Abstract
Background
Adverse neurodevelopmental and neuropsychiatric outcomes have been established as signs of nutrient deficiencies and may be applicable to insufficient dietary intakes of omega-3 long chain fatty acids (n-3 LCFAs).
Objective
Consider if statistical definitions for Daily Reference Intakes can be applied to n-3 LCFAs intakes during pregnancy for maternal and neurodevelopmental deficiencies.
Design
Data was prospectively collected from women during pregnancy and children up to age 8 y participating in the Avon Longitudinal Study of Parents and Children (ALSPAC). Statistical analyses took social and lifestyle factors into account.
Results
During pregnancy, n-3 LCFA intakes from seafood that putatively meet statistical definitions of an Estimated Average Requirement ranged from 0.05 –0.06 en % (111–139 mg/d/2,000 Cal) for suboptimal fine motor control at 42 m and 0.065-0.08 en% (114–181 mg/d/2,000 Cal) for suboptimal verbal IQ at age 8 y and 0.18–0.22 en% (389–486 mg/d/2,000 Cal) for maternal depression at 32 w. Intakes of n-3 ranging from 0.2–0.41 en% (445 – 917 mg/d/2,000 Cal) prevented both increased risk of maternal depression and adverse neurodevelopmental outcomes for children among 97.5% of the population. No upper limit for safety was found.
Conclusion
During pregnancy, a n-3 LCFA intake of 0.40 en% (900 mg/d/2,000 Cal) from seafood is likely to meet the nutritional requirements for 97.5 % of the mothers and children of this population. These considerations do not constitute DRI’s for docosahexaenoic acid and n-3 LCFAs, but may contribute to their formulation.
Introduction
Estimation of the daily requirements of docosahexaenoic acid (DHA) necessary to maintain optimal health was a central objective of the Workshop on Docosahexaenoic acid (DHA) as a Required Nutrient, convened by Martek Biosciences on June 23, 2008. This workshop considered the criteria of Daily Recommended Intakes (DRI’s) for nutrients which are issued by The Food and Nutrition Board of the Institute of Medicine using established statistical criteria and an extensive deliberative process. Here we hope to contribute to this process by utilizing the statistical definition of Estimated Daily Requirement’s (EAR) in considering dietary intakes of DHA and other omega-3 long chain fatty acids (n-3 LCFA: eicosapentaenoic acid, docosapentaenoic acid n-3 and docosahexaenoic acid). The Food and Nutrition Board defines an EAR as: “The average daily intake level that is estimated to meet the nutrient requirements of half the healthy individuals in a particular life state and gender group” (1). “When available, data on a nutrient’s safety and role in health are considered in the formulation of a recommendation, taking into account the potential reduction in the risk chronic degenerative disease or developmental abnormality, rather than just the absence of signs of deficiency.” (1). To meet these definitions, the relationship of n-3 LCFA intakes to signs of deficiency, chronic degenerative disease and developmental abnormalities must be understood in terms of population risk. Optimally, data from graded dose response interventions trials, evaluating hundreds of doses, each in a representative population, each dose given for the decades necessary for the development of chronic diseases would be available. Unfortunately, such data has not available for most nutrients in establishing DRI’s, such as for fiber. An evaluation of the Hill criteria for causality of n-3 LCFA deficiency in depressive symptoms and neurodevelopmental deficits is included in the discussion.
The absence of signs of deficiency related to critical target tissues has been considered in formulation of required dietary intakes. DHA is selectively concentrated in synaptic neuronal membranes and comprises nearly 14% of all brain fatty acids and is necessary for optimal neurological function (2). Thus, deficit intakes of DHA or n-3 LCFA’s supporting DHA are likely to manifest as signs or symptoms of neural dysfunction including neurodevelopmental and neuropsychiatric impairments. Neurodevelopmental impairments are identified as signs and symptoms of deficiency in setting the DRI’s (1) for biotin, folate , iodine and iron. Psychiatric and neurocognitive impairments are also identified as signs and symptoms of deficiency in setting the DRI’s (1) for vitamin B6; depression and confusion, vitamin B12; mood changes, confusion, insomnia and cognitive impairments, biotin; depression, lethargy and hallucinations, folate; irritability and difficulty concentrating, niacin; depression and apathy, panothenic acid; irritability, restlessness, apathy and malaise, thiamin; apathy, irritability, confusion decreased short term memory, iodine; hypothyroidism and learning impairments and iron; impaired cognition and decreased work capacity. Severe symptoms of major depression may be used as a categorical diagnosis and can potentially be used to calculate an EAR. Major depression is commonly a chronic degenerative disease. Neurodevelopmental and neuropsychiatric impairments are clearly identified by the Food and Nutrition Board as signs and symptoms of deficiencies for several nutrients (2), thus setting a precedent potentially applicable to n-3 LCFA’s deficiencies in similar outcome parameters.
In the absence of large scale long term graded intervention trials, dietary exposure data with well defined clinical outcomes from a carefully characterized large normative population can clearly be useful for evaluating statistical definitions for DRI’s. These study criteria are met in ALSPAC (the Avon Longitudinal Study of Parents and Children) which was prospectively designed to identify features of the environment, including dietary exposure in pregnancy, that influenced the health and development of children and their parents (3). The initial population consisted of 14,541 pregnant women who were resident in the Avon health authority area in south-west England and had an expected date of delivery between April 1991 and December 1992. Data were collected using multiple strategies (4). Women with outcomes of foetal or infant death, and those with multiple births were excluded. In this cohort, we have previously reported that lower seafood intake during pregnancy was associated with greater risks neurodevelopmental abnormalities in the children; low verbal IQ, greater risks of fine motor deficits, and suboptimal behavioral problems (5). Here we also consider adverse neuropsychiatric outcomes for the mother, specifically significant depressive symptoms during pregnancy (6), in evaluating n-3 LCFAs intake levels that might meet the statistical definition of an EAR and an RDA.
Methods
Neurodevelopmental outcomes
The assessment of suboptimal verbal IQ by the WISC-III- UK, fine motor deficits by the ALSPAC Developmental Index and suboptimal behavioral outcomes by the Strengths and Difficulties Questionnaire were conducted as previously detailed (6).
Depressive symptoms
The Edinburgh Postnatal Depression Scale (EPDS) was completed by the mother at 18 and 32 weeks gestation. This scale was devised to measure maternal depression after childbirth, but has also been validated for use during pregnancy (7). Symptoms attributable to somatic effects of pregnancy and childbirth (e.g. weight gain, sleeplessness, tiredness) are excluded in this scale. Scores on the EPDS can be categorised by well validated cut-off points; those scoring 13+ are classified as having high levels of depressive symptoms (HLDS) with sensitivities and specificities of 95% and 93% respectively in comparison to the DSM III criteria for depression (8).
Seafood Exposure
Maternal food consumption was estimated at 32 weeks gestation using a self-completion food frequency questionnaire, which was also used to derive n-3 LCFA energy intake (9). Three questions assessed seafood consumption: “How many times nowadays do you eat (a) white fish (cod, haddock, plaice, fish fingers, etc.), (b) dark or oily fish (tuna, sardines, pilchards, mackerel, herring, kippers, trout, salmon, etc.) (c) Shellfish (prawns, crabs, cockles, mussels etc.)?” Each response had five pre-defined categories: Never or rarely, once in 2 weeks, 1–3 times per week, 4–7 times per week, and more than once a day. These were converted to weekly frequencies of consumption (portions per week) of 0, 0.5, 2, 5.5 and 10 respectively. Portion sizes and types of fish were based on typical consumption patterns in Britain at that time. Fatty acid compositions were calculated using British food composition tables (10). Only 221 (2%) of the study women were taking omega-3 supplements. Exclusion did not substantially alter the results. The validity of estimations of the dietary intakes of long chain omega-3 intake from fish in comparison to red blood cell compositions of DHA from dietary and tissue compositional data from this cohort has previously been established (11).
Potential confounders
The following factors were considered as potential confounders either because of an association with depression or with fish eating or both: maternal age (<25, 25+); parity - the number of previous pregnancies resulting in a live birth or a late foetal death (0,1,2+); maternal education based on the highest educational qualification achieved (low - no more than a vocational qualification; medium - O-level or equivalent; high - A-level or higher); housing tenure (owned/mortgaged, council rented (public housing), other); the mothers' life events in childhood scale - a 108 point measure concerning major events (<21, 21+); the scales of 44 recent life events occurring in pregnancy (<90th, ≥90th centile); chronic stress as measured by a Family Adversity Index (FAI) (<90th centile, 90th centile +); maternal smoking (no, yes) at each time point; alcohol (none, any) at each time point; maternal ethnic origin (white, non-white). We examined the influence of both 12 food groups and of 24 derived nutrients on neurodevelomental risks and found these nutrients to be non-contributory (5). We performed similar analyses for the effect of these nutrients and foods for the outcomes of maternal depression and found them to be non-contributory. Potential confounding factors for maternal depression were evaluated as previously described (6).
Statistics
An EAR is defined as “an estimated median intake such that the level of intake at which half the health individual at a particular stage of life fall short of the nutrient requirements and half exceed their nutrient requirements.” (1). This does not mean that 50% of the population shows signs of deficiency, but rather that “the EAR is the intake at which the risk of inadequacy is 0.5 (50% to an individual)” (1). Operationally this corresponds to an equal risk of signs or symptoms of deficiency (a probability or odds ratio, (OR) of 0.5) comparing groups above or below the cut point (1). For example, a population of 4,000 persons may have 40 persons with manifested signs of deficiency. If 3,000 were below and 1,000 were above an ERA cut point, then signs of deficiency would be manifest in 30 persons above, and 10 persons below, that set point for a 50:50 risk of inadequacy. Progressively higher cut-points of intake were iteratively tested until the risk of deficiency symptoms was nearly equal comparing groups above and below the cut-off point. This risk was expressed as an adjusted odds ratio closest to β= 1.0, using the group below the cut-off point as the reference (figure 1). Multivariate logistic regression was used here to adjust for the potential confounding variables when comparing groups of mothers above and below each cut point tested (SPSS, version 11.0.1). This adjustment accounted for an unequal distribution of other factors the increase risk of manifesting signs of deficiency. For suboptimal verbal IQ and suboptimal fine motor development, and maternal severe depressive symptoms adjusted odds ratios included the potential confounding variables previously described (5).
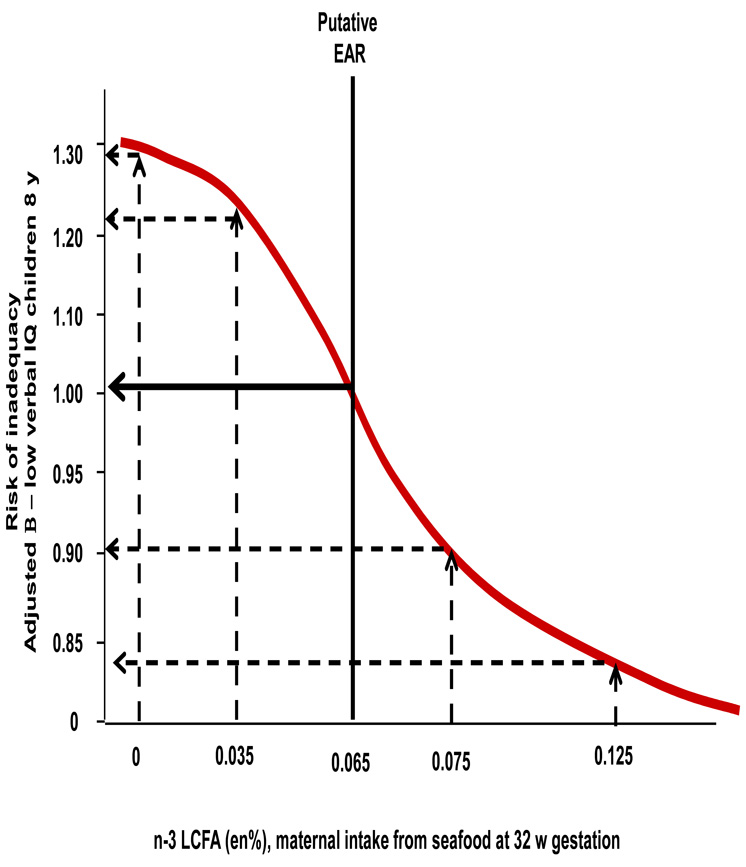
Figure 1. Estimation of Average Requirements comparing -3 LCFA intakes to risk of inadequacy.
illustrates the risk curve the level of maternal n-3 LCFA intake at 32 w gestation to the risk of suboptimal verbal IQ for children at age 8 y among the ALSPAC population. The putative Estimated Average requirement is the cut point of maternal intake where the risk of inadequacy for the children is nearly equal for the group below the cut point compared to the group above the cut point. Multivatiate logistic regression models were used to adjust for potential confounding variables among the groups of mothers above and below each cut point. An adjusted odds ratio β= 1.0 would indicate a precisely 50:50 risk of inadequacy comparing the groups above and below the cut point. A dietary intake of 0.065 en% provide the best fit with an adjusted odds ratio β= 1.02.
Results
Possible DRI’s are presented in table 1, based on the ALSPAC cohort. The Recommended Daily Allowance (RDA) is defined as “an estimate of the daily average intake that meets the nutritional requirements of nearly all (97–98%) of healthy members of a particular life stage and gender group.” (1). When the nutrient requirement has a statistically normal distribution, the RDA is derived from the EAR by adding two standard deviations in nutrient requirement to the EAR. The SD of the variance in nutrient requirement is defined as the EAR times a coefficient of variance (CV).
Table 1. Putative Daily Intakes for n-3 LCFA’s during pregnancy.
Putative Estimated Average Requirements (ERAs) and Recommended Daily Requirements (RDA’s) for n-3 LCFAs (eicosapentaenoic acid, docosapentaenoic acid n-3 and docosahexaenoic acid) were calculated following definitions described by the Food and Nutrition Board, but should be considered solely the opinion of the authors. Range reflects n-3 LCFA derived from seafood intake, lower number and estimation of 25% correction for other food sources. Suboptimal fine motor skills were assessed by the ALSPAC Developmental Index as previously described (2). Low verbal IQ was assessed by administration of the WISC III- UK as described (2). Significant maternal depressive symptoms at 18 and 32 w gestation were assessed by the Edinburgh Postnatal Scale of Depression
| Putative EAR range |
Putative RDA range |
|||
|---|---|---|---|---|
| Inadequacy model | en% | mg/d 2,000 Cal |
en% | mg/d 2,000 Cal |
| Suboptimal fine motor skills, child, 42 m | 0.050– 0.06 | 111– 139 | 0.20– 0.26 | 456– 570 |
| Low verbal IQ child 8 y | 0.065– 0.08 | 114– 181 | 0.22– 0.28 | 590– 611 |
| Maternal depression 18 w gestation | 0.15– 0.19 | 333– 417 | 0.30– 0.38 | 777– 850 |
| Maternal depression 32 w gestation | 0.18– 0.22 | 389– 486 | 0.33– 0.41 | 830– 917 |
In the absence of adequate data, the coefficient of variance of the nutrient requirement is assumed to be 10 % of the EAR (1). When using a CV of 10% for suboptimal fine motor control, which has an EAR of 0.05 en % (n-3 LCFA), the RDA would be 0.06 en% (n-3 LCFA) (putative-RDA model, table 1). However, at this level of intake nearly an equal number of the population of children above and below this set point would still manifest this symptom of deficiency. Thus, a CV for n-3 LCFA assumed to be 10% fails at face value to meet the nutrient requirements of 97.5 % of the population and thus fails to meet the definition of an RDA.
Since the ALSPAC cohort as large and representative of a UK population, it represents a normative population distribution in the variance in nutrient requirements during pregnancy. Thus in this cohort, the variance of the nutrient requirement (2 SD’s) can be directly derived from the intake distribution data. The frequency distribution of n-3 LCFA’s was Z – score transformed to calculate 2 SD’s (0.155 en%) as described in the IOM methodology (1). 2 SD’s were added to each putative EAR to derive a putative RDA’s for each deficiency model (Table 1). Since other food sources, such as eggs and meat are sources of n-3 LCPUFAs (12), the range of values presented assumes a possible upward limit of correction of 25% to account for n-3 LCFA from these other dietary sources.
Discussion
To our knowledge this is the first calculation of intakes of n-3 LCFAs that approaches established statistical criteria and definitions for DRI’s. We wish clarify that the putative considerations presented here are solely the opinions of the authors and should not be confused with Dietary Reference Intakes issued under authority of the Food and Nutrition Board. We estimated that for reduction of neurodevelopmental and psychiatric risks, a putative EAR for n-3 LCFA in pregnancy is 0.175 en% in a Western population with a background intake of approximately 4–6 en% LA. A higher requirement for the mother is consistent with the observation that DHA is preferentially transferred by the placenta to the fetus (13). Specific requirements for DHA in isolation for other n-3 LCFAs could not be established from this population based analysis. We also recognized that other foods such as meats may contribute as much as 30% of dietary n-3 LCFAs and thus a putative EAR of 0.175 en% is likely to be a low estimate (12). Ranges that include a 25% upward error are thus presented in table 1. Putative RDA’s are difficult to estimate due to uncertainties regarding the variance in nutrient requirements and skewing of the population distribution. A coefficient of variance in nutrient requirements for n-3 LCFAs that is larger than 10% is consistent with observation that nearly 10-fold greater intakes of n-3 LCFAs are needed to achieve the same n-3 LCFA tissue compositions due to a 10-fold variance in background intakes of n-6 fatty acids that compete for elongation, desaturation and phospholipid incorporation (15). In our prior publication we estimated that intakes of 0.350 en% of n-3 LCFA from seafood would reduce risk in 12 models of chronic disease for >98% of the populations, but did not utilize the statistical definitions of DRI’s (14). Despite two divergent statistical methodologies and population datasets, the results are remarkably convergent for neurodevelopmental and psychiatric outcomes, but reduction in mortality from cardiovascular disease, stroke and all cause mortality in men and women (14).
Upper limit of intake
We found no evidence for an upper limit of intakes n-3 LCFA seafood among the ALSPAC cohort. We are not aware of data indicating that an excess of n-3 LCFA intake contributes to neurodevelopmental or psychiatric risks. Intakes of linoleic acid greater that 1–2 en% impair the ability dietary intakes of n-3 LCFA to reach EAR targets for the tissue compositions of n-3 LCFAs due to completion for elongation and desaturation (15). Lowering background intakes of the omega-6 linoleic acid, especially below 3 energy %, elevates tissue compositions of n-3 LCFAS (1) and would likely lower dietary requirements for n-3 LCFAs (16).
Consideration of the Hill Criteria for Causality
The viewpoints of Sir Austin Bradford Hill are routinely evaluated in assessing the strength of the body of epidemiological and other evidence for possible relationships to causality (17). While an epidemiological study of a representative population was utilized to calculate the putative DRI’s presented here, a single epidemiological study is insufficient to establish causality. The causal relationships between n-3 LCFA deficits and neurodevelopmental abnormalities have recently been reviewed in the consensus recommendations on behalf of the European Commission research projects on Perinatal Lipid Metabolism and Early Nutrition Programming (18). Here we utilize the “Hill Criteria to evaluate the postulation that DHA and n-3 LCFA deficiencies are causally related to an increase risk of psychiatric disorders, specifically major depression.
1. Strength
The ecological and epidemiological associations between low seafood and n-3 LCFA intake are strong. Ecological studies indicate that in comparison to countries with the highest consumption, low seafood consumption is associated with: a 65-fold higher risk for lifetime prevalence of major depression (r= − 0.84, p<0.0001) (19), a 50-fold higher risk for postnatal depression, (r= − 0.81, p<0.0001) (20), a 30-fold higher risk for bipolar spectrum disorder (r= − 0.80, p<0.0003) (21) and a 10-higher risk of death from homicide mortality (r= − 0.63, p<0.0006) (22). Correlation coefficients in these ranges are considered to be strong.
2. Consistency
Consistent findings observed by different persons in different places with different samples strengthens the likelihood of an effect” (17). We find relationship of low n-3 LCFA status and greater risk of affective illnesses to be consistent across ecological studies, epidemiological studies, case control studies and biological tissue sample studies. In particular, epidemiological studies have reported strong associations between low seafood intake and greater risk of depression with a high degree of consistently. Among 1,767 subjects in Northern Finland, Tanskanen et al (23) found that both the risk of being depressed (OR=0.63; 95% CI, 0.43 to 0.94; P=0.02) and the risk of having suicidal ideation (OR= 0.57; 95% CI 0.35 to 0.95; P=0.03) were significantly lower among frequent lake-fish consumers compared with more infrequent consumers. In a birth cohort of 5,689 Finnish subjects, the risk of depression was 2.6-fold (95% CI 1.4 to 5.1) greater and risk of suicidal thinking was 1.5 fold (95% CI 1.0 to 3.0) greater comparing females with rare fish consumption to regular consumers (24). Finnish fishermen (n=6,410) consume twice as much fish but have lower risk of mortality from alcohol-related diseases (OR= 0.59; 95% CI 0.41 to 0.82) and suicides (OR= 0.61; 95%CI 0.39 to 0.91) compared to the general population, after adjustment for confounding variables (25). In contrast, Hakkarainen et al (26) found no associations between dietary intakes of omega-3 fatty acids (or fish consumption) and self reports of depressed mood, hospitalization for a major depressive episode or suicide among 29,133 Finnish men. However, there was a high covariance with fish and omega-6 linoleate consumption, which was 20-fold higher than n-3 LCFA from fish (27). Thus, it is difficult to determine which factor was specifically associated with increased risk of depression. Among 21,835 Norwegians, users of cod liver oil were significantly less likely to have depressive symptoms than non-users after adjusting for multiple possible confounding factors (OR=0.71; 95% CI 0.52 to 0.97) (28). In a longitudinal follow up study of 13,017 French subjects, subjects consuming fatty fish, or having an n-3 LCFA intake greater than 1 en%, had significantly reduced risk of single or recurrent depressive episodes (29). In the Zupthen Study of the Elderly, high intakes of n-3 LCFAs (mean = 407 mg/d) were associated with lower risk of depressive symptoms (OR= 0.46; 95% CI 0.22 to 0.95), compared with low intakes= 21 mg/d) (30). Among 7,903 Spanish subjects, moderate consumption of fish had a relative risk reduction of more than 30% (31). Among 10,602 men from Northern Ireland and France, greater depressed mood is associated with lower fish intake in a nonlinear relationship (32). A similar non-linear relationship between greater depression and lower fish intake was described among a UK population (n=2,982) (33). Jack et al (34) also found no association between fish consumption and depression defined by DSM-III criteria in New Zealand population (n= 755). Murakami et al found no association between fish intake and lower risk of depressive symptoms among 618 adults. However the mean n-3 LCFA intake was approximately 0.37 en% far above the putative EAR’s presented here (35), thus the majority of the population may have adequate intakes. We conclude that epidemiological studies utilizing dietary intake measures find associations between low n-3 LCFA intake and significant depressive symptoms with good consistency to these data (6).
3. Specificity
Tissue compositional studies have fairly consistently reported a lower n-3 LCFA status and/or a higher n-6 LCFA status among depressed subjects. The most specific evidence of tissue compositional deficits is the finding that DHA was 22% lower in the postmortem orbitofrontal cortex of patients with major depressive disorder (36). A similar deficit of DHA was found in the orbitofrontal cortex of patients with bipolar disorder (37). The pathophysiology of depressive disorders is thought to involve deficits in orbitofrontal cortex function (38). These specific deficits in brain composition, combined with the epidemiologically based tissue compositional studies, indicate that deficits of n-3 LCFA, in particular EPA and DHA, are associated with depressive illnesses. Adams et al (37) found a significant positive correlation between to the severity of depression and both the erythrocyte phospholipid arachidonic acid (AA) and EPA ratio and erythrocyte EPA alone. Maes et al (39) found lower n-3 LCFAs in serum phospholipids and cholesterol esters of depressed patients compared to controls. Edwards et al (40) reported lower EPA and DHA concentrations in erythrocytes depressed compared to control subjects. They also noted a biological gradient with lower erythrocyte DHA correlated with greater severity of symptoms (r= 0.80, p<0.01). Peet et al (41) also reported a nearly 50% reduction in DHA in the erythrocytes of depressed subjects. Among a community sample of the elderly in Bordeaux, plasma EPA alone was inversely associated with severity of depressive symptoms (42). Among adolescents in Crete (n=90), depressive symptoms were negatively associated with EPA and positively associated with the omega-6 fatty acid dihomo-gamma linolenic acid in adipose tissue (43). Among 247 healthy males in Crete, mildly depressed subjects had significantly reduced (−34.6%) adipose tissue DHA levels compared to non-depressed subjects. Multiple linear regression analysis indicated that depression related negatively to adipose tissue DHA levels (44). Among 3884 elderly subjects in Rotterdam, n-3 LCFAs were significantly lower (5.2% vs. 5.9% P = 0.02) and ratios of n-6 to n-3 LCFAs were higher (7.2 vs. 6.6, P = 0.01) among subjects with depressive disorders compared to controls (45). Among a US community sample (n=207), higher plasma AA and lower EPA concentrations were associated with greater depression and neuroticism (46) and erythrocyte n-3 LCFAs were decreased among patients with social anxiety disorder (47). EPA levels in erythrocyte were significantly lower in suicide attempters compared to control subjects (48). When the highest and lowest quartiles of EPA in RBC were compared, the odds ratios of suicide attempt was 0.12 in the highest quartile (95% CI 0.04 to 0.36, p for trend =0.0001) after adjustment for possible confounding factors (48). A bias against publications failing to find tissue compositional differences may exist, however most published studies indicate a lower n-3 LCFA body composition status among depressed subjects.
Depression associated with other medical disorders
Among patients with acute coronary syndromes depressed subjects had significantly lower concentrations of total omega-3 and DHA and higher ratios of AA/DHA and AA/EPA, compared to controls, (49). A second study of patients with acute coronary syndromes reported that higher depression severity scores were significantly associated with lower DHA levels (50). Similar, but non-significant trends were observed for EPA and total n-3 LCFA levels. Consistent with these reports, Schins et al (51) found higher plasma AA/EPA ratios among depressed subjects compared to non-depressed subjects in a study of 50 post myocardial infarction patients. Kobayakawa et al (52) found no differences comparing depressed and non-depressed lung cancer patients, but used very low cut point to define significant depression. Among patients with multiple sclerosis, no differences in n-3 LCFA compositions were found comparing depressed to non-depressed patients (53).
4. Temporality
Deficits in n-3 LCFA tissue status can be caused by either low intakes of n-3 LCFA and or excessive intakes of competing omega-6 fatty acids; for example reducing dietary intakes of n-6 linoleic acid, from 8 en% to 1 en %, results in 10-fold higher tissue concentrations of n-3 LCFA (14). Strong temporal relationships has been reported between increasing intakes of the omega-6 fatty acid, linoleic acid and greater prevalence rates of major depression (54) and homicide mortality in 5 different countries 1960 and 2000 (20-fold higher risk, r=0.94, p<0.0001) (55).
5. Biological gradient
A biological gradient is apparent in the reduction of risk of depression in evident in ecological studies and epidemiological studies progressively greater exposure to n-3 LCFA from fish consumption generally lead to progressively lower incidence of psychiatric symptoms and illness as reviewed above. Direct compositional analyses of tissue composition usually report a similar biological gradient, as reviewed above. In ecological and epidemiological studies, negative exponential equations consistently best describe the biological gradient relationships (14).
6. Plausibility
DHA is a required nutrient for neurological development, cannot be substituted by any other molecule (2, 56). The multiple interacting mechanisms linking n-3 LCFA and depressive symptoms have recently been reviewed (57, 58). Plausible biological mechanisms linking dietary deficiencies of n-3 LCFAs with psychiatric illness include: depletion of serotonin and dopamine levels by 50% in animal models, impaired neuronal migration, connectivity, timed apoptosis, and dendritic arborization, such that there is an irreversible disruption in the neuronal pathways that regulate behavior (58) neuroinflammatory processes (39,59) and dysregulation of the hypothalamic pituitary adrenal axis (60). N-3 LCFAs may prevent vascular contributions to depression (61). Inadequate serotinergic and dopaminergic function has long been recognized in the pathophysiology of depression and is the target of most pharmaceutical treatments. Concentrations of serotonin and dopamine were nearly doubled in the frontal cortex of piglets among piglets fed infant formula supplemented with DHA and AA for 18 days (62). Unconditioned mild stress induced a significant decrease in the tissue levels of serotonin decreased in the frontal cortex, striatum and hippocampus in the range of 40% to 65%. Interestingly, the n-3 LCFA supplementation reversed this stress-induced reduction in 5-HT levels and decrease aggressive behavior (63). One generation of n-3 LCFA deficiency markedly increased depressive and aggressive behaviors in rats (64). Consistent with these animal studies Hibbeln et al (65) found the lower plasma DHA concentrations were correlated with lower concentrations of the metabolites of both serotonin and dopamine in cerebrospinal fluid among healthy controls. Low cerebrospinal fluid concentrations of these metabolites have been repeatedly reported among suicidal and impulsive patients. Chronic alcohol use, in the context of a low omega-3 diet depleted DHA levels by 50% in Rhesus frontal cortex, (14% to 7%), suggesting that depression and impulsive behaviors associated with alcohol may be attributable, in part, to depletion of n-3 LCFAs (66). Alcohol induced depletion of neural tissues may contribute to the high rates of violence and depression among alcoholics (54). We conclude that many known biological mechanisms plausibly link n-3 LCFA deficiencies to depressive and aggressive pathologies.
7. Coherence
Coherence between epidemiological and laboratory findings increases the likelihood of an effect (17). One example of the coherence is the role of n-3 LCFA in relationship to depressive symptom in pregnancy. We posited that since maternal DHA is selectively transported to the fetus, mother without sufficient dietary intakes may become depleted, leaving them more vulnerable to depression symptoms during or after pregnancy (20). A cross national ecological study supported this proposition linking both low maternal milk DHA composition and low seafood consumption with higher rates of postnatal depression (20). In a series of animal experiments Levant et al (67–69) have demonstrated the depletion of regionally specific brain DHA in a single reproductive cycle, with multiple parity and alterations of dopamine and dopamine related behaviors. Decreased brain DHA was associated with decreased hippocampal brain derived neurotropthic factor, increased corticostrerone responses to stress and increased immobility on the forced swim test (70). Otto et al (71) found that postpartum depression symptoms were associated with a slower recovery of DHA plasma status. A recent randomized controlled trial among pregnant women reported significantly lower depression scores and higher rates of clinical response (62%) to 3.5 g/d of omega-3 fatty acids compared to placebo (27%) (72). Another randomized controlled trial reported a trend towards efficacy, but was described by the authors as underpowered (73). Recent non-blinded trials of EPA and DHA supplementation have also reported a reduction of depressive symptoms related to pregnancy of 50%(74.75). Two prior epidemiological studies (from New Zealand and Japan) reported no association between seafood consumption and pregnancy-related depressive symptoms. The New Zealand study included only 80 women (76) and consequently did not have sufficient power. Although the Japanese study had much larger numbers (n=865) it used a cut point of 9+ (rather than 13+) on the Edinburgh Postnatal Depression Scale and nearly all subjects had a relatively high intake of oily fish with very few subject consuming zero omega-3 from seafood (77). In contrast, Sontrop et al (78) found that among smoking and single women, low intakes of omega-3’s from seafood were associated depressive symptoms in pregnancy. In summary, the status of studies in pregnancy related depression is coherent and promising: animal studies, adequately powered intervention and epidemiological studies do support the proposition that n-3 LCFAs may have therapeutic benefit.
8. Experiment
The specificity and efficacy of n-3 LCFAs in reducing significant depressive symptoms has been assessed in the meta-analyses of randomized placebo controlled trials. Three recent meta-analyses of up to 11 randomized placebo controlled trials of omega-3 fatty have each reported large treatment effect sizes of n-3 LCFA in reducing significant depressive symptoms (75, 79, 80). In 2006 the accumulation of data was sufficient enough for the American Psychiatric Association to issue treatment recommendations for n-3 LCFAs (75). Since that time several confirmatory studies have also been published. Jazayeri et al (81) reported similar response rates (defined as a 50% reduction in depressive symptoms) comparing patients receiving 1 g of EPA alone (50%) to fluoxetine (56%) but significantly better rates (81%) when patients received both EPA and fluoxetine in combination. Antypa et (82) found a reduction of depression related cognitive symptoms even among healthy controls. Mischoulon et al (83) reported antidepressant efficacy of DHA 1gm/d, but not at higher doses. Dinan et al (84) reported that n-LCFA levels and ratios of EPA to AA predicted clinical responses to antidepressants. Freund-Levi et al (2007) found that reduction of aggressive and depressive symptoms in Alzheimer’s patient to n-3 LCFAs appeared to depend upon their APOEω4 genotype. Some randomized controlled trials have failed to find treatment effects (85–88). However, Elkin et al (89) established that if clinical trials include subjects without a sufficiently high initial severity of depression, antidepressants and antidepressant treatments were unlikely to demonstrate treatment effects because of inadequate power and floor effects inherent in the clinical study of depression (89). A common feature of the clinical trials that have failed to find antidepressive effects of n-3 LCFAs has been the enrollment of patients below the cut points of depressive symptom severity established by Elkin et al (89) with one exception (86). Thus, these insufficiently powered trials should be not considered as definitively negative trials. The demonstration of efficacy in randomized trials appears to depend upon adequate symptom severity, baseline n-LCFA status perhaps the relative amounts of EPA and DHA and perhaps allelic variance. The evidence from the currently published placebo controlled randomized trials and meta-analyses of these trials indicate that n-3 LCFAs are effective in treating severe depression.
9. Analogy
Nutritional deficiencies in vitamin B6, vitamin B12, niacin, folate, panothenic acid iodine and iron results in reversible neuropsychiatric symptoms. Deficiency symptoms for these nutrients are rarely confined to one organ system. Deficiencies in n-3 LCFAs may similarly increase risk of several chronic diseases. We have previously proposed that increased risk of cardiovascular disease and increased risk of affective disorders are two different manifestations of a common deficiency of n-3 LCFA’s (54).
Conclusion
After consideration of these viewpoints, commonly referred to as the Hill Criteria, we conclude that the relationship between low n-3 LCFA status and increased risk of major depression is highly likely to be causal. This conclusion is consistent with the statement of treatment recommendations for n-3 LCFAs issued by the American Psychiatric Association (90) and by a United Kingdom Parliamentary Inquiry Report on Nutrients in Mental Health (91). We conclude that neuropsychiatric and neurodevelopmental endpoints have precedent for use deficiency outcome measure and that Dietary Reference Intake’s for n-3 LCFAs can be established after careful consideration.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council, the Wellcome Trust and the University of Bristol currently provide core support for ALSPAC. This research was supported in part by the Intramural Research Program of the NIH, NIAA. At the time of data collection in pregnancy support was obtained from a variety of sources include the UK Department of the Environment and the Ministry of Agriculture, Fisheries and Food. This manuscript does not represent any policy or position of the National Institutes of Health or DHHS and is solely the scientific opinion of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Workshop on DHA as Required Nutrient, Baltimore, MD June 20–21, 2008
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.Hellwig JP, Meyers LD. Dietary reference intakes: the essential guide to nutrient requirements. Washington, D.C: National Academy of Sciences Press; 2006. [Google Scholar]
- 2.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 3.Golding J, Pembrey M, Jones R. ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 4.ALSPAC Study. http://www.bristol.ac.uk/alspac/last asseessed 23 April 2009.
- 5.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 6.Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009 Mar 10; doi: 10.1097/EDE.0b013e31819d6a57. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 8.Harris B, Huckle P, Thomas R, Johns S, Fung H. The use of rating scales to identify postnatal depression. Br J Psychiatry. 1989;154:813–817. doi: 10.1192/bjp.154.6.813. [DOI] [PubMed] [Google Scholar]
- 9.Rogers I, Emmett P. Diet during pregnancy in a population of pregnant women in South West England. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Eur J Clin Nutr. 1998;52:246–250. doi: 10.1038/sj.ejcn.1600543. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Agriculture Fisheries and Food and Royal Society of Chemistry. Fatty acid supplement to McCance and Widdowson's the composition of foods. London: Ministry of Agriculture, Fisheries and Foods/ Royal Society of Chemistry; 1998. [Google Scholar]
- 11.Newson R ALSPAC Study Team. Multiple test procedures and smile plots. Stata J. 2003;3:109–132. [Google Scholar]
- 12.Howe P, Meyer B, Record S, Baghurst K. Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition. 2006;22:47–53. doi: 10.1016/j.nut.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Crawford M. Placental delivery of arachidonic and docosahexaenoic acids: implications for the lipid nutrition of preterm infants. Am J Clin Nutr. 2000;71:275S–284S. doi: 10.1093/ajcn/71.1.275S. [DOI] [PubMed] [Google Scholar]
- 14.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 15.Lands WE, Libelt B, Morris A, et al. Maintenance of lower proportions of (n - 6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n - 3) fatty acids. Biochim Biophys Acta. 1992;1180:147–162. doi: 10.1016/0925-4439(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 16.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 17.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- 19.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 20.Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 21.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 22.Hibbeln JR. Seafood consumption and homicide mortality. World Rev Nutr Diet. 2001;85:41–46. doi: 10.1159/000059747. [DOI] [PubMed] [Google Scholar]
- 23.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki H. Fish consumption, depression, and suicidality in a general population. Archives of General Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- 24.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Rasanen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Turunen AW, Verkasalo PK, Kiviranta H, et al. Mortality in a cohort with high fish consumption. Int J Epidemiol. 2008 doi: 10.1093/ije/dyn117. [DOI] [PubMed] [Google Scholar]
- 26.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 27.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J. Food and nutrient intake in relation to mental wellbeing. Nutr J. 2004;3:14. doi: 10.1186/1475-2891-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–249. doi: 10.1016/j.jad.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Astorg P, Couthouis A, Bertrais S, et al. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2008;78:171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Kamphuis MH, Geerlings MI, Tijhuis MA, Kalmijn S, Grobbee DE, Kromhout D. Depression and cardiovascular mortality: a role for n-3 fatty acids? Am J Clin Nutr. 2006;84:1513–1517. doi: 10.1093/ajcn/84.6.1513. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 32.Appleton KM, Woodside JV, Yarnell JW, et al. Depressed mood and dietary fish intake: direct relationship or indirect relationship as a result of diet and lifestyle? J Affect Disord. 2007;104:217–223. doi: 10.1016/j.jad.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Appleton KM, Peters TJ, Hayward RC, et al. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Soc Psychiatry Psychiatr Epidemiol. 2007;42:100–104. doi: 10.1007/s00127-006-0142-3. [DOI] [PubMed] [Google Scholar]
- 34.Jacka FN, Pasco JA, Henry MJ, Kotowicz MA, Nicholson GC, Berk M. Dietary omega-3 fatty acids and depression in a community sample. Nutr Neurosci. 2004;7:101–106. doi: 10.1080/10284150410001710438. [DOI] [PubMed] [Google Scholar]
- 35.Murakami K, Mizoue T, Sasaki S, et al. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–147. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 36.McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 37.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008 doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31 Suppl:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 39.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega-3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Research. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 40.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. Journal of Affect Disorders. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 41.Peet M, Murphy B, Edwards R, Shay J, Horrobin D. Depletion of dososahexaenioc acid in erythrocyte membranes of depressed patients. Biological Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 42.Feart C, Peuchant E, Letenneur L, et al. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr. 2008;87:1156–1162. doi: 10.1093/ajcn/87.5.1156. [DOI] [PubMed] [Google Scholar]
- 43.Mamalakis G, Jansen E, Cremers H, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose and serum cholesteryl ester polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. Eur J Clin Nutr. 2006;60:1016–1023. doi: 10.1038/sj.ejcn.1602413. [DOI] [PubMed] [Google Scholar]
- 44.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 45.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 46.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High {omega}-6 and Low {omega}-3 Fatty Acids are Associated With Depressive Symptoms and Neuroticism. Psychosom Med. 2007 doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 47.Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol. 2006;16:107–113. doi: 10.1016/j.euroneuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Huan M, Hamazaki K, Sun Y, et al. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Parker GB, Heruc GA, Hilton TM, et al. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Res. 2006;141:279–286. doi: 10.1016/j.psychres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Schins A, Crijns HJ, Brummer RJ, et al. Altered omega-3 polyunsaturated fatty acid status in depressed post-myocardial infarction patients. Acta Psychiatr Scand. 2007;115:35–40. doi: 10.1111/j.1600-0447.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 52.Kobayakawa M, Yamawaki S, Hamazaki K, Akechi T, Inagaki M, Uchitomi Y. Levels of omega-3 fatty acid in serum phospholipids and depression in patients with lung cancer. Br J Cancer. 2005;93:1329–1333. doi: 10.1038/sj.bjc.6602877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aupperle RL, Denney DR, Lynch SG, Carlson SE, Sullivan DK. Omega-3 fatty acids and multiple sclerosis: relationship to depression. J Behav Med. 2008;31:127–135. doi: 10.1007/s10865-007-9139-y. [DOI] [PubMed] [Google Scholar]
- 54.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: When cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Hibbeln JR, Nieminen LR, Lands WE. Increasing homicide rates and linoleic acid consumption among five Western countries, 1961–2000. Lipids. 2004;39:1207–1213. doi: 10.1007/s11745-004-1349-5. [DOI] [PubMed] [Google Scholar]
- 56.Salem N, Jr, Niebylski CD. The nervous system has an absolute molecular species requirement for proper function. Mol Membr Biol. 1995;12:131–134. doi: 10.3109/09687689509038508. [DOI] [PubMed] [Google Scholar]
- 57.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr. 2007;16 Suppl 1:391–397. [PubMed] [Google Scholar]
- 59.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 60.Hibbeln JR, Bissette G, Umhau JC, George DT. Omega-3 status and cerebrospinal fluid corticotrophin releasing hormone in perpetrators of domestic violence. Biol Psychiatry. 2004;56:895–897. doi: 10.1016/j.biopsych.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Teper E, O'Brien JT. Vascular factors and depression. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.2020. [DOI] [PubMed] [Google Scholar]
- 62.de la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. Journal of Nutrition. 1999;129:2088–2093. doi: 10.1093/jn/129.11.2088. [DOI] [PubMed] [Google Scholar]
- 63.Vancassel S, Leman S, Hanonick L, et al. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N., Jr Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biological Psychiatry. 1998;44:235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 66.Pawlosky RJ, Bacher J, Salem N., Jr Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res. 2001;25:1758–1765. [PubMed] [Google Scholar]
- 67.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J Nutr. 2006;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- 68.Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- 69.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 70.Levant B, Ozias MK, Davis PF, et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69:237–243. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 72.Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 73.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42:199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- 74.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: A randomized placebo-controlled study. J Affect Disord. 2008;110:142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006;113:31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 76.Browne JC, Scott KM, Silvers KM. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. J Affect Disord. 2006;90:131–139. doi: 10.1016/j.jad.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Miyake Y, Sasaki S, Yokoyama T, et al. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychol Med. 2006;36:1727–1735. doi: 10.1017/S0033291706008701. [DOI] [PubMed] [Google Scholar]
- 78.Sontrop J, Campbell MK. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med. 2006;42:4–13. doi: 10.1016/j.ypmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84:1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 80.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 81.Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 82.Antypa N, Van der Does AJ, Smelt AH, Rogers RD. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol. 2008 doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 83.Mischoulon D, Best-Popescu C, Laposata M, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 84.Dinan T, Siggins L, Scully P, O'Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: Focus on cytokines and polyunsaturated fatty acids. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 87.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72:211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 89.Elkin I, Gibbons RD, Shea MT, et al. Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1995;63:841–847. doi: 10.1037//0022-006x.63.5.841. [DOI] [PubMed] [Google Scholar]
- 90.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 91.The Associate Parliamentary Food and Health Forum. The Influence of Nutrition on Mental Health. London: Parliament of the United Kingdom; 2008. The Links Between Diet and Behaviour. [Google Scholar]