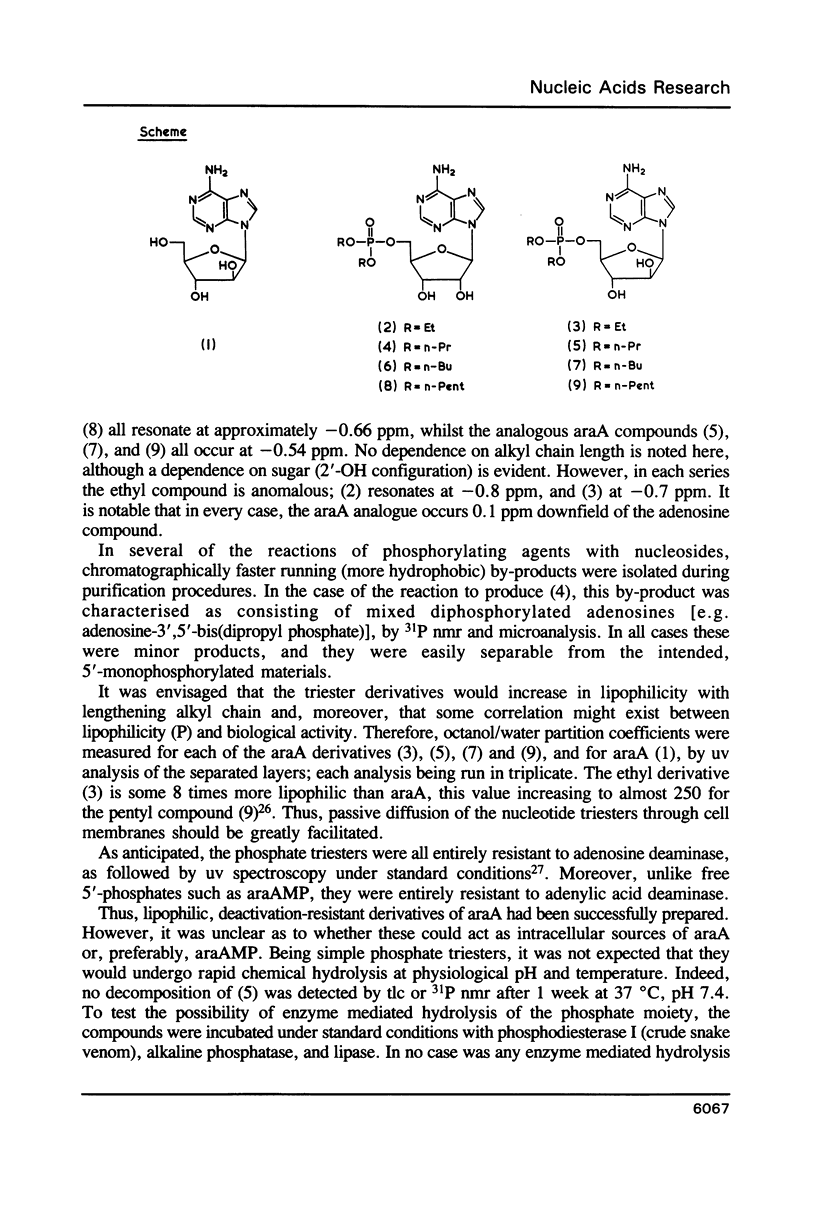
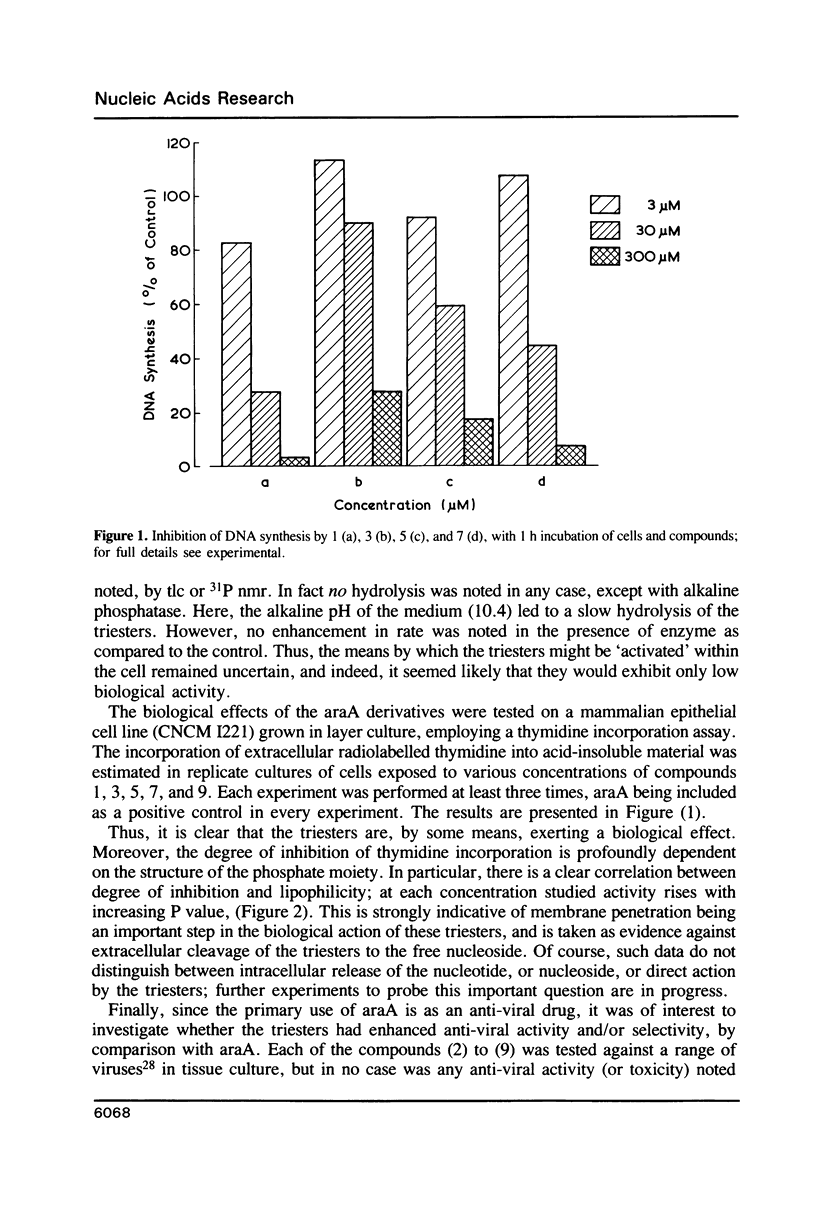
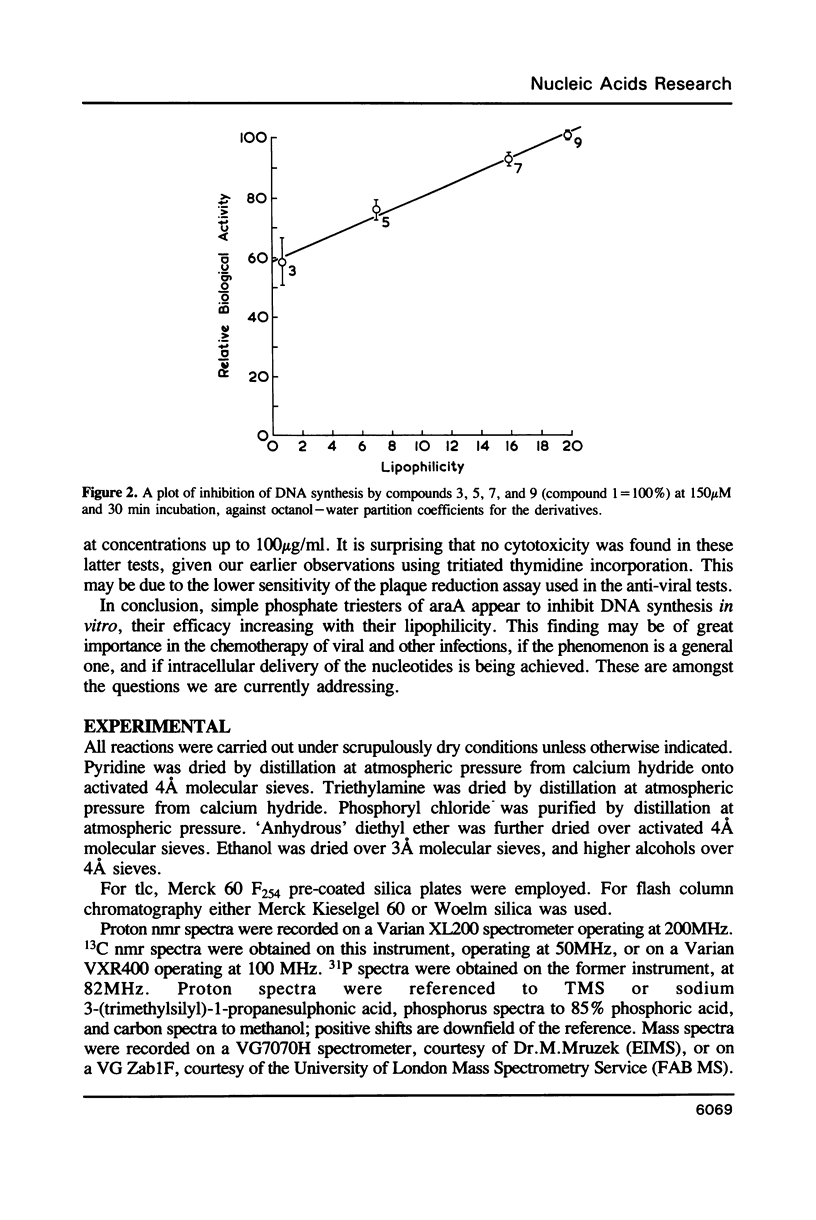
Abstract
A number of novel phosphate triester derivatives of the anti-viral nucleoside analogue araA have been prepared by a rapid 2-step procedure, not necessitating prior sugar protection. Spectroscopic and lipophilicity data have been collected on these compounds, and they have been assayed with a range of hydrolytic enzymes. The compounds have been found to be highly resistant to hydrolysis at physiological pH, enzymatic or otherwise. An in vitro assay indicated inhibition of DNA synthesis by mammalian cells, by each of these compounds, in the range 3-300 microM. Moreover, the degree of inhibition showed a close correlation to chemical structure; in particular, there was a direct relationship between inhibition of thymidine incorporation and lipophilicity. These results suggest cellular penetration by the phosphate triesters and intracellular hydrolysis, by an unspecified mechanism, to the free nucleotide or nucleoside.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bapat A. R., Zarow C., Danenberg P. V. Human leukemic cells resistant to 5-fluoro-2'-deoxyuridine contain a thymidylate synthetase with lower affinity for nucleotides. J Biol Chem. 1983 Apr 10;258(7):4130–4136. [PubMed] [Google Scholar]
- Bryson Y. J., Connor J. D. In vitro susceptibility of varicella zoster virus to adenine arabinoside and hypoxanthine arabinoside. Antimicrob Agents Chemother. 1976 Mar;9(3):540–543. doi: 10.1128/aac.9.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R. R., Freed J. J., Hampton A. Bis(m-nitrophenyl) and bis(p-nitrophenyl) esters and the phosphorodiamidate of thymidine 5'-phosphate as potential sources of intracellular thymidine 5'-phosphate in mouse cells in culture. J Med Chem. 1984 Dec;27(12):1733–1736. doi: 10.1021/jm00378a036. [DOI] [PubMed] [Google Scholar]
- Elion G. B. Symposium on immunosuppressive drugs. Biochemistry and pharmacology of purine analogues. Fed Proc. 1967 May-Jun;26(3):898–904. [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunston R. N., Jones A. S., McGuigan C., Walker R. T., Balzarini J., De Clercq E. Synthesis and biological properties of some cyclic phosphotriesters derived from 2'-deoxy-5-fluorouridine. J Med Chem. 1984 Apr;27(4):440–444. doi: 10.1021/jm00370a005. [DOI] [PubMed] [Google Scholar]
- LEIBMAN K. C., HEIDELBERGER C. The metabolism of P32-labeled ribonucleotides in tissue slices and cell suspensions. J Biol Chem. 1955 Oct;216(2):823–830. [PubMed] [Google Scholar]
- LEPAGE G. A. BASIC BIOCHEMICAL EFFECTS AND MECHANISM OF ACTION OF 6-THIOGUANINE. Cancer Res. 1963 Sep;23:1202–1206. [PubMed] [Google Scholar]
- LICHTENSTEIN J., BARNER H. D., COHEN S. S. The metabolism of exogenously supplied nucleotides by Escherichia coli. J Biol Chem. 1960 Feb;235:457–465. [PubMed] [Google Scholar]
- Prusoff W. H., Goz B. Potential mechanisms of action of antiviral agents. Fed Proc. 1973 Jun;32(6):1679–1687. [PubMed] [Google Scholar]
- REICHARD P., SKOLD O., KLEIN G., REVESZ L., MAGNUSSON P. H. Studies on resistance against 5-fluorouracil. I. Enzymes of the uracil pathway during development of resistance. Cancer Res. 1962 Feb;22:235–243. [PubMed] [Google Scholar]
- Repta A. J., Rawson B. J., Shaffer R. D., Sloan K. B., Bodor N., Higuchi T. Rational development of a soluble prodrug of a cytotoxic nucleoside: preparation and properties of arabinosyladenine 5'-formate. J Pharm Sci. 1975 Mar;64(3):392–396. doi: 10.1002/jps.2600640307. [DOI] [PubMed] [Google Scholar]



