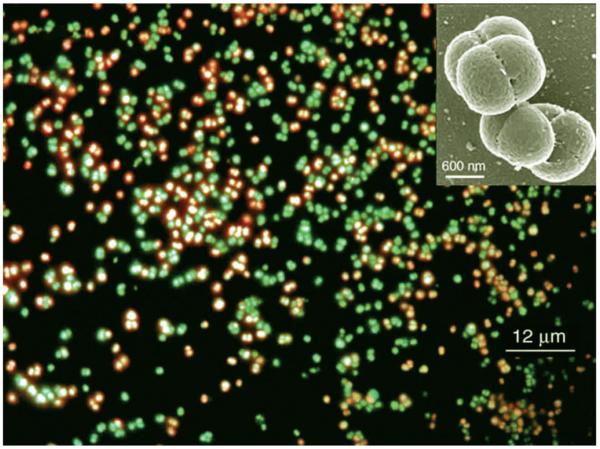
Halococcus dombrowskii H4 DSM 14522T grows in most media as small aggregates of 2–8 cells (see Fig. 1, insert). After exposure to simulated martian UV light and staining with the LIVE/DEAD BacLight kit (henceforth referred to as LIVE/DEAD kit), which contains nucleic acid dyes, cells can be distinguished by their color under a fluorescence microscope. Viable cells possess intact membranes, which allow permeation of the dye SYTO 9, and thus appear green in the fluorescence micrograph. Cells with damaged membranes, however, are penetrated by the red dye propidium iodide and are therefore considered nonviable (Haugland, 2002), which has been verified for haloarchaea (Leuko et al., 2004). Figure 1 is a representative fluorescence microscope photograph of cells of Hcc. dombrowskii subsequent to staining with the LIVE/DEAD kit after exposure to UV radiation. Such stained cells were used for the quantitative determination of viability of haloarchaeal cells, which was expressed as percent of cells that fluoresced green.
FIG. 1.
Cells of Halococcus dombrowskii following exposure to a simulated martian UV flux and subsequent staining with the LIVE/DEAD kit. Red: nonviable cells due to damaged membranes; green: viable cells with intact membranes. Insert: Scanning electron micrograph (photo by Chris Frethem, CharFac, University of Minnesota) of a typical small aggregate of the coccoid cells of Hcc. dombrowskii.
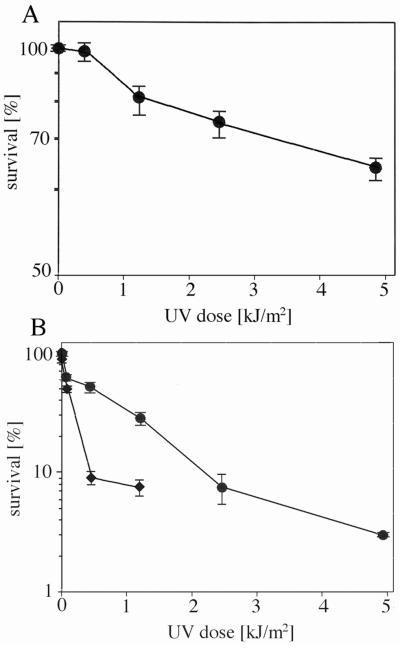
The data in Fig. 2 show that no loss of viability of Hcc. dombrowskii occurred following exposure of up to 21 kJ/m2 and that about 75% of viable cells were still observed following exposure for 1 h, which equalled a dose of 148 kJ/m2. The exposure time to the Mars-UV simulator lamp was limited to one hour; extrapolation of the survival curve yielded an estimated D37 (dose of 37% survival) in the range of at least 420 kJ/m2 for Hcc. dombrowskii. The potential survival of Hcc. dombrowskii in halite may therefore be up to a dose of at least 3000 kJ/m2 or higher. The process of embedding cultures of Hcc. dombrowskii in artificial halite, dissolving the salt, and resuspending the cells in high-salt buffer apparently resulted in damage to a portion of the culture. The recovery was 65% ± 3% viable cells, compared to the liquid starting culture. This number was highly reproducible and probably represented a portion of cells that were aging and close to dying, as is common in a culture of high density; therefore, the amount of viable cells in the control after recovery was taken as 100% for the survival experiments (Fig. 2). Aliquots of all exposed samples were incubated in growth medium M2, which contained nutrients and 4 M NaCl (Tomlinson and Hochstein, 1976). Onset of growth was delayed by 12–14 days, occasionally by several weeks, but doubling times in the logarithmic phase and final densities of cells (30–35 h, OD600nm 1.0–1.1, respectively) were essentially identical to those of unexposed controls, once growth had resumed (data not shown).
FIG. 2.
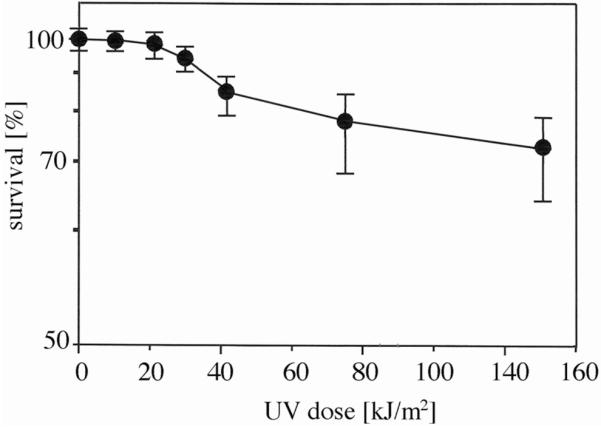
Survival of halite-entrapped Hcc. dombrowskii, following exposure to increasing doses of UV irradiation. Numbers of survivors were determined following staining with the LIVE/DEAD BacLight kit (see Fig. 1 and Materials and Methods) and are given as averages ± standard deviation, indicated by error bars (number of experiments: 5–8).
Ultraviolet exposure in liquid and on semi-solid medium
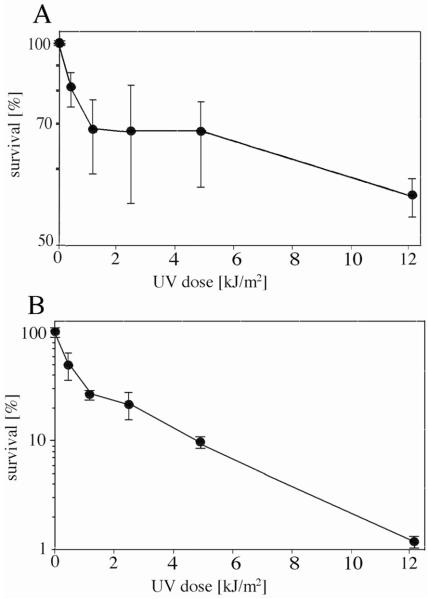
Most published data on haloarchaeal survival of UV irradiation was acquired from exposure of cells while in liquid medium or buffer. Exposure of Hcc. dombrowskii in liquid medium to the Mars-UV simulator lamp resulted in no loss of viability of up to a dose of 0.41 kJ/m2 (Fig. 3A), when LIVE/DEAD staining data were considered. Extrapolation of the survival curve yielded an estimated D37 of about 10 kJ/m2, which is 2 orders of magnitude less than the D37 obtained with halite-embedded Hcc. dombrowskii. The determination of colony-forming units (CFUs) yielded a D37 of about 1 kJ/m2 (Fig. 3B), which is lower than the value obtained from LIVE/DEAD staining but still in the same order of magnitude. Given that the number of CFUs obtained with cultures of Hcc. dombrowskii are likely less precise than with singly growing microorganisms due to cell aggregation (see Fig. 1), we used a multiplication factor of 4 for this strain. To explore the relationship between the environment of haloarchaea and the organisms’ ability to survive exposure to UV flux further, Hcc. dombrowskii was exposed to the Mars-UV simulator lamp while spread on the surface of agar plates (semi-solid medium). Figure 3B shows the CFUs following growth on the same agar plates. The D37 value was about 0.4 kJ/m2, which indicates an even higher sensitivity toward UV radiation than cells exposed in a liquid environment. For comparison with other haloarchaea, essentially the same exposure experiments shown in Figs. 2 and 3 were performed with Halobacterium salinarum NRC-1 and Haloarcula japonica. Following exposure, a portion of cells were immediately stained with the LIVE/DEAD kit, and viable cells in micrographs were counted, while CFUs of another portion of exposed cells were determined after incubation for 6–7 days. The D37 of Hbt. salinarum NRC-1 was approximately 0.7 kJ/m2 for cells irradiated while on agar plates, 1.5 kJ/m2 for cells irradiated in liquid medium, and at least 148 kJ/m2 for cells embedded in halite during exposure (data not shown). The ratios of these survival rates were thus similar to those given above for Hcc. dombrowskii. The D37 for Hbt. salinarum NRC-1 was in a similar range as published data, and a D37 of 212 J/m2 was reported for a strain of Hbt. salinarum (Shahmohammadi et al., 1997). A D37 of approximately 250 J/m2 was calculated from Fig. 1 of Baliga et al. (2004), who had used Hbt. salinarum NRC-1. The D37 of Har. japonica, as estimated from LIVE/DEAD staining, was about 16 kJ/m2; from CFUs, the value for D37 was 1 kJ/m2 (Fig. 4A, B). These data were in a similar range as those determined for Hcc. dombrowskii and Hbt. salinarum.
FIG. 3.
Survival of Hcc. dombrowskii following exposure to increasing doses of UV radiation while in liquid medium (●) or spread on agar plates (◆). Numbers of survivors were determined following staining with the LIVE/DEAD BacLight kit (A) and by counting of CFUs (B). Error bars, representing standard deviation from 5–6 experiments each, are indicated.
FIG. 4.
Survival of Har. japonica following exposure to increasing doses of UV radiation while in liquid medium. Number of survivors were determined following staining with the LIVE/DEAD BacLight kit (A) and by counting of CFUs (B). Error bars, representing standard deviation from 5 experiments each, are indicated.
Preparation of samples and localization of cells within halite
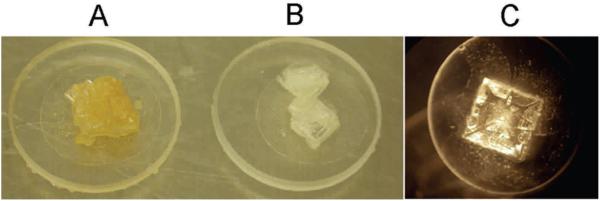
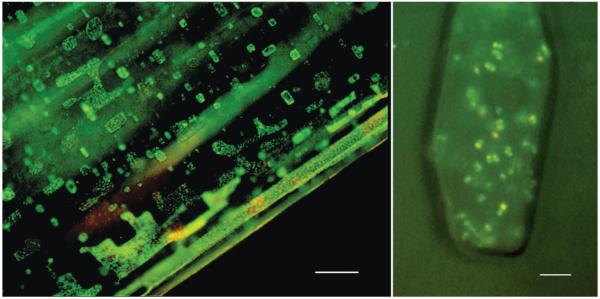
In this study, we attempted to simulate “natural conditions,” i.e., cell suspensions of Hcc. dombrowskii were left to dry at ambient temperature, which results in the formation of halite crystals. Even when cells are spread thinly on a quartz disc, salt crystals form as a cubic mineral (Fig. 5C); and cells will be trapped inside, mostly in fluid inclusions (Fig. 6; Fendrihan and Stan-Lotter, 2004). Therefore, a “crowding effect” within the fluid inclusion will likely sometimes occur, with several layers of cells on top of each other; and partial shielding against irradiation could take place, as was demonstrated for spores of Bacillus subtilis (Mancinelli and Klovstad, 2000). On the other hand, such somewhat in-homogeneous samples are characteristic of natural samples from evaporation events.
FIG. 5.
Halite crystals on 11 mm quartz discs, after (A) and before (B) irradiation with 1.5 × 105 kJ/m2 of UV light 200–400 nm and SOL 2000 (this experiment was performed at the DLR Köln, Germany). (C) typical halite crystal on quartz disc with entrapped cells of Hcc. dombrowskii as used in this study.
FIG. 6.
Localization of pre-stained haloarchaea in fluid inclusions. Low magnification of cells of Halobacterium salinarum NRC-1 (left) in halite and high magnification of cells of Halococcus dombrowskii (right) in an individual fluid inclusion. Cells were stained with the LIVE/DEAD kit (Stan-Lotter et al., 2006) prior to embedding in halite; epifluorescence microscopy was performed following entrapment of cells for 3 days. Bars: 25 μm (left panel) and 5 μm (right panel).
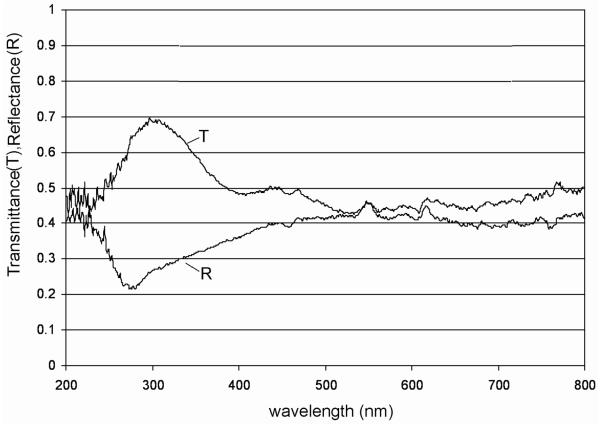
Reflectance and transmittance of UV by halite
The transmittance of halite on quartz disc (Fig. 7) was higher in the UV region, between 250 and 350 nm, and dropped to 40–50% in the region between 350 and 800 nm. The drop was probably caused by defects (vacancies) in the halite crystal and the related absorption of incoming light by these so-called color centers [a trapping energy level occupied by an electron—or an ion different from the original one—within the large band gap of halite; see Marder (2000), Kittel (2006)].
FIG. 7.
Transmittance (T) of a halite crystal on a quartz disc, and reflectance (R) of a similar halite sample in which cells of Hcc. dombrowskii were embedded.
In a case such as this, the emitted light is shifted to longer wavelengths (Stokes shift) and scattered in all directions. These crystal lattice vacancies become enhanced when halite is subject to high-energy radiation, which leads to increased light absorption and enhanced colored (yellow) appearance of the crystal (an example is shown in Fig. 5A). The reflectance curve (Fig. 7) of the halite sample with embedded Hcc. dombrowskii shows a rather low reflectance in the UV region between 250 and 350 nm, which increases to about 40–50% in the region between 350 and 800 nm. As soon as the light of shorter wavelength becomes absorbed by the color centers, the emitted light becomes Stokes-shifted and scattered in all directions, which thus increases the reflectance.
Discussion
Halite-embedded cells of Hcc. dombrowskii on small quartz discs survived exposure to the UV spectrum from 200–400 nm produced by the Mars surface radiation simulator for doses as high as 104 kJ/m2. This range of doses is similar to the UV dose that Haloarcula sp. and an osmophilic cyanobacterium survived during 2 weeks in Earth orbit (Mancinelli et al., 1998). In addition, preliminary data from ground experiments of the ADAPT project (http://www.nasa.gov/mission_pages/station/science/experiments/Expose.html#overview; Baglioni et al., 2007) with a similar UV irradiation device (wavelength range 200–400 nm) and a solar lamp (SOL2000) suggest survival of halite-embedded Hcc. dombrowskii of a dose of 1 × 105 kJ/m2 (Fendrihan and Polacsek, unpublished data.).
Survival of cells of Hcc. dombrowskii that were exposed to the UV spectrum while in liquid medium was lower by about 2 orders of magnitude than when cells were embedded in halite fluid inclusions during exposure; even less survival was observed when cells were spread on the surface of agar plates and exposed to UV radiation. Less mobility of cells on agar plates than is the case for cells in liquid culture may result in more cell damage and account for the approximately 2.5-fold difference in survival of UV radiation. Given the novel nature of these results, several phylogenetically different strains of haloarchaea were subjected to essentially the same UV exposure procedures. Apart from some differences in the estimated values for D37, the results were similar for all strains, which suggests that cells entrapped in fluid inclusions of halite receive some degree of protection from radiation.
In this study, an estimation of archaeal survivors of exposure to UV radiation was attempted for the first time by using a non-growth-based method—staining with the dyes of the LIVE/DEAD kit, which provides a measure of membrane intactness. This is a very rapid method for assessment of viability, compared to growth-based methods, such as determination of CFUs, which can require weeks of incubation with some strains. The limitations and advantages of several non-growth methods for the determination of stress-exposed cells of Escherichia coli, including UV radiation, were reviewed recently by Villarino et al. (2000). The data obtained with the LIVE/DEAD kit for haloarchaea show a decline of cell viability, concomitant with increase of radiation dose (Figs. 3 and 4). This result suggests membrane damage by UV radiation, which probably affects the transport systems. Koch et al. (1976) suggested that UV radiation resulted in protein damage within the transport system of Escherichia coli and concomitant leakiness of membranes. Recently, extensive oxidation of prokaryotic proteins during irradiation was reported for Deinococcus radiodurans (Daly et al., 2007) and Shewanella oneidensis (Qiu et al., 2006). It is now believed that protein damage produces even more deleterious affects within cells than does DNA damage due to radiation. Though our results suggest that the LIVE/DEAD kit can be used as a suitable method for the determination of relative numbers of survivors in parallel experiments, we found that the number of survivors obtained with the LIVE/DEAD kit were consistently higher than those obtained from CFUs of aliquots of the same samples (Figs. 3 and 4). We suspect the organisms may reach a viable, though nonculturable state, which can manifest itself in extended lag phases, as was observed in our liquid cultures following UV radiation (data not shown). It is worth noting that the mechanisms for inactivation of haloarchaea by UV radiation are not well known and still need to be elucidated (see Goosen and Moolenaar, 2008, for a review).
The marked increase in survival of salt-embedded haloarchaeal cells is attributed to the properties of halite, which cause attenuation of the UV radiation due to the presence of color centers in halite’s crystal structure that lead to a partial absorption of the light of shorter wavelength and re-emission at longer wavelengths. Color centers do not appear in liquids; therefore, the absorption due to crystal vacancies (color centers) is probably one of the main reasons why the survival rates of Hcc. dombrowskii and Hbt. salinarum were increased in the crystal when compared to survival rates for the liquid and the agar plate. Drying of natural brines populated by halophilic microorganisms will lead to the formation of cubic crystals of different sizes that contain minerals, fluid inclusions, and various amounts of cells within fluid inclusions. The physicochemical “system” where halophilic archaea and bacteria tend to be found today is, indeed, very old, since fluid inclusions in the microliter range were detected in billion-year-old halite within meteorites (Zolensky et al., 1999). In addition, nonhalophilic microorganisms could also become embedded in halite and possibly remain there for extended times, as was shown recently by Adamski et al. (2006). Dispersal of halite crystals can occur by wind, as was suggested by Wheeler (1985), who found cubes of halite among Sahara Desert sands that had blown over Britain in the year 1984. The well-documented dust storms on Mars might provide conditions for dispersal of crystals within which microorganisms could potentially reside, regardless of whether they are indigenous or a result of forward contamination. Such crystals, on the inhospitable surface of Mars, could provide sufficient shielding from UV radiation that might allow the microorganisms within them to remain viable.
Acknowledgments
This work was supported by the Austrian Science Foundation (FWF), projects P16260-B07 and P18256-B06, and by the Austrian Research Foundation (FFG), ASAP project 815141 HALOSPACE. We thank Petra Rettberg, Elke Rabbow, and Corinna Panitz, all at DLR Köln, for help with exposure experiments.
Abbreviations
- CFUs
colony-forming units
- OD
optical density
References
- Adamski JC, Roberts JA, Goldstein RH. Entrapment of bacteria in fluid inclusions in laboratory-grown halite. Astrobiology. 2006;6:552–562. doi: 10.1089/ast.2006.6.552. [DOI] [PubMed] [Google Scholar]
- Baglioni P, Sabbatini M, Horneck G. Astrobiology experiments in low Earth orbit: facilities, instrumentation and results. In: Horneck G, Rettberg P, editors. Complete Course of Astrobiology. Wiley VCH; Weinheim: 2007. pp. 273–319. [Google Scholar]
- Baliga NS, Bjork SJ, Bonneau R, Pan M, Iloanusi C, Kottemann MCH, Hood L, DiRuggiero J. Systems level insights into the stress response to UV radiation in the halophilic archaeon Halobacterium NRC-1. Genome Res. 2004;14:1025–1035. doi: 10.1101/gr.1993504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérces A, Kovács G, Lammer H, Kolb C, Rontó G. Life and the solar UV environment on early Earth [abstract COSPAR2006-A-02493]. 36th COSPAR Scientific Assembly; Paris: Committee on Space Research; 2006. [Google Scholar]
- Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Cockell CS. Biological effects of high ultraviolet radiation on early Earth—a theoretical evaluation. J. Theor. Biol. 1998;193:717–729. doi: 10.1006/jtbi.1998.0738. [DOI] [PubMed] [Google Scholar]
- Cockell CS, Brack A, Wynn-Williams DD, Baglioni P, Brandstätter F, Demets R, Edwards HG, Gronstal AL, Kurat G, Lee P, Osinski GR, Pearce DA, Pillinger JM, Roten CA, Sancisi-Frey S. Interplanetary transfer of photosynthesis: an experimental demonstration of a selective dispersal filter in planetary island biogeography. Astrobiology. 2007;7:1–9. doi: 10.1089/ast.2006.0038. [DOI] [PubMed] [Google Scholar]
- Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li SM, Kemner KM, Fredrickson JK. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5(4):e92. doi: 10.1371/journal.pbio.0050092. doi:10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner EBM, McGenity TJ, Busse H-J, Wanner G, Grant WD, Stan-Lotter H. Halococcus salifodinae sp. nov., an archaeal isolate from an Austrian salt mine. Int. J. Syst. Bacteriol. 1994;44:774–780. [Google Scholar]
- Fendrihan S, Stan-Lotter H. Survival of halobacteria in fluid inclusions as a model of possible biotic survival in martian halite. In: Teodorescu HN, Griebel HS, editors. Mars and Planetary Science and Technology. Performantica Press; Iasi, Romania: 2004. pp. 9–18. Selected papers from EMC’04. [Google Scholar]
- Fendrihan S, Legat A, Gruber C, Pfaffenhuemer M, Weidler G, Gerbl F, Stan-Lotter H. Extremely halophilic archaea and the issue of long term microbial survival. Rev. Environ. Sci. Biotechnol. 2006;5:1569–1605. doi: 10.1007/s11157-006-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsterer S, Musso M, Asenbaum A, Fürnkranz D. Reflectance measurements of glossy petals of Ranunculus lingua (Ranunculaceae) and of non-glossy petals of Heliopsis helianthoides (Asteraceae) Plant Biol. 1999;1:70–78. [Google Scholar]
- Gooding JL. Soil mineralogy and chemistry on Mars: possible clues from salts and clays in SNC meteorites. Icarus. 1992;99:28–41. [Google Scholar]
- Goosen N, Moolenaar GF. Repair of UV damage in bacteria. DNA Repair (Amst) 2008;7:353–379. doi: 10.1016/j.dnarep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Grant WD, Gemmell RT, McGenity TJ. Halobacteria: the evidence for longevity. Extremophiles. 1998;2:279–287. doi: 10.1007/s007920050070. [DOI] [PubMed] [Google Scholar]
- Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse H-J, Stan-Lotter H. Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permo-Triassic salt deposit, classification of Halobacterium sp. NRC-1 as a strain of Halobacterium salinarum and emended description of Halobacterium salinarum. Extremophiles. 2004;8:431–439. doi: 10.1007/s00792-004-0403-6. [DOI] [PubMed] [Google Scholar]
- Haugland RP. LIVE/DEAD BacLight bacterial viability kits. In: Gregory J, editor. Handbook of Fluorescent Probes and Research Products. 9th ed Molecular Probes; Eugene, Oregon: 2002. pp. 626–628. [Google Scholar]
- Horneck G. Responses of Bacillus subtilis spores to space environment: results from experiments in space. Orig. Life Evol. Biosph. 1993;23:37–52. doi: 10.1007/BF01581989. [DOI] [PubMed] [Google Scholar]
- Horneck G, Rettberg P, Reitz G, Wehner J, Eschweiler U, Strauch K, Panitz C, Starke V, Baumstark-Khan C. Protection of bacterial spores in space, a contribution to the discussion of panspermia. Orig. Life Evol. Biosph. 2001;31:527–547. doi: 10.1023/a:1012746130771. [DOI] [PubMed] [Google Scholar]
- Kittel C. Introduction to Solid State Physics. John Wiley and Sons, Inc.; New York: 2006. [Google Scholar]
- Koch AL, Doyle RJ, Kubitschek HE. Inactivation of membrane transport in Escherichia coli by near-ultraviolet light. J. Bacteriol. 1976;126:140–146. doi: 10.1128/jb.126.1.140-146.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C, Abart R, Bérces A, Garry JRC, Hansen AA, Hohenau W, Kargl G, Lammer H, Patel MR, Rettberg P, Stan-Lotter H. An ultraviolet simulator for the incident martian surface radiation and its applications. Int. J. Astrobiology. 2005;4:241–249. [Google Scholar]
- Langanger M, Jokl S, Musso M. UV-reflectance in flowers of Nymphea alba L. and Nuphar lutea (L.) Sm. (Nymphaeaceae) Aquat. Bot. 2000;67:13–21. [Google Scholar]
- Leuko S, Legat A, Fendrihan S, Stan-Lotter H. Evaluation of the LIVE/DEAD BacLight kit for extremophilic archaea and environmental hypersaline samples. Appl. Environ. Microbiol. 2004;70:6884–6886. doi: 10.1128/AEM.70.11.6884-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli RL, Klovstad M. Martian soil and UV radiation: microbial viability assessment on spacecraft surfaces. Planet. Space Sci. 2000;48:1093–1097. [Google Scholar]
- Mancinelli RL, White MR, Rothschild LJ. Biopan survival I: exposure of the osmophile Synechococcus sp. (Nägeli) and Haloarcula sp. to the space environment. Adv. Space Res. 1998;22:327–334. [Google Scholar]
- Marder MP. Condensed Matter Physics. John Wiley and Sons, Inc.; New York: 2000. [Google Scholar]
- McCready S. The repair of ultraviolet light-induced DNA damage in the halophilic archaebacteria, Halobacterium cutirubrum, Halobacterium halobium and Haloferax volcanii. Mutat. Res. 1996;364:25–32. doi: 10.1016/0921-8777(96)00018-3. [DOI] [PubMed] [Google Scholar]
- McGenity TJ, Gemmell RT, Grant WD, Stan-Lotter H. Origins of halophilic micro-organisms in ancient salt deposits (MiniReview) Environ. Microbiol. 2000;2:243–250. doi: 10.1046/j.1462-2920.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, Shukla HD, Lasky SR, Baliga NS, Thorsson V, Sbrogna J, Swartzell S, Weir D, Hall J, Dahl TA, Welti R, Goo YA, Leithauser B, Keller K, Cruz R, Danson MJ, Hough DW, Maddocks DG, Jablonski PE, Krebs MP, Angevine CM, Dale H, Isenbarger TA, Peck RF, Pohlschröder M, Spudich JL, Jung KW, Alam M, Freitas T, Hou S, Daniels CJ, Dennis PP, Omer AD, Ebhardt H, Lowe TM, Liang P, Riley M, Hood L, DasSarma S. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12176–12181. doi: 10.1073/pnas.190337797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet EG, Sleep NH. The habitat and nature of early life. Nature. 2001;409:1083–1091. doi: 10.1038/35059210. [DOI] [PubMed] [Google Scholar]
- Patel MR, Bérces A, Kerékgyárto T, Rontó G, Lammer H, Zarnecki JC. Annual solar UV exposure and biological effective dose rates on the martian surface. Adv. Space Res. 2004;33:1247–1252. doi: 10.1016/j.asr.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Qiu X, Daly MJ, Vasilenko A, Omelchenko MV, Gaidamakova EK, Wu L, Zhou J, Sundin GW, Tiedje JM. Transcriptome analysis applied to survival of Shewanella oneidensis MR-1 exposed to ionizing radiation. J. Bacteriol. 2006;188:1199–1204. doi: 10.1128/JB.188.3.1199-1204.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radax C, Gruber C, Stan-Lotter H. Novel haloarchaeal 16S rRNA gene sequences from Alpine Permo-Triassic rock salt. Extremophiles. 2001;5:221–228. doi: 10.1007/s007920100192. [DOI] [PubMed] [Google Scholar]
- Rieder R, Gellert R, Anderson RC, Bruckner J, Clark BC, Dreibus G, Economou T, Klingelhöfer G, Lugmair GW, Ming DW, Squyres SW, d’Uston C, Wanke H, Yen A, Zipfel J. Chemistry of rocks and soils at Meridiani Planum from the Alpha Particle X-ray Spectrometer. Science. 2004;306:1746–1749. doi: 10.1126/science.1104358. [DOI] [PubMed] [Google Scholar]
- Rontó G, Bérces A, Lammer H, Cockell CS, Molina-Cuberos GJ, Patel MR, Selsis F. Solar UV irradiation conditions on the surface of Mars. Photochem. Photobiol. 2003;77:34–40. doi: 10.1562/0031-8655(2003)077<0034:suicot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sancho LG, de la Torre R, Horneck G, Ascaso C, de los Rios A, Pintado A, Wierzchos J, Schuster M. Lichens survive in space: results from the 2005 LICHENS experiment. Astrobiology. 2007;7:443–454. doi: 10.1089/ast.2006.0046. [DOI] [PubMed] [Google Scholar]
- Schidlowski M. Search for morphologigal and biochemical vestiges of fossil life in extraterrestrial settings: utility of terrestrial evidence. In: Horneck G, Baumstark-Khan C, editors. Astrobiology. The Quest for the Conditions of Life. Springer Verlag; Berlin: 2002. pp. 373–386. [Google Scholar]
- Shahmohammadi HR, Asgarani E, Terato H, Ide H, Yamamoto O. Effects of 60Co gamma-rays, ultraviolet light, and mitomycin C on Halobacterium salinarium and Thiobacillus intermedius. J. Radiat. Res. 1997;38:37–43. doi: 10.1269/jrr.38.37. [DOI] [PubMed] [Google Scholar]
- Squyres SW, Knoll AH, Arvidson RE, Clark BC, Grotzinger JP, Jolliff BL, McLennan SM, Tosca N, Bell JF, III, Calvin WM, Farrand WH, Glotch TD, Golombek MP, Herkenhoff KE, Johnson JR, Klingelhöfer G, McSween HY, Yen AS. Two years at Meridiani Planum: results from the Opportunity Rover. Science. 2006;313:1403–1407. doi: 10.1126/science.1130890. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H, McGenity TJ, Legat A, Denner EBM, Glaser K, Stetter KO, Wanner G. Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits. Microbiology. 1999;145:3565–3574. doi: 10.1099/00221287-145-12-3565. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H, Pfaffenhuemer M, Legat A, Busse H-J, Radax C, Gruber C. Halococcus dombrowskii sp. nov., an archaeal isolate from a Permo-Triassic alpine salt deposit. Int. J. Syst. Evol. Microbiol. 2002;52:1807–1814. doi: 10.1099/00207713-52-5-1807. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H, Leuko S, Legat A, Fendrihan S. The assessment of the viability of halophilic microorganisms in natural communities. In: Oren A, Rainey F, editors. Methods in Microbiology. Extremophiles. Vol. 35. Elsevier; Oxford: 2006. pp. 569–584. [Google Scholar]
- Tomlinson GA, Hochstein LI. Halobacterium saccharovorum sp. nov., a carbohydrate-metabolizing, extremely halophilic bacterium. Can. J. Microbiol. 1976;22:587–591. doi: 10.1139/m76-087. [DOI] [PubMed] [Google Scholar]
- Treiman AH, Gleason JD, Bogard DD. The SNC meteorites are from Mars. Planet. Space Sci. 2000;48:1213–1230. [Google Scholar]
- Villarino A, Bouvet OM, Regnault B, Martin-Delautre S, Grimont PAD. Exploring the frontier between life and death in Escherichia coli: evaluation of different viability markers in live and heat- or UV-killed cells. Res. Microbiol. 2000;151:755–768. doi: 10.1016/s0923-2508(00)01141-4. [DOI] [PubMed] [Google Scholar]
- Westall F, de Ronde CEJ, Southam G, Grassineau N, Colas M, Cockell CS, Lammer H. Implications of a 3.472–3.333 Gyr-old subaerial microbial mat from the Barberton Greenstone Belt, South Africa for the UV environmental conditions on the early Earth. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2006;361:1857–1875. doi: 10.1098/rstb.2006.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DA. An analysis of the aeolian dustfall on Eastern Britain, November 1984. Proceedings of the Yorkshire Geological Society. 1985;45:307–309. [Google Scholar]
- Zhou P, Wen J, Oren A, Chen M, Wu M. Genomic survey of sequence features for ultraviolet tolerance in haloarchaea (family Halobacteriaceae) Genomics. 2007;90:103–109. doi: 10.1016/j.ygeno.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Zolensky ME, Bodnar RJ, Gibson EK, Nyquist LE, Reese Y, Shih CY, Wiesman H. Asteroidal water within fluid inclusion-bearing halite in an H5 chondrite, Monahans (1998) Science. 1999;285:1377–1379. doi: 10.1126/science.285.5432.1377. [DOI] [PubMed] [Google Scholar]