Abstract
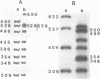
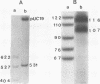
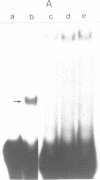
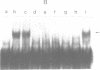
DNA bending has been suggested to play a role in the regulation of gene expression, initiation of DNA-replication, site specific recombination, and DNA packaging. In the human mitochondrial DNA we have found a DNA curvature structure within the 3'-region of ther URF2 sequence in front of the L-strand origin of replication. This structure interacts specifically with a protein factor isolated from mitochondria. Based on the localization of this DNA curvature structure and the known function of such structures the data suggest a model in which this DNA signal sequence and its specific protein binding is involved in the regulatory initiation event of L-strand replication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N. Detection, sequence patterns and function of unusual DNA structures. Nucleic Acids Res. 1986 Nov 11;14(21):8513–8533. doi: 10.1093/nar/14.21.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Better M., Lu C., Williams R. C., Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Yoza B. K. Accurate in vitro transcription of Xenopus laevis mitochondrial DNA from two bidirectional promoters. Mol Cell Biol. 1986 Jul;6(7):2543–2550. doi: 10.1128/mcb.6.7.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun G., Vannier P., Scovassi I., Callen J. C. DNA topoisomerase I from mitochondria of Xenopus laevis oocytes. Eur J Biochem. 1981 Aug;118(2):407–415. doi: 10.1111/j.1432-1033.1981.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987 Mar 6;235(4793):1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Cordonnier A. M., Dunon-Bluteau D., Brun G. A DNA binding protein showing sequence specificity for a region containing the replication origin of Xenopus laevis mitochondrial DNA. Nucleic Acids Res. 1987 Jan 26;15(2):477–490. doi: 10.1093/nar/15.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote G. J., Wang Z., Chiu J. F. alpha-Fetoprotein gene DNA-binding proteins. Arch Biochem Biophys. 1985 Nov 15;243(1):320–324. doi: 10.1016/0003-9861(85)90802-1. [DOI] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Koff A., Tsui S., Tegtmeyer P. The adenine-thymine domain of the simian virus 40 core origin directs DNA bending and coordinately regulates DNA replication. Mol Cell Biol. 1986 Dec;6(12):4578–4584. doi: 10.1128/mcb.6.12.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann S., Zarling D. A. Unique poly(dA).poly(dT) B'-conformation in cellular and synthetic DNAs. Nucleic Acids Res. 1987 Aug 11;15(15):6063–6074. doi: 10.1093/nar/15.15.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon-Bluteau D., Cordonnier A., Brun G. DNA synthesis in a mitochondrial lysate of Xenopus laevis oocytes. H strand replication in vitro. J Mol Biol. 1987 Sep 20;197(2):175–185. doi: 10.1016/0022-2836(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Studies on mitochondrial type I topoisomerase and on its function. Biochim Biophys Acta. 1985 Jan 29;824(1):45–57. doi: 10.1016/0167-4781(85)90028-4. [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Clayton D. A. A transcription factor required for promoter recognition by human mitochondrial RNA polymerase. Accurate initiation at the heavy- and light-strand promoters dissected and reconstituted in vitro. J Biol Chem. 1985 Sep 15;260(20):11330–11338. [PubMed] [Google Scholar]
- Geuskens M., Hardt N., Pedrali-Noy G., Spadari S. An autoradiographic demonstration of nuclear DNA replication by DNA polymerase alpha and of mitochondrial DNA synthesis by DNA polymerase gamma. Nucleic Acids Res. 1981 Apr 10;9(7):1599–1613. doi: 10.1093/nar/9.7.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson J. E., Wong T. W., Clayton D. A. Both the conserved stem-loop and divergent 5'-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem. 1986 Feb 15;261(5):2384–2390. [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Functional roles of DNA polymerases beta and gamma. Proc Natl Acad Sci U S A. 1979 May;76(5):2316–2320. doi: 10.1073/pnas.76.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Ryan K. A., Gann K. L., Rauch C. A., Kang D. S., Wells R. D., Englund P. T. A highly bent fragment of Crithidia fasciculata kinetoplast DNA. J Biol Chem. 1986 Aug 25;261(24):11302–11309. [PubMed] [Google Scholar]
- Koepsel R. R., Khan S. A. Static and initiator protein-enhanced bending of DNA at a replication origin. Science. 1986 Sep 19;233(4770):1316–1318. doi: 10.1126/science.3749879. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Nucleosomes will not form on double-stranded RNa or over poly(dA).poly(dT) tracts in recombinant DNA. Nucleic Acids Res. 1981 Dec 21;9(24):6869–6888. doi: 10.1093/nar/9.24.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin C. J., Zimmerman S. B. A DNA ligase from mitochondria of rat liver. Biochem Biophys Res Commun. 1976 Mar 22;69(2):514–520. doi: 10.1016/0006-291x(76)90551-9. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Barat M. Characterization of a Xenopus laevis mitochondrial protein with a high affinity for supercoiled DNA. Nucleic Acids Res. 1986 Aug 11;14(15):5969–5980. doi: 10.1093/nar/14.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Barat M., Marsault J., Mounolou J. C. Mitochondrial DNA-binding proteins that bind preferentially to supercoiled molecules containing the D-loop region of Xenopus laevis mtDNA. Biochem Biophys Res Commun. 1983 Nov 30;117(1):99–107. doi: 10.1016/0006-291x(83)91546-2. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Patel I., Bastia D. Conformational changes in a replication origin induced by an initiator protein. Cell. 1985 Nov;43(1):189–197. doi: 10.1016/0092-8674(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Prunell A. Nucleosome reconstitution on plasmid-inserted poly(dA) . poly(dT). EMBO J. 1982;1(2):173–179. doi: 10.1002/j.1460-2075.1982.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Inducible binding of a factor to the c-fos enhancer. Cell. 1986 Dec 5;47(5):777–784. doi: 10.1016/0092-8674(86)90520-9. [DOI] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Stenzel T. T., Patel P., Bastia D. The integration host factor of Escherichia coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell. 1987 Jun 5;49(5):709–717. doi: 10.1016/0092-8674(87)90547-2. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N. Curved DNA. CRC Crit Rev Biochem. 1985;19(2):89–106. doi: 10.3109/10409238509082540. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., Pavco P. A. The rat liver mitochondrial DNA-protein complex: displaced single strands of replicative intermediates are protein coated. J Cell Biol. 1985 Jan;100(1):251–257. doi: 10.1083/jcb.100.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. In vitro transcription of human mitochondrial DNA. Identification of specific light strand transcripts from the displacement loop region. J Biol Chem. 1983 Jan 25;258(2):1268–1275. [PubMed] [Google Scholar]
- Welter C., Meese E., Blin N. Rapid step-gradient purification of mitochondrial DNA. Mol Biol Rep. 1988;13(2):117–120. doi: 10.1007/BF00539059. [DOI] [PubMed] [Google Scholar]
- Williams J. S., Eckdahl T. T., Anderson J. N. Bent DNA functions as a replication enhancer in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2763–2769. doi: 10.1128/mcb.8.7.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. DNA primase of human mitochondria is associated with structural RNA that is essential for enzymatic activity. Cell. 1986 Jun 20;45(6):817–825. doi: 10.1016/0092-8674(86)90556-8. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. Isolation and characterization of a DNA primase from human mitochondria. J Biol Chem. 1985 Sep 25;260(21):11530–11535. [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Binding and bending of the lambda replication origin by the phage O protein. EMBO J. 1985 Dec 16;4(13A):3605–3616. doi: 10.1002/j.1460-2075.1985.tb04124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Direct evidence for DNA bending at the lambda replication origin. Science. 1987 Apr 24;236(4800):416–422. doi: 10.1126/science.2951850. [DOI] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Sequence-induced DNA curvature at the bacteriophage lambda origin of replication. Nature. 1985 Oct 3;317(6036):451–453. doi: 10.1038/317451a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Chen S. M., Bolden A., Weissbach A. Mitochondrial DNA replication does not involve DNA polymerase alpha. J Biol Chem. 1980 Dec 25;255(24):11847–11852. [PubMed] [Google Scholar]