Abstract
The marine ecosystem is governed by a multitude of environmental cycles, all of which are linked to the periodical recurrence of the sun or the moon. In accordance with these cycles, marine species exhibit a variety of biological rhythms, ranging from circadian and circatidal rhythms to circalunar and seasonal rhythms. However, our current molecular understanding of biological rhythms and clocks is largely restricted to solar-controlled circadian and seasonal rhythms in land model species. Here, we discuss the first molecular data emerging for circalunar and circatidal rhythms and present selected species suitable for further molecular analyses. We argue that a re-focus on marine species will be crucial to understand the principles, interactions and evolution of rhythms that govern a broad range of eukaryotes, including ourselves.
Keywords: chronobiology, ecology, lunar periodicity, marine biology, photobiology
Introduction
Life evolved in the sea, an ecosystem that is governed by a multitude of environmental cycles (Box 1). These include not only the daily day-night cycle, but also cycles with shorter or longer periods, such as tides, lunar/semi-lunar cycles or the seasons. Organisms in the marine environment have adapted to these steady cycles over millions of years, and use them for synchronization on many levels. Depending on the species, behaviour, reproduction, physiology or even cell divisions are tuned to environmental cycles with differing periods, resulting in a range of biological rhythms (Box 1). Periodic changes of external stimuli – such as light or pressure – provide cues for these rhythms. In all known cases, these zeitgeber (Box 1) stimuli are either directly or indirectly linked to the periodical recurrence of sun or the moon (Fig. 1). A number of molecular players have now been uncovered that explain how species receive zeitgeber stimuli, and what constitutes the underlying molecular networks. But to date, these molecular data almost exclusively cover the rhythms under solar control.
Box 1.
Concepts and terminology
Environmental cycle: Periodically re-occurring natural conditions (e.g. day/night, high/low tides, moon phases, seasons).
Biological rhythm: Periodically re-occurring specific conditions within an organism (e.g. behavioural activity, metabolic state, gonadal maturity, etc.). Rhythms are typically in accordance with environmental cycles (see Fig. 1).
Zeitgeber: Environmental stimulus that serves as a synchronization cue.
Molecular clock: Molecular system that is able to maintain a given biological rhythm even under free-running conditions, i.e. in the absence of a zeitgeber. The molecularly best understood clock is the circadian clock. A unifying principle of eukaryotic circadian clocks is the existence of transcriptional/translational autoregulatory feedback loops 3.
Entrainment: Synchronization of a clock with the respective environmental cycle.
Periodicity: Cycles, rhythms and clocks are distinguished by their respective period length (common periodicities are listed in Fig. 1).
Figure 1.
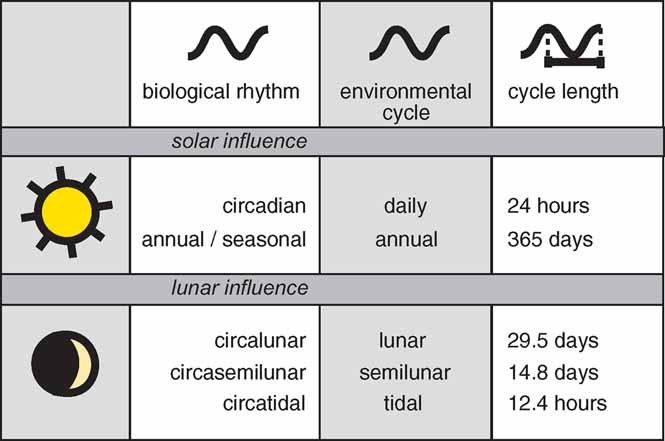
Common biological rhythms under solar and lunar influence. The overview lists a given biological rhythm (left), along with the corresponding environmental cycle (middle) and the periodicity of this environmental cycle (right). For simplicity, no distinction is made between the synodic (29.5 days) and the sidereal (27.3 days) lunar period. For additional terminology, see Box 1.
In contrast to their marine relatives, terrestrial model species, such as the fruitfly, the mouse or the thale cress, only display overt circadian and, in some instances, seasonal, rhythms. Therefore, our molecular understanding of these two types of rhythms is most advanced. A unifying principle of eukaryotic circadian rhythms is that they rely on negative transcriptional/translational feedback loops formed by a set of regulatory genes 1, 2. Importantly, this molecular system is able to maintain rhythmic circadian changes even under free-running conditions, i.e. in the absence of outside stimuli, and is therefore called circadian clock (Box 1). A number of studies now provide a detailed mechanistic understanding of how circadian clocks operate. This includes the role of protein turnover, specific post-translational modifications by de-actylation, methylation and phosphorylation, as well as the involvement of signalling cascades (reviewed in ref. 3).
However, free-running rhythms have not only been observed in the context of circadian clocks, but exist also for typical ‘marine rhythms’ of shorter and longer periods, like tidal or lunar rhythms (representative species listed in Fig. 2). This implies that the respective species possess multiple clock-like systems. Like their circadian counterparts, they allow species to anticipate important regular environmental changes; and they can do this even under circumstances when the environmental cues would be misleading (e.g. due to weather conditions).
Figure 2.
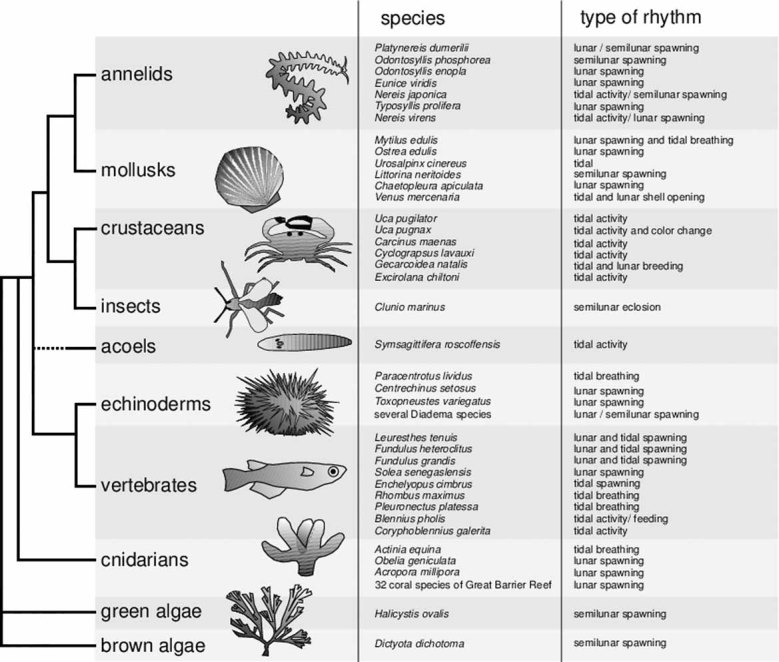
Lunar-controlled rhythms are widespread and of fundamental importance for marine organisms. Simplified phylogeny of metazoan groups with representatives exhibiting moon-controlled rhythms. In most mentioned cases evidence for a free-running lunar clock mechanism exist. Note that it is likely that all the mentioned species also possess a circadian clock and many also exhibit seasonality. All mentioned groups represent marine species. Lunar-controlled rhythms have also been described outside metazoans in green and brown algae (bottom) 9, 11–13, 58, 62, 86, 87.
In this article, we focus on the value of marine model species for molecular chronobiology. We first highlight important lunar rhythms, which are commonly found among marine species, but are far less, if at all, reliably described in terrestrial species. Next, we review how species adjusting to both lunar and solar cycles coordinate the underlying clocks, again a phenomenon only well known for marine species. Finally, we discuss the value of marine model species to shed light on the evolution and diversification of known molecular clocks.
Throughout the article, our main emphasis is on molecular aspects of ‘marine’ rhythms; for their behavioural or physiological dimensions, we refer the interested reader to recent overviews 1, 2, 4–6.
Rhythms under lunar control
Historically, the remarkable discrepancy in our molecular understanding of solar and lunar rhythms can be traced back to the beginning of modern chronobiology. Biased by the availability of molecular model systems, molecular chronobiology has so far almost entirely focused on the analyses of a few selected land model species. Although nocturnal light can have slight effects on the daily rhythm of some land species 7, none of them display lunar-controlled rhythms. Consequently, no molecule has so far been clearly implicated in a moon-entrained clock. In the following sections, we present selected marine species that could serve as molecular models for two of the most common lunar-controlled rhythms – circatidal and circasemilunar/circalunar rhythms.
Circatidal rhythms and clocks
Tides are caused by the gravitational forces of the sun and moon on the earth's water masses. Coastal areas can either exhibit semidiurnal tides (two high and two low waters per day) or diurnal tides (one high and one low water per day) where the former has a period of 12.4 h (i.e. half a lunar day), and the latter 24.8 h (i.e. a lunar day).
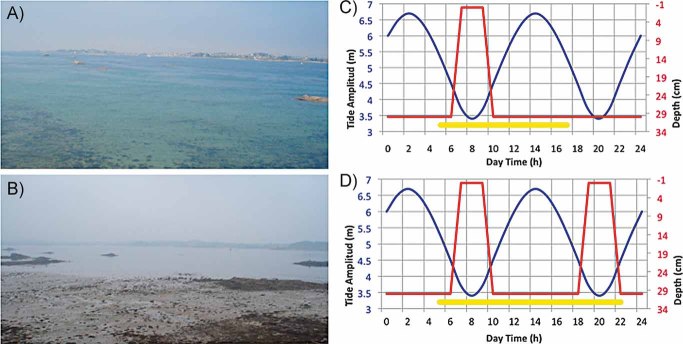
The rise and fall of water has a significant impact on the organisms living in the tidal zones. Humidity, salinity, temperature, oxygen levels, water current, sun irradiation, food availability, hydrostatic pressure and also predator exposure change due to the tides. Therefore, molecular clock mechanisms that allow a given species to anticipate the tidal change can be of critical importance to avoid exposure to life-threatening conditions. Indeed, the existence of circatidal clocks is evident from a number of species (Fig. 2). The first organism for which such a clock was described was the acoel flatworm Symsagitiffera roscoffensis 8–10 (Fig. 3; formerly in the genus Convoluta). Notwithstanding the uncertainty of the acoels' phyletic position (reviewed in ref. 11), their interest for chronobiology is undisputed, as they allow the circatidal clock to be molecularly assessed. EST and BAC sequences for the animal are forthcoming (P. Martinez and X. Bailly, personal communication).
Figure 3.
Habitat and rhythms of S. roscoffensis. A-B: Shallow coastal areas in Roscoff (France) during high and low tide periods, either covering or exposing the natural habitat of S. roscoffensis. C: During normal tide fluctuations over 24-hour periods (blue line), S. roscoffensis migrate to the surface of the sand or stay burrowed during low and high tides, respectively (red line). This process is dependent on the presence of sunlight (yellow line). D: In summer months, when long days overlap two low tide periods, S. roscoffensis migrate twice a day to expose themselves to sunlight. (C, D: after 88).
S. roscoffensis live in groups of thousands of translucent individuals in shallow coastal areas in the southern part of the English Channel. The obligate endosymbiotic association with the microalga Tetraselmis convolutae dictates the tidal activity cycles of S. roscoffensis. During low tides, acoels emerge from the sand and expose themselves and their photosynthetic endosymbionts to the sunlight. Prior to the tide rising, acoels burrow again into the darkness of the sand. During the summer months, this migration cycle follows a circatidal rhythm of 12.4 h (Fig. 3). Experiments from the early 20th century showed that when adult animals were removed from their environment and placed in a glass container in the laboratory, they maintained free-running movement cycles for 5 days 8–10. This observation provides the first evidence for the existence of a circatidal clock in a marine organism.
Physiological and behavioural circatidal rhythms have been studied in a variety of other marine organisms, e.g. in sea spiders 12, crabs, crustaceans, sea anemones and various molluscs (see Fig. 2 and associated references). For most of those, it has also been shown that they are under the control of a tidal clock 13.
In the mussel Mytilus californianus, a first systematic study has been performed to identify molecular changes associated with the tidal cycle 14. Expression levels of cell division and metabolism genes appear to be anti-correlated in this species, suggesting that these cellular functions occur at separate time points, probably reflecting different favourable environmental conditions. Any clear association between the tidal cycle and gene expression levels, however, is missing, possibly owing to the low resolution of the study 14.
Circalunar rhythms and clocks
Classical authors – starting with Aristotle – had already seen a connection between the different phases of the moon and the size of certain marine invertebrates. Zoological descriptions of the early and mid 20th century have since re-established the connection between the apparent size of marine animals with moon. More precisely, these studies have revealed that the maturation of gonads in these animals depends on a lunar cycle (see e.g. 15, 16 and Fig. 2). In species where the gonads contribute to a large proportion of the body mass, such as sea urchins, this effect is particularly prominent.
In contrast to the short period rhythms of the tides that most obviously manifest themselves in behavioural changes of animals, semi-lunar and lunar cycles are most prominently involved in reproductive events such as mass synchronous spawnings. This has been documented for a broad range of marine metazoans that include corals, sea urchins, bristle worms, molluscs and fish, as well as non-metazoans, such as green and brown algae (Fig. 2 and associated references). Lunar-controlled reproductive rhythms may be even further widespread than currently known, as in many species, the time of reproduction has not been regularly documented.
Typically, species reproducing with a circalunar or -semilunar rhythm rely on external fertilization, releasing the gonadal products of each individual into the open water. Whereas species with internal fertilization can tolerate differences in the maturation of each partner, successful fertilization in such broadcast spawners critically relies on tight synchronization between male and female maturation and the release of germ products. The regular cycle of the moon provides these organisms with a steady timeframe that can be used for synchronization even across widespread populations.
Towards a molecular understanding of light-entrained circalunar rhythms and clocks
Coral reefs represent a habitat that is characterized by extensive lunar reproductive rhythmicity. In the Great Barrier Reef, more than 30 coral species display synchronous spawning in accordance with the lunar phase 17. Likewise, several coral reef fish species follow lunar reproductive rhythms (see below). One of the coral species that has been studied in more detail is Acropora millipora. It spawns once a year, at a defined lunar phase. A recent study suggests that an Acropora cryptochrome (Cry) molecule is influenced by the lunar rhythm 18. Members of the cry gene family are present throughout all kingdoms and play a crucial role in circadian rhythms 2, 19, 20. In mammals, vertebrate-type Crys act as transcriptional repressors that control the circadian clock 21, whereas the blue light/UV-A photoreceptor activity of Crys acts as input in the circadian clock of insects 22, 23 and plants 19.
Acropora harbours at least three different members of the cry/phl family, a cry-dash, cry1 and cry2 18. Of those, the RNA and protein levels of Cry2 are affected by exposure to lunar light, leading the authors to suggest that Cry2 might be a lunar light sensor 18. The apparent changes in Cry levels, along with the evolutionary conservation of the molecule, make it an attractive candidate for a molecule involved in circalunar rhythms. However, it is not yet clear if Acropora Cry2 can actually act as a light receptor and hence mediate a lunar light stimulus.
Coral reef fishes represent another group in which circalunar rhythms have been described (reviewed in ref. 24). Among those, rabbitfish species display cycles of gonadal maturation and spawning in accordance with the lunar cycle. Molecular studies have shown that the observed histological changes accompanying gonad differentiation correlate with fluctuations of steroid hormones known to play a role in gonadal development 24–27.
Another set of observations has shown the influence of moonlight on components of the circadian clocks in rabbitfishes 28, 29. Melatonin acts as a major output molecule of the circadian clock in vertebrates, with levels typically peaking during the night. In Siganus guttatus and Siganus canaliculatus, plasma levels of melatonin have been found to be elevated in new moon nights compared to full moon nights. Similarly, pineal mRNA levels of Siganus guttatus per2, a likely core component of the circadian clock, differed significantly between full moon and new moon nights 30. Along with additional lunar changes in the mRNA levels of a candidate S. guttatus melatonin receptor (referred to in ref. 31), and measurements on cultured pineal tissue from the same species 32, these data suggest that lunar light is received by the rabbitfish pineal and thereby impacts on the circadian fluctuations of melatonin.
However, caution has to be used for the interpretation of these molecular studies. Also in Drosophila, which does not exhibit any biological circalunar rhythm, dim light of in the range of lunar light intensity can directly influence expression of circadian clock genes 7. Therefore, in the absence of free-running experiments, it remains unclear if the observed changes in levels of Acropora Cry2, Siganus per2, melatonin receptor or melatonin are caused by an endogenous lunar clock or directly result from illumination differences. Indeed, a study in soles suggests rather the latter to be the case 33. Furthermore, these observations do not necessarily imply that any of these molecules are functionally involved in a circalunar rhythm or clock.
Polychaetes (bristle worms) are a particularly attractive group to study lunar periodicity. Not only are the mass spawnings of polychaete species, such as the bioluminescent fire worm of Bermuda (Odontosyllis), or the famous Palolo worms in the Southern Pacific (Palolo viridis), spectacular and fascinating natural phenomena, in selected animals from this group, lunar reproductive periodicity also has a long history of scientific research 16, 34–41. Among these species is Platynereis dumerilii (Fig. 2), the first organism for which the entrainment to a lunar cycle was shown to only depend on nocturnal light stimuli 42–44. Classical studies, including one free-running experiment, indicate that Platynereis possesses an endogenous circalunar clock, and that lunar light acts as a zeitgeber for this clock 42. Notably, Platynereis has recently started to emerge as a novel molecular model system with large sequence resources and molecular tools 45–50. These resources, along with its ancestral-type nervous system 51–54, now make this organism an ideal starting point not only to unravel the molecular principles of its circalunar clock, but also to place it in an evolutionary context.
Another invertebrate that appears very promising for the molecular analyses of circalunar rhythms is the marine midge Clunio marinus. Similar to Platynereis, a wealth of classical studies attests to its circalunar rhythm of reproduction 55–58. Clunio marinus lives in the tidal zones of rocky beaches, spending most of its life cycle underneath the water. Only the few hours from eclosion to reproduction need to take place outside the water. At the end of this period, females deposit their eggs on algae that only emerge from the water during spring tides, and are otherwise covered by water 59. In accordance with the critical importance of this final stage of their life cycle, Clunio displays a strong preference to hatch and reproduce just during the spring tides, when the low water levels reach their extremes. The synchronization of these midges therefore does not primarily affect gonadal maturation, as is the case of the bristle worm, but the time point of their eclosion.
Similar to Platynereis, but in contrast to many other marine organisms, Clunio can be easily grown in an in-land laboratory. Free-running experiments indicate that Clunio possesses a circalunar clock that is able to anticipate the correct time window for eclosion 56. Importantly, naturally occurring Clunio strains display different hatching cycles that are precisely adapted to the tidal times in the different coastal areas from which the strains are isolated. Their comparison reveals that they only evolved in the relatively short time period after the last ice age 60. This makes Clunio a highly attractive model for population genetics to unravel the molecular players that determine differences of hatching peaks of different strains.
How are different rhythms and clocks interconnected?
Projecting beyond the molecular mechanisms of single circadian, circalunar, circatidal or seasonal/circaannual rhythms, the next level of functional and evolutionary understanding will be to discern how these different types of rhythms are molecularly connected, and to what extent underlying molecular clocks share the same components. Interconnections between different rhythms are evident from many examples (Figs. 2 and 3 and 13, 61, 62). For instance, in species like Clunio, the light-sensory system involved in the entrainment of the circalunar clock is controlled in a circadian manner. This specific regulation of the sensory system offers an attractive explanation for how the animals can discriminate nocturnal light from daylight, and hence recognize the correct moon phases in the absence of obvious differences, e.g. in spectral composition of sun- and moonlight 58. Furthermore, lunar reproduction can be modulated by seasonal factors, restricting reproduction to only few reproductive months 16, 63. Likewise, most species that show lunar reproduction also have a defined circadian time window in which reproduction occurs. In exemplary cases, this interconnection allows precise timing of reproduction to specific hours of specific days of the reproductive month(s) 13, 14, 41, 64, 65. These intricate connections between different types of rhythms argue for the existence of common molecular regulators, for instance hormones or signalling metabolites, which mediate crosstalk between different molecular processes or even clocks within the same species.
The connections between different clocks have been intensively debated for circatidal and circadian rhythms in intertidal crabs. Two main hypotheses have been put forward to explain the circatidal locomotor activity of these crabs and their performance under constant laboratory conditions. The first hypothesis, the ‘circatidal/circadian clock model’, was developed by Naylor 66, supported by experiments showing a decoupling of circadian and circatidal rhythmicity in crabs 67. In essence, it postulates that locomotor activity is promoted by a circadian oscillator (24.8 h) and suppressed by an overlapping circatidal oscillator (12.4 h) (reviewed in refs. 68, 69). In the crab Carcinus maenas, the circadian oscillator would modulate the activity levels and by this determine that night activity is generally higher than day activity. The circatidal clock would suppress this general activity, leading to activity bursts (of different height) every 12.4 hours.
The second hypothesis, the ‘circalunidian-clock model’ originates from Palmer and associates in the late 80s (reviewed in ref. 70). It rejects the idea of a circatidal clock, instead suggesting two circalunar clocks at a period of 24.8 hours. Normally, these clocks would run in 180° antiphase to each other, generating activity bursts of the animals every 12.4 hours. Changes in the period length of either one of the two circalunar clocks would entail irregularities in the 12.4 hour cycles (such as a drift or splitting of the activity peaks) that occur under constant conditions in species like Uca pugnax or Helice crassa and are hard to reconcile with the circatidal/circadian clock model. The prediction is that in the absence of external stimuli, the coupling of both clocks can get lost. This provides a relative straightforward explanation how circatidal locomotor activity peaks under constant conditions can drift or temporarily disappear, a frequently observed phenomenon.
Supporting evidence for both models has been collected over the years (summarized in refs. 5, 6). In the polychaete Nereis virens, the acquired data fit both models 62, amphipod researchers have favoured the circatidal/circadian clock model 71, while for other organisms like the horseshoe crab and intertidal crabs data support the circalunidian-clock model 72, 73. Both models provide testable hypotheses for the underlying molecular machinery. It is thus likely that molecular research in those species will soon discriminate between both scenarios.
Evolution and diversification of ‘marine’ rhythms and clocks
Given that the marine biosphere is the ancient environment in which life, with all its rhythms, evolved, marine species can provide crucial input on the origin and diversification of rhythms and their underlying clocks. This starts with the sampling of species used for chronobiological research. In light of the diverse tree of eukaryote phylogeny 74, it is obvious that the current molecular chronobiological models only represent small and specialized subsets of species. The study of other, molecularly unexplored groups is therefore promising to reveal additional strategies to cope with periodic environmental changes, and also delineate more precisely which aspects of the extant clocks are of ancient origin.
This principle can be well exemplified for the circadian clock. Within the eumetazoans, work on organisms other than Drosophila and mouse, such as honeybees, butterflies and sea urchins has already led to the novel insight that ancient Bilateria had clock molecules that were previously believed to be only present in either Drosophila or vertebrates, such as vertebrate-type and Drosophila-type cryptochromes (crys), timeless and timeout 75, 76. These findings have two implications: First, the eumetazoan ancestor (most likely a marine species) possessed a complex repertoire of circadian clock genes. Second, the current animal models use secondary simplifications of this ancestral circadian clock machinery.
The comparison between clock systems is not only informative in animals. Photosynthetic algae reveal details of the evolution of circadian clocks in plants. Research in the marine unicellular green alga Ostreococcus has served to reveal ancient aspects of the Arabidopsis circadian clock. Orthologs of two Arabidopsis core clock genes, timing of cab expression1 (TOC1) and circadian clock associated1 (CCA1), also play a central role in the alga. In contrast, the minimalistic genome of Ostreococcus, a picoeukaryote with only 13 Mbp of sequence, lacks other components of the Arabidopsis circadian clock, and therefore allows a core set of clock genes to be studied in a simpler context 77.
Marine species also provide insight into ancient correlations between clocks and the physiology of species. For instance, researchers studying regenerating mouse liver uncovered an unexpected link between the entry of cells into the G2 cell cycle phase and the circadian clock (reviewed in ref. 78). This finding might be less surprising in light of the fact that unicellular marine organisms, such as diatoms or green algae, synchronize their cell divisions in a circadian manner 79, 80. This coordination ensures harmonization with the algae's photosynthetic activity. The need to coordinate cell division with the organism's physiology might well represent an ancient marine condition still maintained in today's vertebrate liver cells. Similarly, in the diatom Phaeodactylum tricornutum, a single Cryptochrome molecule combines DNA repair function, light perception and circadian regulation. This Cryptochrome therefore appears to provide a missing link between the different functions of Cryptochromes in more diversified organisms 81.
Another example where ‘marine rhythms’ provide interesting physiological connections is the coupling of lunar reproduction and regeneration in several polychaetes. Especially in nereidids, the ability to regenerate is often anti-correlated with the production of eggs or sperm 82–85. This anti-correlation probably reflects the economy of energy expenditure in an organism with extreme fecundity, but it might also yield general insight into the mechanisms by which regenerative ability is restricted in other species, including vertebrates.
In summary, these examples attest to the value of marine species for understanding how molecular rhythms and clocks evolved and diversified along different evolutionary lineages. Moreover, they indicate that selected marine species have a strong potential to reveal new insights into the crosstalks between molecular clocks and physiological or cell biological events. With the advent of new molecular insight into marine non-circadian rhythms, it is likely that we will also learn more about the correlates or remnants of these rhythms in terrestrial models. Is it possibly more than sheer coincidence that the female reproductive cycle in humans lasts around a lunar month, or could this instead reflect some regulatory left-over from our evolutionary past? In this sense, the study of rhythms in our marine relatives can shed more light onto our own evolutionary past.
Whereas we have mostly focused on the interest of ‘marine rhythms’ for chronobiology, their molecular dissection will also advance studies in other areas of marine biology: Molecules involved in ‘marine rhythms’ will be useful to measure the effects of climate change or light pollution on the marine ecosystem and guide the search for improvement, e.g. by limiting light pollution to specific wavelengths or phases of the night. Finally, a better molecular knowledge on marine spawning rhythms could help to improve aquaculture and re-growth of species like corals.
Acknowledgments
The authors are grateful to three anonymous referees for their constructive criticism.
The authors also wish to thank the Tessmar and Raible labs for continuous helpful discussion on the topic of lunar periodicity. This work was supported by MFPL start-up funds to F.R. and K.T.-R., an FWF START award (#AY0041321) and HFSP YIG (#RGY0082/2010) to K.T.-R.
References
- 1.Roenneberg T, Merrow M. Circadian clocks – the fall and rise of physiology. Nat Rev Mol Cell Biol. 2005;6:965–71. doi: 10.1038/nrm1766. [DOI] [PubMed] [Google Scholar]
- 2.Stillman B, Stewart D, Grodzicker T, editors. Cold Spring Harb. Symp. Quant. Biol. Clocks and Rhythms. Cold Spring Harbor Laboratory: CSHL Press; 2007. [Google Scholar]
- 3.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–76. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 4.Palmer JD. The clocks controlling the tide-associated rhythms of intertidal animals. BioEssays. 2000;22:32–7. doi: 10.1002/(SICI)1521-1878(200001)22:1<32::AID-BIES7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Wilcockson D, Zhang L. Circatidal clocks. Curr Biol. 2008;18:R753–5. doi: 10.1016/j.cub.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Naylor E. Chronobiology of Marine Organisms. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 7.Bachleitner W, Kempinger L, Wulbeck C, Rieger D, et al. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104:3538–43. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohn G. Sur les movements oscillatoires des Convoluta roscoffiensis. C R Acad Sci Paris. 1903;139:576–8. [Google Scholar]
- 9.Gamble FW, Keeble F. The bionomics of Convoluta roscoffensis, with special reference to its green cells. Proc R Soc Lond. 1903;72:93–8. [Google Scholar]
- 10.Gamble FW, Keeble F. The bionomics of Convoluta roscoffensis, with special reference to its green cells. Q J Microsc Sci. 1904;47:363–431. [Google Scholar]
- 11.Deutsch JS. Do acoels climb up the “Scale of Beings”? Evol Dev. 2008;10:135–40. doi: 10.1111/j.1525-142X.2008.00220.x. [DOI] [PubMed] [Google Scholar]
- 12.Isaac MJ, Jarvis JH. Endogenous tidal rhythmicity in the littoral pycnogonid Nymphon gracile (Leach) J Exp Mar Biol Ecol. 1973;13:83–90. [Google Scholar]
- 13.Fingerman M. Lunar rhythmicity in marine organisms. Am Naturalist. 1957;91:167–78. [Google Scholar]
- 14.Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, et al. Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol. 2008;18:1501–7. doi: 10.1016/j.cub.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Fox HM. Lunar periodicity in reproduction. Proc R Soc Lond S Biol. 1924;95:523–50. [Google Scholar]
- 16.Korringa P. Relations between the moon and periodicity in the breeding of marine animals. Ecol Monogr. 1947;17:347–81. [Google Scholar]
- 17.Harrison PL, Babcock RC, Bull GD, Oliver JK, et al. Mass spawning in tropical reef corals. Science. 1984;223:1186–9. doi: 10.1126/science.223.4641.1186. [DOI] [PubMed] [Google Scholar]
- 18.Levy O, Appelbaum L, Leggat W, Gothlif Y, et al. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science. 2007;318:467–70. doi: 10.1126/science.1145432. [DOI] [PubMed] [Google Scholar]
- 19.Somers DE, Devlin PF, Kay SA. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science. 1998;282:1488–90. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- 20.Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6:220. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem. 2004;279:34079–82. doi: 10.1074/jbc.R400016200. [DOI] [PubMed] [Google Scholar]
- 22.Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, et al. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Sauman I, Yuan Q, Casselman A, et al. Cryptochromes define a novel circadian clock mechanism in Monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takemura A, Rahman S, Nakamura S, Park YJ, et al. Lunar cycles and reproductive activity in reef fishes with particular attention to rabbitfishes. Fish Fisheries. 2004;5:317–28. [Google Scholar]
- 25.Rahman MS, Takemura A, Takano K. Correlation between plasma steroid hormones and vitellogenin profiles and lunar periodicity in the female golden rabbitfish, Siganus guttatus (Bloch) Comp Biochem Phys B. 2000;127:113–22. doi: 10.1016/s0305-0491(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 26.Rahman MS, Takemura A, Takano K. Lunar synchronization of in vitro steroidogenesis in ovaries of the golden rabbitfish, Siganus guttatus (Bloch) Gen Comp Endocr. 2002;125:1–8. doi: 10.1006/gcen.2001.7708. [DOI] [PubMed] [Google Scholar]
- 27.Ayson FG. Induced spawning of rabbitfish, Siganus guttatus (Bloch) using human chorionic gonadotropin (HCG) Aquaculture. 1991;95:133–7. [Google Scholar]
- 28.Takemura A, Susilo ES, Rahman MDS, Morita M. Perception and possible utilization of moonlight intensity for reproductive activities in a lunar-synchronized spawner, the golden rabbitfish. J Exp Zool A Comp Exp Biol. 2004;301:844–51. doi: 10.1002/jez.a.105. [DOI] [PubMed] [Google Scholar]
- 29.Rahman MS, Kim BH, Takemura A, Park CB, et al. Effects of moonlight exposure on plasma melatonin rhythms in the seagrass rabbitfish, Siganus canaliculatus. J Biol Rhythm. 2004;19:325–34. doi: 10.1177/0748730404266712. [DOI] [PubMed] [Google Scholar]
- 30.Sugama N, Park JG, Park YJ, Takeuchi Y, et al. Moonlight affects nocturnal Period2 transcript levels in the pineal gland of the reef fish Siganus guttatus. J Pineal Res. 2008;45:133–41. doi: 10.1111/j.1600-079X.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 31.Takemura A, Rahman MS, Park YJ. External and internal controls of lunar-related reproductive rhythms in fishes. J Fish Biol. 2010;76:7–26. doi: 10.1111/j.1095-8649.2009.02481.x. [DOI] [PubMed] [Google Scholar]
- 32.Takemura A, Ueda S, Hiyakawa N, Nikaido Y. A direct influence of moonlight intensity on changes in melatonin production by cultured pineal glands of the golden rabbitfish, Siganus guttatus. J Pineal Res. 2006;40:236–41. doi: 10.1111/j.1600-079X.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira C, Duncan NJ, Pousao-Ferreira P, Mananos E, et al. Influence of the lunar cycle on plasma melatonin, vitellogenin and sex steroids rhythms in Senegal sole, Solea senegalensis. Aquaculture. 2010;306:343–7. [Google Scholar]
- 34.Rumphius GE. D'Amboinsche Rariteitenkamer. Vol. 64. Amsterdam: Hooftdeel; 1705. pp. 51–54. Boek I. [Google Scholar]
- 35.Collin A. Bemerkungen über den essbaren Palolowurm, Lysidice viridis (Gray) Kiel und Leipzig: Verlag von Lipsius & Tischer; 1897. [Google Scholar]
- 36.Krämer A. Palolountersuchungen. Biol Centralblatt. 1899;19:15–30. [Google Scholar]
- 37.Horst R. Over the “Wawo” van Rumphius (Lysidice oele n.sp.) Kolon Mus. Haarlem: Rumphius Gedenkboek; 1902. pp. 105–8. [Google Scholar]
- 38.Corney BG. Abstract of a paper on the periodicity of the swarming Palolo (Eunice viridis Gr.) J Torquay Nat Hist Soc. 1922;3:126–30. [Google Scholar]
- 39.Fage L, Legendre R. Les danses nuptiales de quelques Nereidiens. Comptes Rendus Acad Sci Paris. 1923;177:1150–2. [Google Scholar]
- 40.Fage L, Legendre R. Rythmes lunaires de quelques Nereidiens. Comptes Rendus Acad Sci Paris. 1923;177:982–5. [Google Scholar]
- 41.Ranzi S. Ricerche sulla biologia sessuale degli Anellidi. Pubbl Staz Zool Napoli. 1931;11:271–92. [Google Scholar]
- 42.Hauenschild C. Lunar periodicity. Cold Spring Harb Symp Quant Biol. 1960;25:491–7. doi: 10.1101/sqb.1960.025.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Hauenschild C. Hormonale Hemmung der Geschlechtsreife und der Metamorphose bei dem Polychaeten Platynereis dumerilii. Z Naturforsch. 1956;11/b:125–32. [Google Scholar]
- 44.Hauenschild C. Ueber die lunarperiodische Schwaermen von Platynereis dumerilii in Laboratorienzuchten. Naturwissenschaften. 1955;42:556–7. [Google Scholar]
- 45.Tessmar-Raible K, Steinmetz RHS, Snyman H, Hassel M, et al. Fluorescent two color whole-mount in situ hybridization in Platynereis dumerilii (Polychaeta, Annelida), an emerging marine molecular model for evolution and development. BioTechniques. 2005;39:460. doi: 10.2144/000112023. 462, 464. [DOI] [PubMed] [Google Scholar]
- 46.Raible F, Tessmar-Raible K, Osoegawa K, Wincker P, et al. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science. 2005;310:1325–6. doi: 10.1126/science.1119089. [DOI] [PubMed] [Google Scholar]
- 47.Jekely G, Arendt D. Cellular resolution expression profiling using confocal detection of NBT/BCIP precipitate by reflection microscopy. BioTechniques. 2007;42:751–5. doi: 10.2144/000112462. [DOI] [PubMed] [Google Scholar]
- 48.Jekely G, Colombelli J, Hausen H, Guy K, et al. Mechanism of phototaxis in marine zooplankton. Nature. 2008;456:395–9. doi: 10.1038/nature07590. [DOI] [PubMed] [Google Scholar]
- 49.Christodoulou F, Raible F, Tomer R, Simakov O, et al. Ancient animal microRNAs and the evolution of tissue identity. Nature. 2010;463:1084–8. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dray N, Tessmar-Raible K, Le Gouar M, Vibert L, et al. Hedgehog signaling regulates segment formation in the annelid Platynereis. Science. 2010;329:339–42. doi: 10.1126/science.1188913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, et al. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–71. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- 52.Tessmar-Raible K. The evolution of neurosecretory centers in bilaterian forebrains: insights from protostomes. Semin Cell Dev Biol. 2007;18:492–501. doi: 10.1016/j.semcdb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Denes AS, Jekely G, Steinmetz PR, Raible F, et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–88. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 54.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell. 2010;142:800–9. doi: 10.1016/j.cell.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 55.Caspars H. Rhythmische Erscheinungen in der Fortpflanzung von Clunio marinus (Dipt. Chiron.) und das Problem der lunaren Periodizitat bei Organismen. Arch Hydrobiol Suppl. 1951;18:415–594. [Google Scholar]
- 56.Neumann D. Die lunare und tägliche Schlüpfperiodik der Mücke Clunio. J Comp Physiol A. 1966;53:1–61. [Google Scholar]
- 57.Neumann D. Die Steuerung einer semilunaren Schlüpfperiodik mit Hilfe eines künstlichen Gezeitenzyklus. J Comp Physiol A. 1968;60:63–78. [Google Scholar]
- 58.Neumann D. Physiologische Uhren von Insekten Zur Ökophysiologie lunarperiodisch kontrollierter Fortpflanzungszeiten. Naturwissenschaften. 1995;82:310–20. [Google Scholar]
- 59.Tessmar K, Kaiser TS, Zantke J. Mondlicht als natürlicher Zeitgeber für die Meeresfauna. In: Posch T, Freyhoff A, Uhlmann T, editors. Das Ende der Nacht. Weinheim: Wiley-VCH; 2009. p. 119-132. [Google Scholar]
- 60.Kaiser TS, Neumann D, Heckel DG, Berendonk TU. Strong genetic differentiation and postglacial origin of populations in the marine midge Clunio marinus (Chironomidae, Diptera) Mol Ecol. 2010;19:2845–57. doi: 10.1111/j.1365-294X.2010.04706.x. [DOI] [PubMed] [Google Scholar]
- 61.deCoursey PJ. In: Biological rhythms in the marine environment. deCoursey PJ, editor. Columbia, South Carolina: University of South Carolina Press; 1976. [Google Scholar]
- 62.Last KS, Bailhache T, Kramer C, Kyriacou CP, et al. Tidal, daily, and lunar-day activity cycles in the marine polychaete Nereis virens. Chronobiol Int. 2009;26:167–83. doi: 10.1080/07420520902774524. [DOI] [PubMed] [Google Scholar]
- 63.Franke H-D. Endocrine mechanisms mediating light-temperature effects on male reproductive activity in Typosyllis prolifera (Polychaeta, Syllidae) Dev Genes Evol. 1983;192:95–102. doi: 10.1007/BF00848485. [DOI] [PubMed] [Google Scholar]
- 64.Hauenschild C, Fischer A, Hofmann DK. Untersuchungen am pazifischen Palolowurm Eunice viridis (Polychaeta) in Samoa. Helgoländer Wiss Meeresunters. 1968:254–95. [Google Scholar]
- 65.Franke HD. On a clocklike mechanism timing lunar-rhythmic reproduction in Typosyllis prolifera (Polychaeta) J Comp Physiol A. 1985;156:553–61. [Google Scholar]
- 66.Naylor E. Tidal and diurnal rhythms of locomotory activity in Carcinus maenas (L) J Exp Biol. 1958;35:602–10. [Google Scholar]
- 67.Reid DG, Naylor E. Are there separate circatidal and circadian clocks in the shore crab Carcinus maenas. Mar Ecol Prog Ser. 1989;52:1–6. [Google Scholar]
- 68.Naylor E. Crab clockwork: The case for interactive circatidal and circadian oscillators controlling rhythmic locomotor activity of Carcinus maenas. Chronobiol Int. 1996;13:153–61. doi: 10.3109/07420529609012649. [DOI] [PubMed] [Google Scholar]
- 69.Naylor E. Crab clocks rewound. Chronobiol Int. 1997;14:427–30. doi: 10.3109/07420529709001462. [DOI] [PubMed] [Google Scholar]
- 70.Palmer JD. Review of the dual-clock control of tidal rhythms and the hypothesis that the same clock governs both circatidal and circadian rhythms. Chronobiol Int. 1995;12:299–310. [Google Scholar]
- 71.Rossano C, Gambineri S, Fanini L, Durier V, et al. Behavioural adaptations in talitrids from two Atlantic beaches. Estuar Coast Shelf S. 2009;85:573–84. [Google Scholar]
- 72.Thurman CL. Unravelling the ecological significance of endogenous rhythms in intertidal crabs. Biol Rhythm Res. 2004;35:43–67. [Google Scholar]
- 73.Ramberg Pihl NC, Watson WH, Chabot CC. Do circatidal or circalunidian clocks control locomotor rhythms in the American horseshoe crab? Integr Comp Biol. 2009;49:E293–E293. [Google Scholar]
- 74.Baldauf SL. The deep roots of eukaryotes. Science. 2003;300:1703–6. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- 75.Rubin EB, Shemesh Y, Cohen M, Elgavish S, et al. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 2006;16:1352–65. doi: 10.1101/gr.5094806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–55. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 77.Corellou F, Schwartz C, Motta JP, Djouani-Tahri el B, et al. Clocks in the green lineage: comparative functional analysis of the circadian architecture of the picoeukaryote Ostreococcus. Plant Cell. 2009;21:3436–49. doi: 10.1105/tpc.109.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schibler U. Circadian rhythms. Liver regeneration clocks on. Science. 2003;302:234–5. doi: 10.1126/science.1090810. [DOI] [PubMed] [Google Scholar]
- 79.Harding LWJ, Heinbokel JF. Periodicities of photosynthesis and cell division: behavior of phase-lagged replicate cultures of Ditylum brightwellii in a diurnally varying photic regime. Mar Ecol Prog Ser. 1984;15:225–32. [Google Scholar]
- 80.Moulager M, Corellou F, Verge V, Escande M-L, et al. Integration of light signals by the retinoblastoma pathway in the control of S phase entry in the picophytoplanktonic cell Ostreococcus. PLoS Genet. 2010;6:e1000957. doi: 10.1371/journal.pgen.1000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coesel S, Mangogna M, Ishikawa T, Heijde M, et al. Diatom PtCPF1 is a new cryptochrome/photolyase family member with DNA repair and transcription regulation activity. EMBO Rep. 2009;10:655–61. doi: 10.1038/embor.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofmann DK. Analyse der Beziehungen zwischen Regenerationsleistung, Geschlechtsreifung und endokrinem System bei Platynereis dumerilii (Annelida: Polychaeta) Verh Dtsch Zool Ges. 1975:314–9. [Google Scholar]
- 83.Hofmann DK. Regeneration and endocrinology in the polychaete Platynereis dumerilii. An experimental and structural study. Roux Arch Dev Biol. 1976;180:47–71. doi: 10.1007/BF00848884. [DOI] [PubMed] [Google Scholar]
- 84.Baskin D. Neurosecretion and the endocrinology of nereid polychaetes. Am Zoologist. 1976;16:107–24. [Google Scholar]
- 85.Lawrence AJ, Soame JM. The endocrine control of reproduction in Nereidae: a new multi-hormonal model with implications for their functional role in a changing environment. Philos Trans R Soc Lond B Biol Sci. 2009;364:3363–76. doi: 10.1098/rstb.2009.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer JD. Biological Clocks in Marine Organisms: The Control of physiological and behavioral tidal rhythms. New York, London, Sydney, Toronto: John Wiley and Sons; 1974. [Google Scholar]
- 87.Coppard SE, Campbell AC. Lunar periodicities of diadematid echinoids breeding in Fiji. Coral Reefs. 2005;24:324–32. [Google Scholar]
- 88.Keeble F. Plant-Animals: A Study in Symbiosis. Cambridge: Cambridge University Press; 1910. p. 163. [Google Scholar]