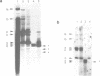Abstract
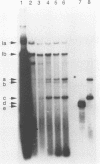
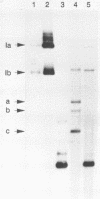
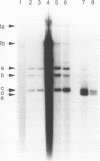
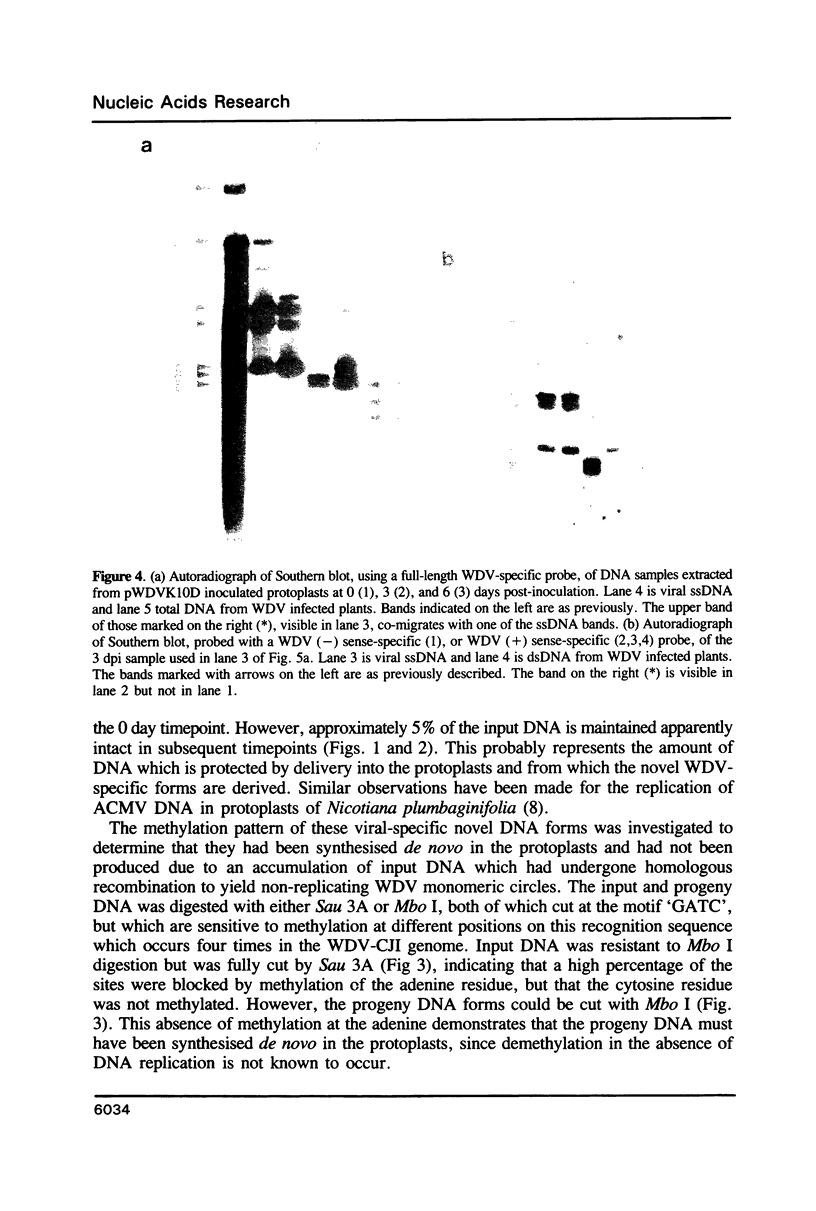
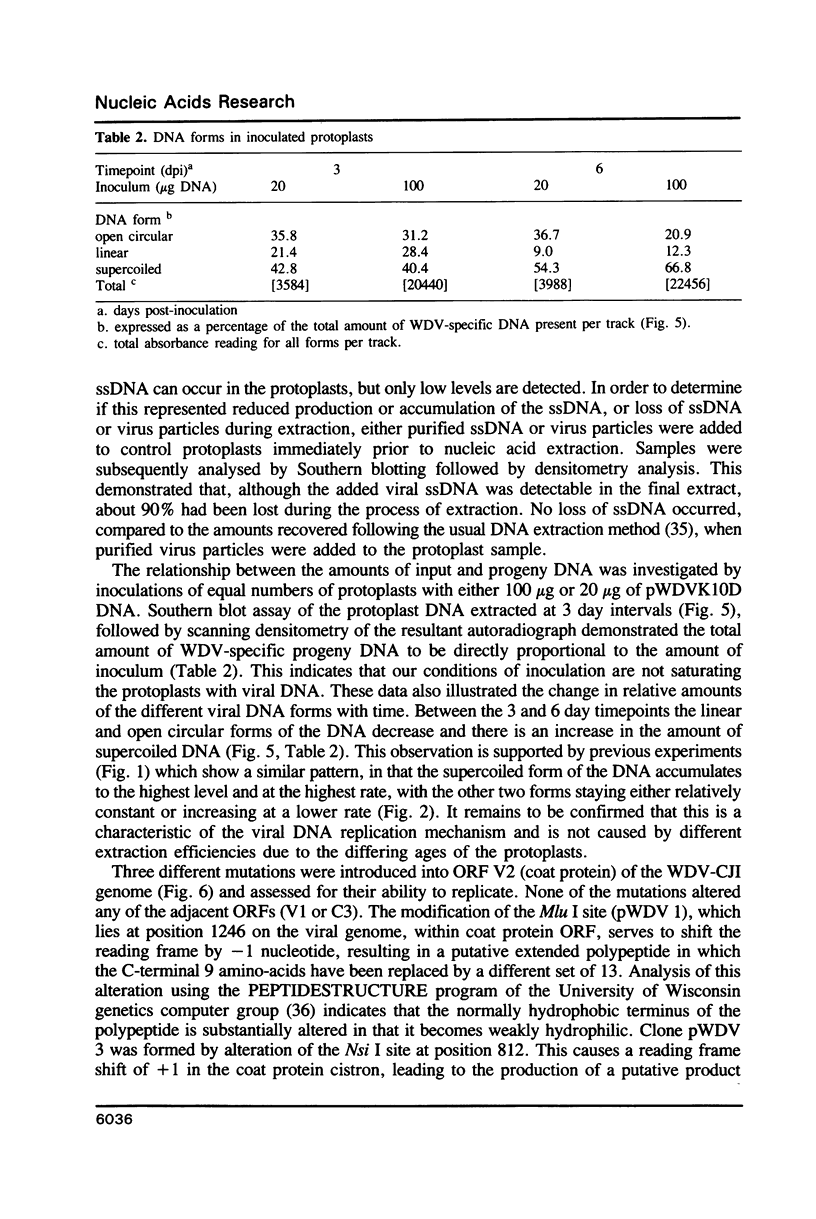
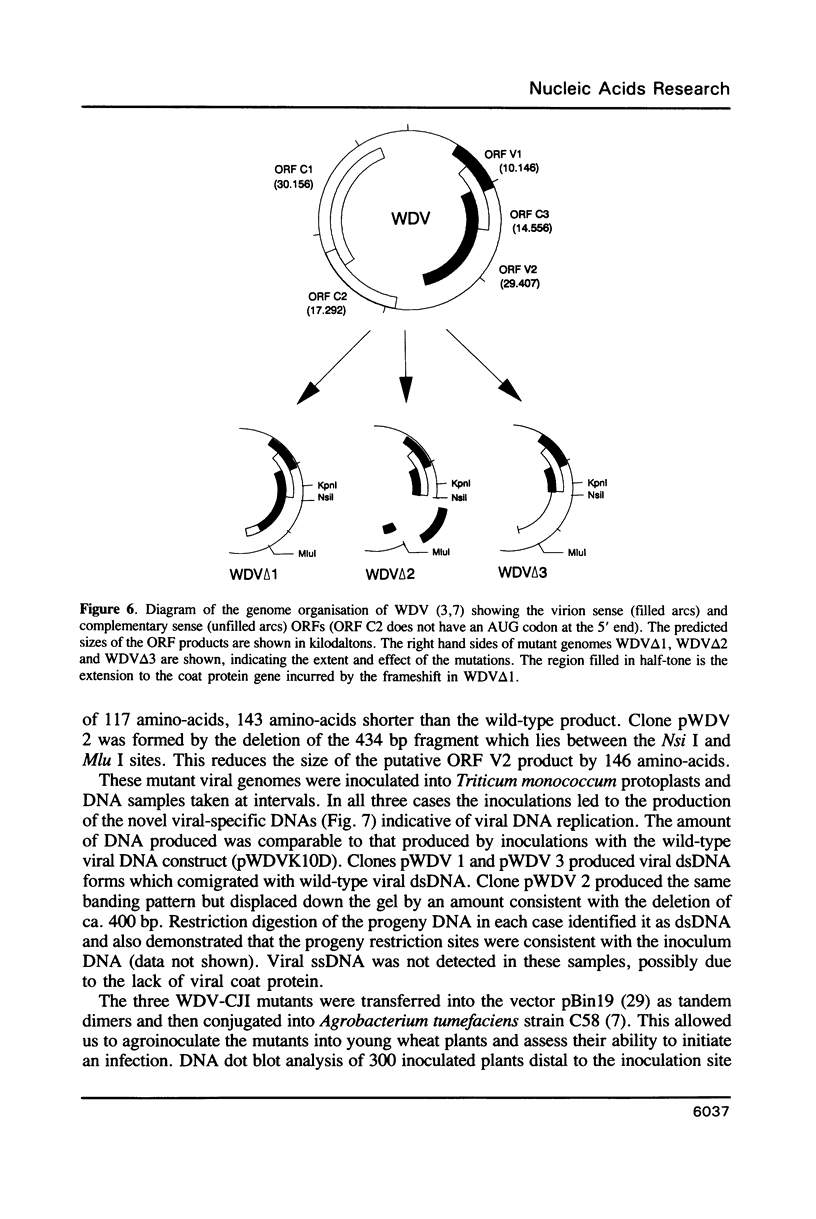
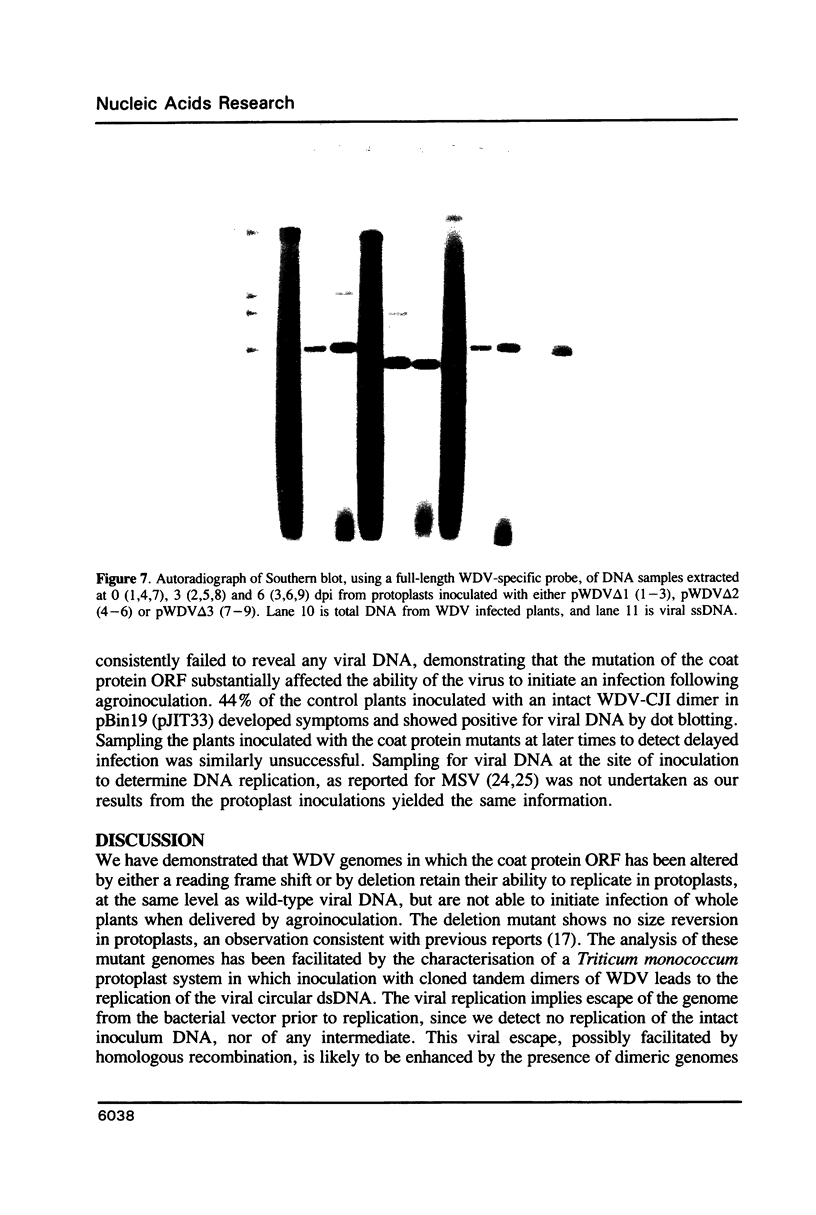
The replication of wheat dwarf virus (WDV) in protoplasts derived from a Triticum monococcum suspension cell system was investigated. The production of circular viral double-stranded DNA (dsDNA) forms consistent with the replication of the viral genome was observed. In comparison to whole plants, the production of viral single-stranded DNA (ssDNA) was reduced, possibly due to only low levels of viral coat protein being produced in the protoplasts. Mutations introduced into the viral coat protein open reading frame (ORF) did not affect the ability of the viral DNA to replicate, and a deletion of ca. 400 bp was tolerated. However, these mutations abolished the infectivity of the viral genome when agroinoculated onto wheat plants, providing evidence that, contrary to the case for the bipartite geminiviruses, the coat protein is essential for infection by WDV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J., Warren M. P. Mother-daughter differences in menarcheal age in adolescent girls attending national dance company schools and non-dancers. Ann Hum Biol. 1988 Jan-Feb;15(1):35–43. doi: 10.1080/03014468800009441. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Stanley J., Donson J., Mullineaux P. M., Boulton M. I. Structure and replication of geminivirus genomes. J Cell Sci Suppl. 1987;7:95–107. doi: 10.1242/jcs.1987.supplement_7.7. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Stanley J. Geminivirus genes and vectors. Trends Genet. 1989 Mar;5(3):77–81. doi: 10.1016/0168-9525(89)90030-9. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J., Gunn H. V., Woolston C. J., Pinner M. S., Boulton M. I., Mullineaux P. M., Davies J. W. Agrobacterium-mediated infectivity of cloned digitaria streak virus DNA. Virology. 1988 Jan;162(1):248–250. doi: 10.1016/0042-6822(88)90416-3. [DOI] [PubMed] [Google Scholar]
- Etessami P., Callis R., Ellwood S., Stanley J. Delimitation of essential genes of cassava latent virus DNA 2. Nucleic Acids Res. 1988 Jun 10;16(11):4811–4829. doi: 10.1093/nar/16.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etessami P., Watts J., Stanley J. Size reversion of African cassava mosaic virus coat protein gene deletion mutants during infection of Nicotiana benthamiana. J Gen Virol. 1989 Feb;70(Pt 2):277–289. doi: 10.1099/0022-1317-70-2-277. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardiner W. E., Sunter G., Brand L., Elmer J. S., Rogers S. G., Bisaro D. M. Genetic analysis of tomato golden mosaic virus: the coat protein is not required for systemic spread or symptom development. EMBO J. 1988 Apr;7(4):899–904. doi: 10.1002/j.1460-2075.1988.tb02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley N., Hohn B., Hohn T., Walden R. "Agroinfection," an alternative route for viral infection of plants by using the Ti plasmid. Proc Natl Acad Sci U S A. 1986 May;83(10):3282–3286. doi: 10.1073/pnas.83.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G. Infectivity and complete nucleotide sequence of the genome of a South African isolate of maize streak virus. Nucleic Acids Res. 1988 Jan 11;16(1):229–249. doi: 10.1093/nar/16.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Pinder A. J., Damsteegt V. D., Rogers S. G. Maize streak virus genes essential for systemic spread and symptom development. EMBO J. 1989 Apr;8(4):1023–1032. doi: 10.1002/j.1460-2075.1989.tb03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDowell S. W., Macdonald H., Hamilton W. D., Coutts R. H., Buck K. W. The nucleotide sequence of cloned wheat dwarf virus DNA. EMBO J. 1985 Sep;4(9):2173–2180. doi: 10.1002/j.1460-2075.1985.tb03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald M., Rutherford M. The treatment of bedsores. Nurs Times. 1973 Oct 11;69(41):1344–1344. [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Stanley J., Townsend R. Infectious mutants of cassava latent virus generated in vivo from intact recombinant DNA clones containing single copies of the genome. Nucleic Acids Res. 1986 Aug 11;14(15):5981–5998. doi: 10.1093/nar/14.15.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Watts J., Stanley J. Synthesis of viral DNA forms in Nicotiana plumbaginifolia protoplasts inoculated with cassava latent virus (CLV); evidence for the independent replication of one component of the CLV genome. Nucleic Acids Res. 1986 Feb 11;14(3):1253–1265. doi: 10.1093/nar/14.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]