Abstract
Follicular helper (TFH) cells provide crucial signals to germinal center B cells undergoing somatic hypermutation and selection that results in affinity maturation. Tight control of TFH numbers maintains self-tolerance. We describe a population of Foxp3+Blimp-1+CD4+ T cells constituting 10-25% of the CXCR5highPD-1highCD4+ T cells found in germinal center after immunization. These follicular regulatory T cells (TFR) share phenotypic characteristics with TFH and conventional Foxp3+ regulatory T cells (Treg) yet are distinct from either. Similar to TFH cells, TFR development depends on Bcl-6, SAP, CD28 and B cells; however TFR originate from thymic-derived Foxp3+ precursors, not naïve or TFH cells. TFR are suppressive in vitro and limit TFH and germinal center B cell numbers in vivo. In the absence of TFR, an outgrowth of non-antigen-specific B cells in germinal centers leads to fewer antigen-specific cells. Thus, Treg cells use the TFH differentiation pathway to produce specialized suppressor cells that control the germinal center response.
Germinal centers are clusters of rapidly-dividing B cells formed in secondary lymphoid tissues in response to T-dependent antigens. Within germinal centers, mutation of the B cell receptor V-region genes together with subsequent selection results in the production of high affinity plasma cells and memory B cells1. Defective selection can result in the production of autoantibodies and a break in self-tolerance2, 3. Germinal center B cell selection can in part be mediated by a specialized helper T cell subset, CXCR5highPD-1high T follicular helper (TFH) cells4. TFH cells develop in a Bcl-6-dependent manner and provide germinal center B cells with survival and selection signals. Limiting the numbers of TFH cells within germinal centers has been shown to be critical to prevent the emergence of autoantibodies5,6. Little is known about TFH control; in mice, Qa-1-restricted CD8 T cells can regulate the TFH compartment7 and in humans, CD4+CD25+CD69− T cells with a suppressive function in vitro have been found in germinal centers8, 9. Regulatory T Cells (Treg) have also been shown enter the primary B cell follicle in mice, but their phenotype, ontogeny and ability to control TFH cells remain unknown10.
Treg that develop in a Foxp3-dependent manner repress the growth and function of CD4+ effector T cells. Humans and mice lacking Foxp3 cannot form Treg and develop fatal autoimmunity11-15. In order to repress TH1-, TH2- and TH17- mediated immune responses, Treg have been shown to co-opt selective aspects of the differentiation programs required for these TH subsets: Tbet/Stat1, IRF-4 or Rorγt signaling respectively16-18. Here we show that Foxp3+ Treg can be diverted to become TFH repressors via expression of Bcl6 and SAP-mediated interaction with B cells. The resulting follicular regulatory T cells (TFR) share features of both TFH and Treg cells, localize to germinal centers, and regulate the size of the TFH cell population and germinal centers in vivo.
Foxp3+ follicular regulatory T cells are distinct from TFH and Treg
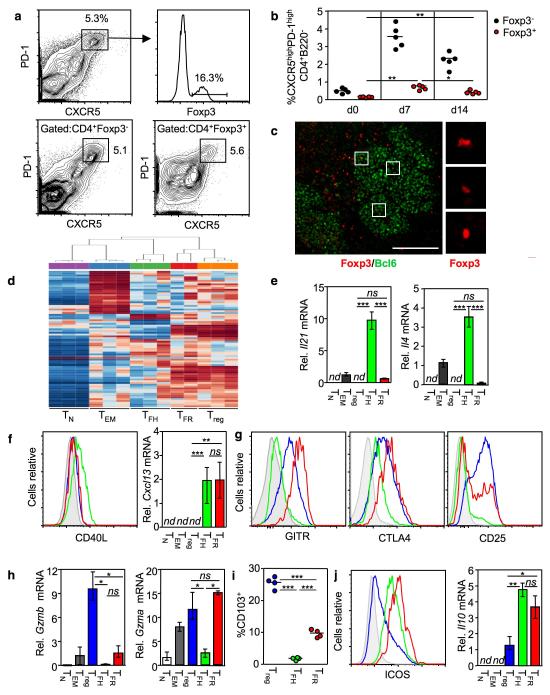
After immunization with a T-dependent antigen we observed that ~10-25% of CD4+CXCR5highPD-1high ‘TFH’ cells expressed the transcriptional regulator of the Treg lineage, Foxp3 (Figure 1a). These cells followed the same formation and resolution kinetics as conventional TFH cells (Figure 1b). Foxp3+ cells could be visualized within germinal centers identified by immunofluorescence staining of frozen spleen sections from immunized mice (Fig. 1c); 17% ± 8% of germinal center CD3+ cells also expressed Foxp3.
Figure 1. A proportion of CXCR5highPD-1highCD4+ cells express the transcription factor Foxp3.
(a,b) After SRBC immunization Foxp3+ cells were identified in the CXCR5highPD-1highCD4+ ‘TFH’ compartment, these cells follow the same kinetics as classic TFH cells. (c) Foxp3+ cells (red) are present within the Bcl6+ germinal center area (green) following SRBC immunization. Scale bar represents 100μm. (d) Heat map comparing the gene expression profiles of different CD4+ T cell subsets from Foxp3GFP mice seven days after immunization. Red: high gene expression; blue: low gene expression. The cells were sorted using the following markers and for simplicity will be referred by the abbreviations in parentheses throughout: CD4+CD44lowFoxp3− naïve (TN) cells, CD4+CD44highCXCR5int/lowPD-1int/lowFoxp3− effector/memory (TEM) cells, CD4+CD44intCXCR5int/lowPD-1int/lowFoxp3+ regulatory T cells (Treg), CD4+CXCR5highPD-1highFoxp3− T follicular helper (TFH) cells and CD4+CXCR5highPD-1highFoxp3+ follicular regulatory (TFR) cells. (e) Il21 and Il4 mRNA measured by quantitative PCR from sorted cells using the strategy described in (d) normalized to Gapdh. Heights of the bars represent the mean and error bars represent the range of expression from 3 biological replicates. nd: gene expression not detected. (f) Left: Intracellular expression of CD40L as determined by flow cytometry in Treg (blue), TFH (green) and TFR (red) cell populations; the grey histogram represents a staining control from an immunized CD40L-deficient mouse. Right: Cxcl13 mRNA measured by quantitative RT-PCR as described in (e). (g) Cell surface expression of GITR, CD25 and intracellular CTLA4 in Treg (blue), TFH (green) and TFR (red) cell populations; grey histograms represent the isotype control. (h) Relative Gzmb and Gzma mRNA determined by quantitative RT-PCR as described in (e). (i) Percentage of CD103+ cells within the Treg, TFH & TFR populations, each symbol represents one mouse. (j) Left: Cell surface expression of ICOS as determined by flow cytometry in Treg (blue), TFH (green) and TFR (red) cell populations; the grey histogram represents staining level of an isotype control. Right: Il10 mRNA detected by quantitative RT-PCR of as described in (e). Flow cytometric and RT-PCR data are representative of at least three independent experiments. In (e)-(i): Statistical significance was determined using a one-way ANOVA analysis with Bonferroni’s multiple testing correction; * P<0.05; **P<0.01; ***P<0.001.
To obtain information about the identity and function of CD4+CXCR5highPD-1high Foxp3+ cells - designated TFR, we performed microarray expression profiling on sorted populations from Foxp3GFP mice19 seven days after SRBC immunization. Treg, TFH, non-TFH effector/memory cells (TEM) and naïve (TN) T cells were also included (sorting strategy is depicted in Supplementary Fig. 1). TFR more closely resembled Treg than TFH, TEM or TN (Fig. 1d and Supplementary Table 1), with elevated expression of many Treg associated genes including Foxp3, Ctla4, Gitr, Klrg1 and Prdm1. Nevertheless, TFR also expressed high amounts of the prototypic TFH genes Cxcr5, Pdcd1, Bcl6, Cxcl13, and Icos. TFR did not express the helper cytokines IL-21 or IL-4 (Fig. 1e) or the costimulatory ligand CD40L (Fig. 1f), but expressed comparably high levels of the ligand for CXCR5, CXCL1320, as TFH cells (Figure 1f). Differential expression of TFH or Treg associated molecules was confirmed by flow cytometry and/or real time PCR (Fig. 1g-i and Supplementary Fig. 2).
TFR expressed numerous molecules characteristically expressed by Treg, such as GITR and CTLA-4, but at higher levels than Treg, consistent with an activated Treg phenotype21 (Figure 1g). Gzma expression was comparable to Treg (Figure 1h) but Gzmb, a reported target of Bcl-6 repression22 was barely detectable. TFR also expressed high levels of Il10 mRNA and surface ICOS protein, which are common to both TFH and Treg cells (Figure 1j and Supplementary Fig. 2). The elevated levels of GITR, IL-10 and ICOS on TFR compared with the rest of the Treg pool is consistent with an effector Treg phenotype23, which suggests TFR have a regulatory function. The phenotypic features shared by TFH cells and TFR may account for their common germinal center localization.
TFR and TFH cells require similar differentiation cues for their formation and maintenance
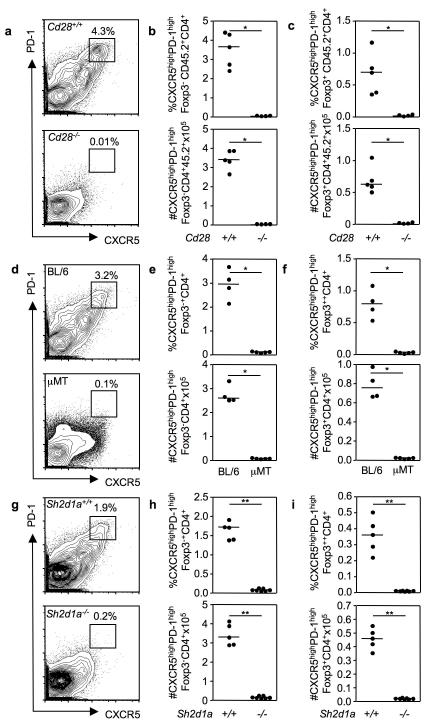
As both TFH cells and TFR co-localize in germinal centers, we sought to determine whether TFR formation was dependent on similar developmental cues. T cell priming through CD28 is one of the first signals required for TFH development24, 25. Enumeration of TFH and TFR in mixed Cd28−/− CD45.2: CD45.1 Cd28+/+ bone marrow chimeras immunized seven days previously with SRBC revealed a complete absence of both TFH and TFR cells in the absence of CD28 signaling (Fig. 2a-c). Consistent with previous reports26, 27, CD28-deficiency moderately reduced peripheral Treg numbers (Supplementary Fig. 3a).
Figure 2. TFR require the same differentiation cues as TFH cells for their development.
Flow cytometric contour plots (a, d, g) and dot plots of TFH (b, e, h) and TFR (c, f, i) cells in the groups of mice described below, seven days after SRBC immunization. (a-c) Mixed bone marrow chimeras generated by sub-lethally irradiating Rag2−/− mice and reconstituting their immune system with a 1:1 ratio of bone marrow cells from CD45.1 Cd28+/+ and CD45.2 Cd28−/− mice or control CD45.1 Cd28+/+ and CD45.2 Cd28+/+. (d-f) C57BL/6 (BL/6) and B-cell deficient μMT mice. (g-i) Sh2d1a+/+ and Sh2d1a−/− mice. Each symbol represents one mouse and horizontal bars represent median values. Figures represent one of 3 independent experiments with similar results. Statistical significance was determined using a Mann-Whitney Test: *P<0.05, **P<0.01.
SAP-dependent interactions of TFH precursors with B cells are required for TFH formation and/or maintenance5, 28-30. We therefore investigated whether interactions with B cells and/or SAP-mediated signals are essential for TFR formation. Neither TFH nor TFR cells formed after SRBC immunization of B cell deficient μMT mice (Fig. 2d-f) whereas Treg formed normally (Supplementary Fig. 3b). In addition, immunization of Sap-deficient (Sh2d1a−/−) mice revealed that, similar to TFH cells (Figure 3g, h), TFR cells are dependent on SAP for their formation (Figure 3i). Treg were only slightly reduced in the absence of SAP (Supplementary Fig. 3c). These data demonstrate that the developmental requirements of TFR are similar to those that govern TFH formation and dispensable for the generation of Treg.
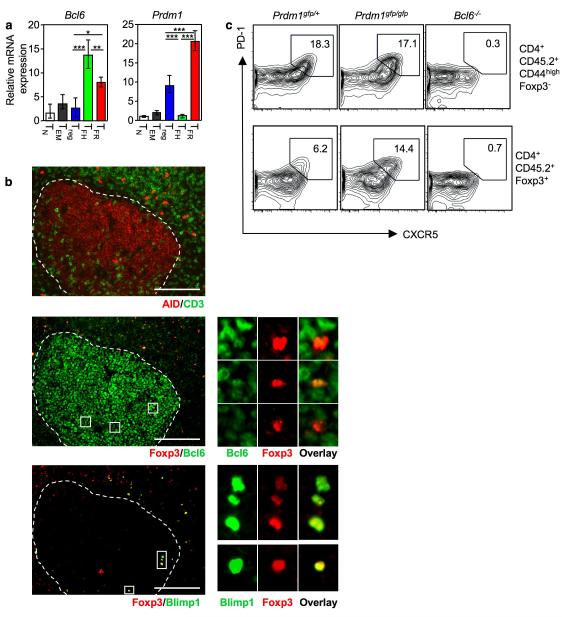
Figure 3. TFR cells express Bcl-6 and Blimp-1.
(a) Bcl6 and Prdm1 mRNA normalized to Gapdh determined by quantitative RT-PCR from sorted cells using the strategy described in Fig. 1d and Supplementary Fig. 1. Heights of the bars represent the mean and error bars represent the range of expression from 3 biological replicates. Statistical significance was determined using a one-way ANOVA analysis with Bonferroni’s multiple testing correction; * P<0.05; **P<0.01; ***P<0.001. Bar graphs are representative of 3 experiments. (b) Immunofluorescence of frozen spleen sections from mice immunized seven days previously with SRBC. The germinal center is demarcated by the white dotted line in the three consecutive sections. Upper panel: AID (red) and CD3 (green); middle panel: Foxp3 (red) and Bcl-6 (green); lower panel: Foxp3 (red) and Blimp1 (green). Scale bar represents 100μm. (c) Flow cytometric contour plots of TFH (upper panels) & TFR (lower panels) formation in the draining (mediastinal) lymph node ten days after intranasal influenza infection of mixed fetal liver chimeras reconstituted with a 1:1 ratio of fetal liver cells from E14.5 CD45.2 Prdm1gfp/gfp : CD45.1 Prdm1+/+ embryos, E14.5 CD45.2 Bcl6−/− : CD45.1 Bcl6+/+ embryos or control E14.5 CD45.2 Prdm1gfp/+ : CD45.1 Prdm1+/+ embryos.
Coordinated Bcl-6 and Blimp-1 expression in TFR cells
Bcl-6, the transcriptional regulator of the TFH subset, regulates key molecules required for follicular localization and function31-33 in a process thought to be counteracted by the transcriptional repressor Blimp-131; Bcl-6 and Blimp-1 also mutually repress each other during B cell differentiation34, 35. We asked whether, similar to TFH cells, TFR cells also expressed Bcl-6 and would be devoid of Blimp-1 expression. Quantitative RT-PCR revealed that Bcl6 was expressed in TFR cells (Fig. 3a). Of note, TFR co-expressed Prdm1, the gene encoding Blimp-1, and its expression on TFR was higher than in any other CD4 T cell subset (Fig. 3a). Expression of Bcl-6 and Blimp-1 protein in Foxp3+ cells within germinal center identified was also confirmed by immunofluorescence staining of spleen sections from SRBC-immunized mice. All Foxp3+ TFr within AID+ germinal center expressed Bcl-6, albeit at low levels (Fig. 3b) and 75% stained positive for Blimp-1 7 days after immunization; this proportion was reduced to 50% by day 14.
Bcl-6 is required for TFR formation and Blimp-1 regulates TFR homeostasis
To determine whether Blimp-1 and/or Bcl-6 play a role in TFR cell formation or homeostasis, we reconstituted sub-lethally irradiated CD45.1 mice with a 1:1 ratio of fetal liver cells from congenically-marked Prdm1+/+ and Prdm1gfp/gfp embryos, Bcl6−/− and Bcl6+/+ embryos or control Prdm1+/+ and Prdm1gfp/+ embryos. Eight weeks after reconstitution the mice were infected intranasally with influenza virus (HKx31), and 10 days later TFR formation was assessed in the mediastinal lymph node. In contrast to published data31, loss of Blimp-1 did not alter the proportion of TFH cells but caused TFR to double (Fig. 3c), suggesting that Blimp-1 limits the size of the TFR population. This is consistent with a recent report showing Blimp-1 limits the numbers of effector Treg through a Bcl-2-dependent mechanism23.
Mixed Bcl6−/−: Bcl6+/+ chimeras confirmed previous reports that TFH cells do not form in the absence of Bcl-6 (Fig. 3c, upper panel). Cells lacking Bcl-6 expression did not give rise to TFR cells despite the presence of germinal centers in the mice (Fig. 3c, lower panel). Similar results demonstrating the requirement for Bcl-6 in splenic TFR generation were obtained after SRBC immunization (Supplementary fig. 4a-c). As reported previously33, Bcl-6 was dispensable for Treg formation (Supplementary fig. 4d). Together, this suggests that Bcl-6 is essential for TFR formation and Blimp-1 expression regulates the size of the TFR population.
Although co-expression of Bcl-6 and Blimp-1 seems paradoxical, there are precedents in which both Prdm1 and Bcl6 are co-regulated; for example in both effector and memory CD8+ subsets36. Blimp-1 has been recently shown to influence Treg function inducing an effector phenotype23,37. It is expressed by Treg at mucosal sites and by a small (8-12%) subset of splenic Treg, which produce IL-10 in a Blimp-1-dependent manner37. Blimp-1+ Treg and TFR cells also share expression of high amounts of IL-10, GITR and ICOS23. TFR are thus likely to be the follicular counterparts of the Blimp-1+ IL-10+ effector Treg found at mucosal surfaces.
TFR cells derive from Treg precursors
The observation that TFR require the same cues as TFH for their differentiation raised a critical question: do TFR represent induced Treg that arise from TFH cells that switch on Foxp3 in the germinal center, or do they derive from Foxp3+ Treg? Plasticity of CD4 helper T cell subsets is well documented38, as is the adoption of TH transcriptional programs by Treg16-18.
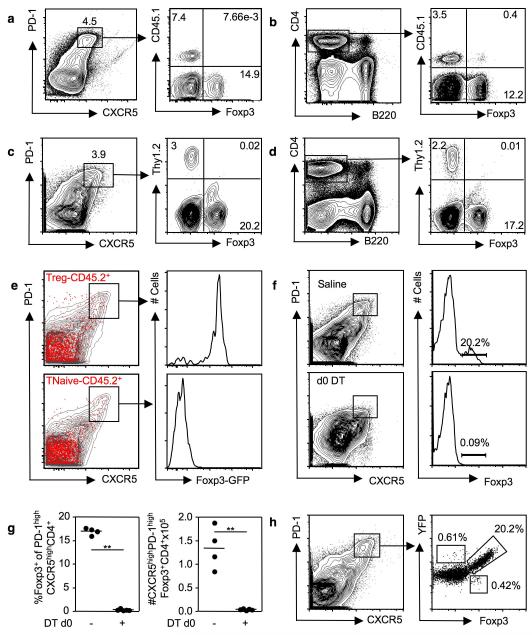
In order to test whether TFR derive from TFH cells that turn on Foxp3 expression we transferred 1×105 naïve cells (CD4+CD44lowCD25−) from CD45.1 mice expressing the 3A9 TCR transgene (TCRHEL), which recognizes hen egg lysozyme (HEL) peptide presented by I-Ak, into congenic CD45.2 B10.BR mice. Seven days after immunization with HEL in alum, 6-10% of TFH cells derived from the donor HEL-TCR T cells, but no donor-origin TFR could be identified; all TFR derived exclusively from the recipients’ cells (Fig. 4a). Between 1-2% of the transferred naïve TCRHEL donor population developed into inducible Treg (Fig. 4b). To exclude that an idiosyncratic effect of the 3A9 transgene had precluded development of TFR cells, this experiment was repeated with OT-II transgenic T cells: Again, OT-II T cells could form TFH but not TFR (Fig. 4c, d), suggesting that TFR do not derive from TFH cells.
Figure 4. TFR derive from Foxp3+ precursors.
Flow cytometric contour plots of splenic CD4+CXCR5highPD-1high cells (a) or CD4+ cells (b) seven days after 1×105 transferred transgenic TCR3A9 HEL-specific CD45.1 T cells were adoptively transferred into congenically distinct CD45.2 B10.Br mice and immunized with HEL in alum. Flow cytometric contour plots of splenic CD4+CXCR5highPD-1high cells (c) or CD4+ cells (d) seven days after adoptive transfer of 1×105 OT-II OVA-specific Thy1.2 T cells into congenically distinct Thy1.1 C57BL6 mice and immunization with OVA in alum. (e) Flow cytometric contour plots of splenic CD4+ T cells from CD45.1 C57BL/6 mice seven days after adoptive transfer of 1×106 sorted naïve CD4+CD44intFoxp3+ Treg (top panel) or CD4+CD44lowFoxp3− naïve T cells (lower panel) from unimmunized CD45.2 Foxp3GFP mice and KLH in Ribi immunization. Transferred CD45.2 cells are shown in red, the endogenous CD45.1 cells are represented by the grey contour plots. Histograms showing Foxp3-GFP expression in transferred CD45.2+CD4+CXCR5highPD-1high cells. (f) Contour plots of splenic CD4+ T cells and quantification of TFR cells (g) from Foxp3DTR mice six days after SRBC immunization and administration of either 0.9% saline (top panel) or DT (lower panel). Histograms show Foxp3+ cells within the CD4+CXCR5highPD-1high compartment. (h) Flow cytometric contour plots of splenic CD4+ cells from Foxp3-cre x ROSA-Stop-flox-YFP mice immunized seven days previously with SRBC (left panel). Enumeration of the proportion of CD4+CXCR5highPD-1high cells that expressed YFP and/or Foxp3 (right panel). Each symbol represents one mouse and horizontal bars represent median values. Figures are representative of 2-4 independent experiments.
To test whether TFR derive from Foxp3+ precursors, 1×106 naïve CD4+CD44lowFoxp3− T cells or CD44intFoxp3+ Treg from unimmunized Foxp3GFP mice were adoptively transferred into congenically-marked mice. Seven days after immunization with KLH in Ribi, ~1-2% of both donor-origin Treg and donor-origin naïve cells had upregulated CXCR5 and PD-1 to high levels (Figure 4e). More than 90% of donor-origin CXCR5highPD-1high Treg cells retained Foxp3 expression, but none of the transferred naïve T cells that became CXCR5highPD-1high after immunization switched on Foxp3 to become TFR cells (Fig. 4e).
We then asked whether thymic Treg (nTreg) could become TFR cells. Thymic Foxp3+CD4SP or Foxp3−CD4SP from Foxp3GFP CD45.2 mice were adoptively transferred into CD45.1 mice. Seven days after SRBC immunization only Foxp3+ CD45.2 cells had become TFR; Foxp3− CD45.2 cells had become TFH but not TFR (Supplementary Fig. 5). Furthermore, ~97% of TFR cells expressed Helios (Supplementary Fig. 5), a transcription factor which has been reported to be expressed by thymic-derived nTref but not Tref induced in the periphery39.
In an alternative strategy to confirm that TFR derive from Foxp3+ precursors we used mice in which the gene encoding for the diphtheria toxin receptor (DTR) has been inserted in the Foxp3 locus (Foxp3DTR), so that treatment with diphtheria toxin (DT) selectively ablates all Treg within 48 hours 40. Foxp3DTR mice were immunized with SRBC and treated with either DT or saline immediately afterwards. Six days after immunization, TFR cells had formed normally in mice that did not receive DT but were absent in DT-treated mice (Fig. 4f, g) demonstrating TFR cannot form if Foxp3+ cells are absent at the time of immunization.
Within Peyer’s patches, but not in the spleen, it has been demonstrated that a proportion of Foxp3− TFR cells derive from Foxp3+ precursors41. To investigate whether splenic TFR cells stably express Foxp3, we immunized Foxp3-Cre42 x ROSA-Stop-flox-YFP mice, in which any cell that has expressed Foxp3 is permanently marked. Seven days after SRBC immunization, the majority (97%) of YFP+ CD4+CXCR5highPD-1high TFR cells coexpressed Foxp3 and less than ~0.6% of CXCR5highPD-1high cells were Foxp3−YFP+ (Fig. 4h), consistent with the observation that Foxp3 expression is stable in the natural Treg population43. These data suggest that TFR cells derive from Foxp3+ thymic Treg that co-opt the TFH cell differentiation program to migrate to germinal center, where they maintain Foxp3 expression.
TFR cells suppress T cell proliferation in vitro and repress TFH cells in vivo
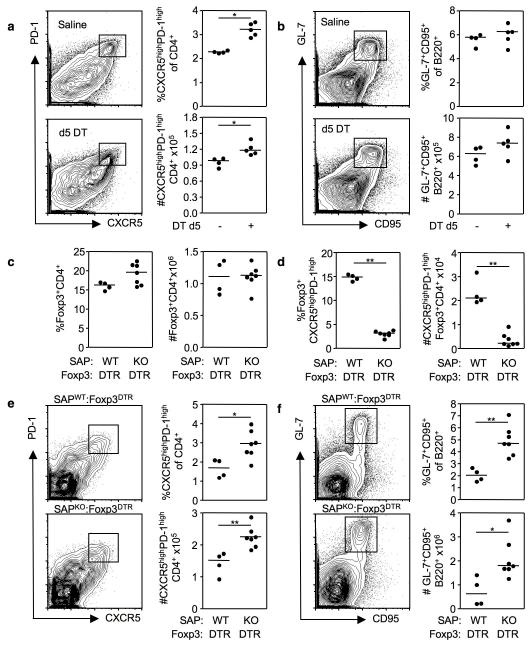
Expression of Foxp3 by CD4+ T cells initiates a transcriptional program that confers suppressor function11-13. TFR and Treg sorted from immunized Foxp3GFP mice displayed comparable suppressive ability in vitro (Supplementary Fig. 6a). To determine whether TFR cells are suppressive in vivo, we sought to ablate TFR cells after the germinal center response had been established. For this, DT was first administered to Foxp3DTR mice 5d after SRBC immunization, when TFH and TFR cells have already formed, but the response has not yet reached its peak44. After 3 days of DT treatment – 8 days after immunization – only 1% of TFR cells and 5% of Treg cells were present compared with vehicle-only-treated mice (Supplementary Fig. 7), and DT-treated mice displayed a significant increase in both the proportion and total number of TFH cells compared to controls (Fig. 5a). At this time point, germinal center B cell numbers were comparable between DT- and vehicle-treated groups (Figure 5b).
Figure 5. TFR regulate the size of the TFH population.
Flow cytometric contour plots and graphs of TFH cells (a) and germinal center B cells (b) from the spleens of Foxp3DTR mice immunized eight days previously (d0) with SRBC. Five days after immunization the mice were treated with either DT or saline. (c-f) Analysis of mixed bone marrow chimeras generated by sub-lethally irradiating Rag2−/− mice and reconstituting their immune system with either a 1:1 ratio of Sh2d1a−/− CD45.2 : Foxp3DTR CD45.1 bone marrow or control Sh2d1a+/+ CD45.2 : Foxp3DTR CD45.1 bone marrow. Eight weeks after reconstitution chimeric mice were immunized with SRBC and treated with 50μg/Kg of DT on one day prior to immunization and d2 and d5 thereafter. Splenocytes were analyzed on d8 for the proportion and total number of CD4+CXCR5highPD-1highFoxp3+ TFR cells (c), CD4+Foxp3+ Treg (d), CD4+CXCR5highPD-1high TFH cells (e) and B220+ GL-7highCD95high germinal center B cells (f). Each symbol represents one mouse and horizontal bars represent median values. Statistical significance was determined using a Mann-Whitney Test: *P<0.05, **P<0.01.
To confirm the in vivo regulatory role of TFR, it is necessary to deplete them while leaving the Treg and TFH compartments intact. To achieve this we generated mixed bone marrow chimeras with a 1:1 ratio of either congenically marked Sh2d1a−/−:Foxp3DTR or Sh2d1a+/+:Foxp3DTR marrow. After immunization and DT treatment, CD45.2+ Sh2d1a−/− cells should not able to form TFH or TFR cells, but should form Treg cells normally, whereas Foxp3DTR cells should form TFH cells, but lack all Foxp3+ cells. Thus, TFH and Treg populations should still be present in both chimeras, but TFR cells will be selectively reduced in Sh2d1a−/− : Foxp3DTR mice (Supplementary Table 2).
Such chimeric mice were generated and treated with DT 1 day prior to, and 2 and 5 days after SRBC immunization. At day 8 after immunization >90% of TFH cells in Sh2d1a−/−:Foxp3DTR chimeras derived from Foxp3DTR cells compared to ~50% in the control Sh2d1a+/+:Foxp3DTR mice (Supplementary Fig. 8a). The size of the Treg population was comparable in both groups of mice (Fig. 5c) with the majority (97%) of Treg being CD45.2+ (Supplementary Fig. 8b). Critically, the number of TFR was reduced by 5 times in Sh2d1a−/−: Foxp3DTR mice compared with the control chimeras (Fig. 5d, Supplementary Fig. 7c). There was also an increase in both the number and proportion of TFH cells (Fig. 5e) and of germinal center B cells (Fig. 5f), indicating that TFR limit the germinal center response during a T-dependent immune reaction in vivo.
TFR limit the outgrowth of non-antigen-specific germinal center B cells
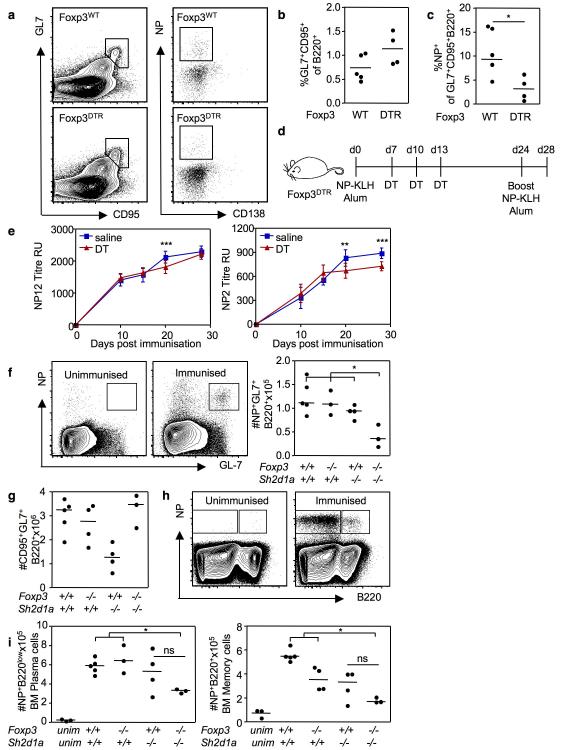
To further characterize the role of TFR during the germinal center response, TFR were transiently depleted at the peak of the germinal center response. For this, Foxp3DTR and control Foxp3WT mice were immunized with NP-KLH in alum, and treated with DT six days later. Ten days after immunization, the fraction of germinal center B cells was not significantly different between the two groups (Fig. 6a, b) but combined Treg and TFR depletion led to a reduction in the proportion of germinal center B cells specific for the dominant epitope of the immunizing antigen, the hapten NP (Fig. 6a, c).
Figure 6. TFR restrict the outgrowth of non-antigen specific clones in the germinal center.
Flow cytometric contour plots (a) and graphs (b) of total GL-7+CD95+ germinal center B cells and (c) NP+ germinal center B cells ten days after immunization of Foxp3WT and Foxp3DTR mice that have been treated with DT 6 days after NP-KLH immunization. Statistical analyses performed using Mann Whitney U-test. Experimental outline (d) of immunization and DT or saline treatment scheme of Foxp3DTR mice (n=8 per group) to examine the antigen specific immunoglobulin response over time, mice were bled prior to, and d10, d15, d20 and d28 after primary immunization. Mice were given a booster immunization 24 days after the primary immunization. (e) ELISA analysis of NP12 and NP2 antibodies in the experiment outlined in (d). Error bars represent the standard error of the mean from eight individual mice from one experiment, representative of two experiments. Statistical analyses in (e) were performed using a two-way ANOVA with Bonferroni post test to compare differences at each time point. Graphs and flow cytometric contour plots of NP+ germinal center B cells (f), total GL-7+CD95+ germinal center B cells (g) and NP+ bone marrow plasma and memory cells (h, i) 21 days after NP-CGG immunization of chimeric mice generated by reconstituting Rag2−/− mice with a 1:1 mix of Sh2d1a−/−:Foxp3−/−, Sh2d1a+/+:Foxp3+/+, Sh2d1a+/+:Foxp3−/− and Sh2d1a−/−:Foxp3+/+ fetal liver. Statistical analyses in (f, g, h and i) were performed using a one-way ANOVA with Bonferroni post test correction. Each symbol represents one mouse and horizontal bars represent median values. *P<0.05, **P<0.01, ***P<0.001.
In order to determine whether this had any long-term impact on the antigen-specific antibody response, Foxp3DTR mice were immunized with NP-KLH in alum and treated with either DT or saline at 7, 10 and 13 days after immunization, then boosted with NP-KLH in alum 24 days after primary immunization (Fig. 6d). High (anti-NP2) & low (anti-NP12) affinity anti-NP antibody titers were assessed by ELISA prior to and d10, 15, 10 and d28 after immunization. Titers of high and low affinity antibodies were comparable until d20, when both were reduced in DT-treated mice compared with controls (Fig 6e). Four days after secondary immunization (day 28 after primary challenge), high affinity antibodies remained lower in DT-treated mice (Fig 6e). This suggests that depletion of TFR and Treg during the germinal center response does not increase antigen-specific antibody production.
To test whether the reduction in the antigen-specific germinal center response was a consequence of TFR depletion rather than general Treg depletion, we generated Sh2d1a−/−:Foxp3−/− mixed fetal liver chimeras that selectively lack TFR cells. Three groups of control chimeras were generated in parallel: Sh2d1a+/+:Foxp3+/+, Sh2d1a+/+:Foxp3−/− and Sh2d1a−/−:Foxp3+/+. Eight weeks after reconstitution, chimeric mice were immunized with alum-precipitated NP-chicken gammaglobulin (NP-CGG). 21 days post-immunization, Sh2d1a−/−:Foxp3−/− chimeras had a reduction in TFR cells compared to all control groups (Supplementary Fig. 9a and b) and an expanded TFH population (Supplementary Fig. 9c). The proportion of Foxp3+ Treg was comparable amongst all 4 groups (Supplementary Fig. 9d). Consistent with a selective defect in TFR and not Treg, circulating TH1 and TH2 cells were not expanded in Sh2d1a−/−:Foxp3−/− mice 14 days post-immunization (Supplementary Fig. 10a and b) nor in the spleen 21 days after immunization (Supplementary Fig. 10c and d). This also confirms that TFR cells are specialized in the regulation of follicular responses, while other Treg effectors control TH1 and TH2 cells.
As observed in Foxp3DTR mice, the proportion and absolute number of antigen-specific (NP+) germinal center B cells was reduced in Sh2d1a−/−:Foxp3−/− mice compared with control chimeras (Fig. 6f), despite formation of abundant germinal centers (Fig. 6g). NP-specific splenic memory B cells and NP-specific bone marrow plasma cells appeared reduced in Sh2d1a−/−:Foxp3−/− chimeras compared with controls 21 days after immunization (Figure 6h and i) and the differences were statistically-significant against all control groups except for the Sh2d1a−/−:Foxp3+/+ group, in which there was greater variability. At this time point there was no difference in anti-NP antibody titers between Sh2d1a−/−:Foxp3−/− mice compared with control chimeras (Supplementary Fig. 11), probably due to a large component being of extrafollicular origin given that abundant NP+ plasma cells were still detectable in the spleen (Supplementary Fig. 11). Together, this suggests that TFR act to limit the outgrowth of non-antigen specific clones in the germinal center.
Discussion
We have shown here that in response to T-dependent antigens a proportion of naïve Treg can turn on Bcl-6, which allows them to adopt the TFH differentiation program and express the follicular homing receptor CXCR5 to localize to the germinal center. Here they exert suppressive functions on TFH cells and the germinal center response. Unlike TFH cells, TFR cells express Blimp-1, which is required to control their numbers in the germinal center. In response to other extracellular stimuli, naïve Foxp3+ cells can turn on Tbet or increase activity of IRF4 or STAT3, required for TH1, TH2 and TH17 cell formation respectively16-18. The specialized regulatory program determined by each of these transcription factors is likely to operate via modification of the Treg chemokine receptor profile in order to allow migration into an anatomical location where Treg are poised to regulate specific T cell responses to prevent autoimmunity or inflammation-associated tissue damage.
Our data suggests TFR cells are specialized in controlling the germinal center reaction through limiting the numbers of TFH cells and inhibiting selection of non-antigen-specific B cells including those carrying self-reactive receptors. Furthermore, the ability of TFR cells to ensure dominance of antigen-specific clones over the germinal center response appears essential for formation of normal numbers of long-lived plasma cells and memory B cells. Although it is likely that TFR control germinal center B cells indirectly, through their ability to limit TFH numbers, it is also possible that TFR also negatively regulate germinal center B cells directly. This would be akin to the description of direct regulation of antigen-presenting cells by Treg45, 46 and reports that Treg can directly regulate B cell function9. Germinal center B cells are the predominant APCs within the germinal center microenvironment, and this makes them attractive candidates for TFR-mediated inhibition.
Selection of cognate germinal center B cells by TFH cells is one of the key mechanisms by which germinal center tolerance is regulated47. Dysregulation of the TFH population has been previously demonstrated to result in autoimmunity3, 5, 7, 48, highlighting the need to tightly control positive selection in germinal centers. Understanding the mechanisms by which TFR are regulated and their TCR specificity will be important for dissecting the pathogenesis of the increasing number of pathologies in which TFH cells appear to play a role, including disease-associated ectopic germinal center formation seen in many autoimmune diseases3, atherosclerosis49, and chronic allograft rejection50. We therefore postulate that TFR cells may represent a critical peripheral tolerance mechanism, essential for preventing germinal center-derived autoimmunity.
Supplementary Material
Acknowledgements
We thank Xin Hu and Jessica Fitch for technical assistance, Dimitra Zotos for help with experiments not included in this manuscript, Alexander Rudensky for kind provision of Foxp3GFP mice and Marion Espeli for helpful discussions. This work was funded by NHMRC program and project grants to CGV, a VIB PI grant to AL and a Wellcome Trust Programme Grant (083650/Z/07/Z) to KGCS. MAL is supported by an EMBO Post-doctoral Long-term Fellowship (ALTF 1041-2009) and a Raymond and Beverly Sackler Junior Research Fellowship, Churchill College, Cambridge; CGV by a Viertel Senior Medical Research Fellowship; KGCS by a Lister Prize Fellowship and TFR by the National Institute of Health Research Cambridge Biomedical Research Centre; and AL by a JDRF Career Development Fellowship and a Marie Curie Reintegration Grant Fellowship.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Smith KGC, Light A, Nossal GJV, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. The EMBO Journal. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo W, et al. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. The Journal of Experimental Medicine. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nature Reviews. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 4.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. The Journal of Experimental Medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 9.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress germinal center-Th cells and germinal center-Th cell-driven B cell responses. The Journal of Clinical Investigation. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields ML, et al. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 13.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature Genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 15.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature Genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 16.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature Immunology. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. The Journal of Experimental Medicine. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida K, et al. Bcl6 controls granzyme B expression in effector CD8+ T cells. European Journal of Immunology. 2006;36:3146–3156. doi: 10.1002/eji.200636165. [DOI] [PubMed] [Google Scholar]
- 23.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature Immunology. 2011 doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 24.Linterman MA, et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Seminars in Immunopathology. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 26.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 28.Cannons JL, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deenick EK, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston RJ, et al. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science. 2009 doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurieva RI, et al. Bcl6 Mediates the Development of T Follicular Helper Cells. Science. 2009 doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu D, et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009 doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 35.Shaffer AL, et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 36.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 37.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nature Immunology. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 42.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan TD, et al. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt EM, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 46.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 47.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nature Reviews. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 48.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunology. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 49.Grabner R, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. The Journal of Experimental Medicine. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wehner JR, et al. B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89:1141–1148. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.