Abstract
Variation in response to antimalarial drugs and in pathogenicity of malaria parasites is of biologic and medical importance. Linkage mapping has led to successful identification of genes or loci underlying various traits in malaria parasites of rodents1-3 and humans4-6. The malaria parasite Plasmodium yoelii is one of many malaria species isolated from wild African rodents and has been adapted to grow in laboratories. This species reproduces many of the biologic characteristics of the human malaria parasites; genetic markers such as microsatellite and amplified fragment length polymorphism (AFLP) markers have also been developed for the parasite7-9. Thus, genetic studies in rodent malaria parasites can be performed to complement research on Plasmodium falciparum. Here, we demonstrate the techniques for producing a genetic cross in P. yoelii that were first pioneered by Drs. David Walliker, Richard Carter, and colleagues at the University of Edinburgh10.
Genetic crosses in P. yoelii and other rodent malaria parasites are conducted by infecting mice Mus musculus with an inoculum containing gametocytes of two genetically distinct clones that differ in phenotypes of interest and by allowing mosquitoes to feed on the infected mice 4 days after infection. The presence of male and female gametocytes in the mouse blood is microscopically confirmed before feeding. Within 48 hrs after feeding, in the midgut of the mosquito, the haploid gametocytes differentiate into male and female gametes, fertilize, and form a diploid zygote (Fig. 1). During development of a zygote into an ookinete, meiosis appears to occur11. If the zygote is derived through cross-fertilization between gametes of the two genetically distinct parasites, genetic exchanges (chromosomal reassortment and cross-overs between the non-sister chromatids of a pair of homologous chromosomes; Fig. 2) may occur, resulting in recombination of genetic material at homologous loci. Each zygote undergoes two successive nuclear divisions, leading to four haploid nuclei. An ookinete further develops into an oocyst. Once the oocyst matures, thousands of sporozoites (the progeny of the cross) are formed and released into mosquito hemoceal. Sporozoites are harvested from the salivary glands and injected into a new murine host, where pre-erythrocytic and erythrocytic stage development takes place. Erythrocytic forms are cloned and classified with regard to the characters distinguishing the parental lines prior to genetic linkage mapping. Control infections of individual parental clones are performed in the same way as the production of a genetic cross.
Protocol
Aseptic techniques must be applied to all materials that will be administrated into animals to avoid inadvertent introduction of exogenous infectious agents into mice that can confound experimental outcomes.
1. Infection of Laboratory Mice with Blood-stage Malaria Parasites
At room temperature, thaw two vials containing the frozen blood stage malaria parasites to be crossed. In this example, two rodent malaria parasite strains used are Plasmodium yoelii yoelii and Plasmodium yoelii nigeriensis.
Fit a syringe with a 22-30 gauge (G) needle and draw up 400 μL of sterile, pharmaceutical grade PBS (pH 7.4).
Using the same syringe, draw up 100 μL of the thawed infected blood; invert the syringe up and down until the content is homogenously mixed. (Optional: a pipette may be used to transfer PBS and infected blood into a collection tube prior to transferring into a syringe for injection).
Inject 200 μL of parasite containing solution directly into the peritoneum of a mouse. When finished, discard the syringe in a sharps bin. (See also Supplementary Materials for maintenance of laboratory mice). In general, the standard size of the inoculum is between 100-500 μL, depending on the levels of parasite infections in the original frozen materials.
Inject the two parasite strains to be crossed into two mice each. Mice will become positive microscopically 3 to 5 days after the injections. When parasitemias reach between 1-5%, continue to the next step.
2. Mixed Clone Infection of Mice
Prior to mixed clone infection, collect tail blood from the two donor mice infected with the different parasite strains for microscopic examination by Giemsa stained thin blood smears (Fig 3), and for measurement of red blood cell density (see Supplementary Materials for detailed procedures).
Calculate the volume of a physiological saline (1.5% w/v trisodium citrate dihydrate, 0.85% w/v sodium chloride) and mouse blood required to produce an inoculum mixture, using the parasitemia and red blood cell density measurements obtained earlier.
Adjust the parasite solution to a final concentration of one million infected red blood cells per 100μl.
Combine the infected blood from the two donors in a 1:1 ratio to obtain a mixed-clone inoculum. When the two parental clones differ in growth rate, adjust the proportions of a faster-growing parental to a slower-growing parental clone to 1:2 or 1:5.
Inject 100 μL of the mixed sample into the peritoneum of each mouse to be infected. (see also step 3.9 to determine the number of mice to be injected)
Using this method, infect two mice each with the single parental strains. The single-clone infections will allow determination of successful transmission of the two parental strains.
3. Feeding Mosquitoes
Prepare three cages containing 300-500 adult (male and females) Anopheles stephensi mosquitoes each, age 5-7 days post emergence from pupae. The containers are covered with netting secure by a plastic or metal lid. Two cages are for feeding on parental clones; the third cage is for preparation of a genetic cross.
Starting four days after the mixed-clone infection, prepare Giemsa-stained thin blood tail smears from the infected mice.
Examine the smears under a microscope by scanning at least 50 microscopic fields at 1000x magnification and determine whether male and female gametocytes are present.
Male and female gametocytes are recognizable due to the presence of large vacuole and large granules (Fig 3). Male and female gametocytes have pink and blue cytoplasm, respectively. If gametocytes are present, follow Step 3.6.
If gametocytes are absent, keep monitoring daily until they appear. If gametocytes are present, continue the following step.
Inject 50 μL of anesthetic drug cocktail containing 33 mg/mL of ketamine and 3 mg/mL of xylazine into the peritoneum of a mouse to anesthetize each infected mouse (per 20 gram mouse body weight). Final dose of ketamine and xylazine is 82.5 and 7.5 mg/Kg, respectively.
Place the mice face down on the top of a cage containing adult Anopheles stephensi mosquitoes that have been starved of sugar supply for 24 hrs and allow the mosquitoes to feed for 30 minutes without interruption.
Maintain the mosquitoes in an insectary under standard conditions (temperature is maintained between 24-26°C; also see Supplementary Methods for maintenance of laboratory mosquitoes).
Use one infected mouse for every 50-100 female mosquitoes. Euthanize the mice immediately by cervical dislocation after the feeding while the mice are still under anesthesia.
4. Dissecting Mosquito Midguts and Counting Oocysts
Nine days after the feeding, the mosquitoes will be examined for oocysts in the insect's midgut. The presence of oocysts in the mosquitoes that feed on single-clone infected mice indicates that gametocytes of the two parental strains are infectious.
To dissect a mosquito midgut, use an aspirator connected to a small container to collect 5 to 10 infected mosquitoes from the cage.
Using CO2 gas, anesthetize the mosquitoes and transfer them into a glass Petri dish on ice. Separate and discard male mosquitoes. The antennae of males are noticeably bushier in comparison to the females.
Fill two syringes with PBS and fit them with 26 gauge half-inch (G½) needles. Deposit about 100 μL of PBS from a syringe onto a glass slide.
Transfer 5 to 10 anesthetized female mosquitoes onto the drop of PBS and place the slide on a dissecting microscope.
Using the same needles, remove each mosquito's wings and legs, then, holding the insect at the thorax and posterior abdomen, pull the body apart until the midgut, a white sac-like body, is released. Detach the midgut and discard the rest of the body.
Add more PBS on the midgut if the slides are dry. The midgut will degenerate if let dried. Place a cover slip over the midguts.
Examine the midguts under a light microscope at a 100x magnification. Count the numbers of oocysts in each midgut (Fig 3). Oocysts are recognizable as thick-wall circular structures that lie on the outer surface of the midguts.
When finished, dispose of syringes, needles, and slide in a sharps bin.
If oocysts are absent in the midguts, feed mosquitoes with infected mice with the higher number of gametocytes and give a longer feeding time. Temperature is critical for this step. Make sure the temperature is set at 24 to 26° C.
5. Collecting Sporozoites from Mosquitoes by Centrifugation
Prepare sporozoite collection tubes as follows: cut the lid of a clean 0.5-mL centrifuge tube and punch a hole (0.1-0.2 mm diameter) at the bottom of the tube using a flamed 19 G needle.
Fill 1/3 of the tube with glass wool. Insert the filter tube into a 1.5-mL collection tube (one tube is sufficient to harvest sporozoites from ~100 mosquitoes)
On day 17 post feeding, collect mosquitoes (Step 4) from the cages into a small container using an aspirator.
Use CO2 gas to anesthetize mosquitoes.
Separate the females from the male mosquitoes. Transfer the anesthetized female mosquitoes from the container to a glass Petri dish on ice.
Prepare two syringes with 26 G½ needles filled with PBS. Deposit about 100 μL of PBS from a syringe onto a glass slide.
Transfer the mosquitoes onto a microscope slide. Remove the mosquitoes' head, wings, legs, and abdomen under a dissecting microscope.
Using fine-tipped forceps transfer the thoraxes into 0.5 mL PBS in a 1.5-mL homogenizer (mortar and pestle). Store the thoraxes on ice (sporozoite remains infective for 2-3 h).
Homogenize the thoraxes on ice to release sporozoites from salivary glands (see Figure 3), and transfer the lysates (homogenates) into the tube filled with glass wool (Step 5.1-5.2) using a glass pipette.
Centrifuge for 10 to 20 sec at 1000 rpm at room temperature.
Collect the flow-through (the purified sporozoite suspension) using a syringe with a 22-30 G needle. (Optional: drop 10-50 μL of the flow-through on a microscopic slide, place a cover slip and visualize the live sporozoites under a standard light microscope, 400X magnification, see also Fig 3)
Inject i.p. 0.1-0.5 mL of the sporozoite suspension into mice (up to 200,000 parasites can be harvested from a single mosquito; see ref 12). In a successful case, the animal will become infected 72 hrs after an injection.
6. Cloning Progeny using Limited Dilution
Seventy-two hrs after the sporozoite injections, collect tail blood from the two donor mice infected with the different parasite strains for microscopic examination after Giemsa staining of thin blood smears and for measurement of red blood cell density (see Supplementary Materials for detailed procedures).
Pick a mouse displaying a 0.1 to 1% parasitemia and whose infected red blood cells contain mostly single parasites as a donor for the cloning procedure. Repeat step 6.1 next days if the mice are microscopically negative. If the animals are not infected within 14 days, repeat Step 2 to 5 and determine the presence of sporozoites in step 5.11).
Dilute the harvested tail blood in a cold 1:1 solution of calf serum and mammalian Ringer's solution (2.7 mM potassium chloride, 1.8 mM calcium chloride, 154 mM sodium chloride) so as to attain a concentration of 0.6 to 1 infected red blood cell per 100 μL, and store the inoculum on ice. In this manner, most of individual mouse should receive either one or zero parasites.
Intravenously inject 100 μL of diluted infected blood in each of 50 to 100 mice.
Five to seven days later, screen the mice for the presence of blood stage parasites by examining Giemsa-stained thin blood smears. In a successful experiment, 20-50% of the mice become infected and will show parasitemia of 0.1-5.0% at day 8 post infection.
7. Characterization of Progeny using Genetic Markers
Collect 20 to 40 μL of mouse tail blood in a 1.5-mL microcentrifuge tube that contains 0.2 mL of chilled physiological citrate saline solution, vortex briefly, and centrifuge for 3 min at 3000 rpm at room temperature to pellet the blood cells. Extract DNA immediately or keep the pellet at -80°C.
Prepare parasite DNA using any standard DNA extraction methods. For rapid isolation of DNA, use a High Pure PCR template preparation kit (Roche Applied Bioscience).
Recombinant progeny are identified after genotyping using microsatellite (MS) or single nucleotide polymorphism (SNP) on different chromosomes that can differentiate the two parental strains of the genetic cross. A simplified method using single fluorescent-labeled primers for MS genotyping of P. yoelii can be used (see ref 8,9).
8. Cryopreservation of Blood-stage Malaria Parasites
Collect 0.5-1 mL of infected blood by cardiac puncture from an anesthetized mouse with parasitemia of 5-20% in 5-10 mL chilled physiological saline solution.
Centrifuge for 5 min at 2000 rpm at room temperature. Discard supernatant.
Add drop-wise 2x volume of Glycerolyte 57 solution (Fenwal Laboratories) onto blood pellets and mix well.
Transfer the suspension to cryotubes (0.2-0.5 mL per tube).
Store the cryopreserved blood at -80 °C (up to 1 month) or in liquid nitrogen.
9. Representative Results:
In a successful experiment, 5-10% of the cloned lines will be independent recombinant progeny.
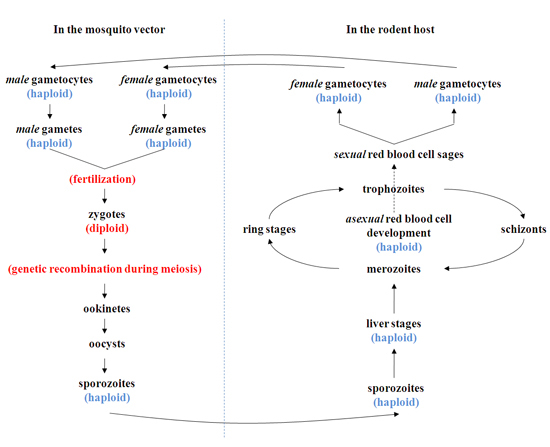 Figure 1. A schematic representation of the life cycle and genetic recombination events during a genetic cross. Genetic recombination events take place during the early phase of development in the mosquito. Following the fertilization of "haploid" male and female gametes of different genetic backgrounds in the midgut of the mosquito, "diploid" zygotes are formed. The zygote constitutes the only diploid stage of the parasite life cycle; meiosis follows within 12 hrs of fertilization, and all subsequent stages in the mosquito and mammals remain haploid. The resulting zygotes develop into ookinetes and oocysts. Growth and mitotic division of each oocyst produces thousands of sporozoites, which are released into mosquitoes' hemoceal. Those sporozoites that migrate to salivary glands are in position to be transmitted to a mammalian host, where development of the liver and red blood cell stages takes place. During the course of the red blood cell cycles, some parasites can differentiate into mature male and female gametocytes. The parasite life cycle is completed when these gametocytes are taken up by another mosquito, perpetuating transmission.
Figure 1. A schematic representation of the life cycle and genetic recombination events during a genetic cross. Genetic recombination events take place during the early phase of development in the mosquito. Following the fertilization of "haploid" male and female gametes of different genetic backgrounds in the midgut of the mosquito, "diploid" zygotes are formed. The zygote constitutes the only diploid stage of the parasite life cycle; meiosis follows within 12 hrs of fertilization, and all subsequent stages in the mosquito and mammals remain haploid. The resulting zygotes develop into ookinetes and oocysts. Growth and mitotic division of each oocyst produces thousands of sporozoites, which are released into mosquitoes' hemoceal. Those sporozoites that migrate to salivary glands are in position to be transmitted to a mammalian host, where development of the liver and red blood cell stages takes place. During the course of the red blood cell cycles, some parasites can differentiate into mature male and female gametocytes. The parasite life cycle is completed when these gametocytes are taken up by another mosquito, perpetuating transmission.
 Figure 2. Inheritance of nuclear genes in malaria parasites and production of recombinant progeny. Genetic recombination can be generated through chromosomal reassortment or by crossover events. Homozygous progeny produced by selfing between gametes of the same parental clone 1 or 2 (A and D), respectively. B) Heterozygous progeny produced by cross-fertilization between gametes of the two different parental clones, recombinant nuclei (blue ovals) being formed by chromosomal reassortment alone. C) Heterozygous progeny produced from cross-fertilization between gametes of the two different parent clones, recombinant nuclei (red ovals) being formed by crossovers between the non-sister chromatids of the homologous chromosomes.
Figure 2. Inheritance of nuclear genes in malaria parasites and production of recombinant progeny. Genetic recombination can be generated through chromosomal reassortment or by crossover events. Homozygous progeny produced by selfing between gametes of the same parental clone 1 or 2 (A and D), respectively. B) Heterozygous progeny produced by cross-fertilization between gametes of the two different parental clones, recombinant nuclei (blue ovals) being formed by chromosomal reassortment alone. C) Heterozygous progeny produced from cross-fertilization between gametes of the two different parent clones, recombinant nuclei (red ovals) being formed by crossovers between the non-sister chromatids of the homologous chromosomes.
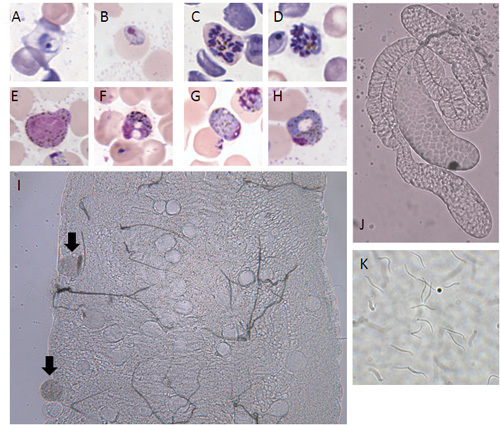 Figure 3. Morphology of the rodent malaria parasite Plasmodium yoelii. A) ring stage, B) trophozoite, C) schizont, D) merozoites, E) immature male gametocyte, F) mature male gametocyte, G) immature female gametocyte, H) mature female gametocyte, I) midgut with oocysts on day 10 post feeding (mature oocysts indicated by arrows; 200x magnification), J) mosquito salivary glands (100x magnification), K) sporozoites (400x magnification).
Figure 3. Morphology of the rodent malaria parasite Plasmodium yoelii. A) ring stage, B) trophozoite, C) schizont, D) merozoites, E) immature male gametocyte, F) mature male gametocyte, G) immature female gametocyte, H) mature female gametocyte, I) midgut with oocysts on day 10 post feeding (mature oocysts indicated by arrows; 200x magnification), J) mosquito salivary glands (100x magnification), K) sporozoites (400x magnification).
Discussion
We demonstrate the techniques for the production of a genetic cross in the rodent malaria Plasmodium yoelii, which is also applicable to production of genetic crosses in other rodent malarias. Infections of mice with single parental clones are usually performed to determine successful transmission of the parental parasites to ensure that the parents are competent in producing functional gametes before performing a cross.
Successful transmission through a mosquito is influenced by multiple factors including temperature of the insectary, species of rodent malaria parasites used, and the doses of blood-stage parasite (inoculum size). While transmission of P. berghei is normally achieved at 19-21°C 13, transmission of P. yoelii and P. chabaudi is routinely performed at 23-25°C 14. Ookinetes of P. berghei fail to develop into oocysts at temperatures lower than 16°C or higher than 24°C 15. In addition to temperature, inoculum size can influence multiplication rate of malaria parasites in the blood. Although injections of a large inoculum size (5 x 106 iRBC or more) will yield higher parasitemia at very early stages of infection, it does not necessarily lead to larger numbers of gametocytes or higher infectivity to mosquitoes. Gadsby and others (2009) 16 showed that gametocytes of P. chabaudi adami are present in the blood from day 3 to day 20 (peak at day 13) post infection of 1 x 106 iRBC; however, day 6 post infection is the only day upon which mosquitoes become infected. Also, administration of a large inoculum size of fast-growing and virulent parasite strains such as clones N67 (nigeriensis), 17XL, and YM of P. yoelii, clone ANKA of P. berghei, and clone DS of P. chabaudi adami often results in severe anemia and pathology in mice, which can cause a significant drop of mouse body temperature. As a result, mice become less infective to mosquitoes (unpublished observations). Nevertheless, transmission of rodent malaria parasites has been conducted with a standard inoculum size (106 iRBC). It has been found that P. berghei and P. yoelii are transmissible to mosquitoes from days 3 to 5 after blood stage-induced infection in mice17, whereas P. chabaudi chabaudi and P. chabaudi adami are only transmissible to mosquitoes on day 6 after blood stage-induced infections in mice16, 18.
Factors that influence production of recombinant progeny include the proportions of gametocytes of the two parental strains in mice. In theory, the maximum production of recombinant progeny occurs when gametocytes of both sexes of the parental clones are in equal number and self-fertilization and cross-fertilization occur at the same frequency. Under this condition, half of the resulting zygotes will be hybrids and the remainder will consist of equal proportions of the two parental clone lines. Production of recombinant progeny clones will be greatly reduced when the proportion of gametocytes is biased toward one parental clone, leading to disproportional self-fertilization. Moreover, parasites often have different growth rates and may have different capability in producing gametocytes16, 19. Therefore, it is necessary to optimize the proportions of parental parasites in the mixture to be injected into the mice for mosquito feeding.
The time to clone parasites from blood after mosquito feeding is also an important factor to consider. It is preferable to clone the progeny of the genetic cross when parasitemia is between 0.1-1.0% (before too many cycles of replication in the blood) to avoid cloning duplicated clones. Fast- or slow-growing parasites may come out in large numbers at certain periods of time, and cloning at the peaks of fast- or slow-growing parasites will end up with clones having the same phenotypes.
In conclusion, performing genetic crosses using rodent malaria parasites is relative easy and much cheaper than performing a genetic cross of the human malaria parasite P. falciparum, which requires infection of a nonhuman primate. Recombinant progeny of the genetic crosses are useful tools for construction of genetic linkage maps and for mapping of important malarial traits including parasite development, drug resistance, and virulence.
Disclosures
Because the authors are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Acknowledgments
We thank Drs Randy Elkins, Robin Kastenmayer, Ted Torrey, Dan Pare and Tovi Lehman for critical reading of manuscripts. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the 973 National Basic Research Program of China, #2007CB513103. We thank NIAID intramural editor Brenda Rae Marshall for assistance.
References
- Hayton K, Ranford-Cartwright LC, Walliker D. Sulfadoxine-pyrimethamine resistance in the rodent malaria parasite Plasmodium chabaudi. Antimicrob Agents Chemother. 2002;46:2482–2489. doi: 10.1128/AAC.46.8.2482-2489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo PV. Genetics of mefloquine resistance in the rodent malaria parasite Plasmodium chabaudi. Antimicrob Agents Chemother. 2003;47:709–718. doi: 10.1128/AAC.47.2.709-718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Cravo PV, Donleavy P, Carlton JM, Walliker D. Chloroquine resistance in Plasmodium chabaudi: are chloroquine-resistance transporter (crt) and multi-drug resistance (mdr1) orthologues involved? Mol Biochem Parasitol. 2004;133:27–35. doi: 10.1016/j.molbiopara.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Yuan J. Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. Nat Chem Biol. 2009;5:765–771. doi: 10.1038/nchembio.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Kirkman LA, Fujioka H, Wellems TE. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- Hayton K. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattaradilokrat S, Cheesman SJ, Carter R. Congenicity and genetic polymorphism in cloned lines derived from a single isolate of a rodent malaria parasite. Mol Biochem Parasitol. 2008;157:244–247. doi: 10.1016/j.molbiopara.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Li J. Hundreds of microsatellites for genotyping Plasmodium yoelii parasites. Mol Biochem Parasitol. 2009;166:153–158. doi: 10.1016/j.molbiopara.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Typing Plasmodium yoelii microsatellites using a simple and affordable fluorescent labeling method. Mol Biochem Parasitol. 2007;155:94–102. doi: 10.1016/j.molbiopara.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D, Carter R, Morgan S. Genetic recombination in malaria parasites. Nature. 1971;232:561–562. doi: 10.1038/232561a0. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Hartley RH. Identification of the meiotic division of malarial parasites. J Protozool. 1985;32:742–744. doi: 10.1111/j.1550-7408.1985.tb03113.x. [DOI] [PubMed] [Google Scholar]
- Ozaki LS, Gwadz RW, Godson GN. Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J Parasitol. 1984;70:831–833. [PubMed] [Google Scholar]
- Yoeli M, Most H, Bone G. Plasmodium berghei: cyclical transmission by experimentally infected Anopheles quadrimachulatus. Science. 1964;144:1580–1581. doi: 10.1126/science.144.3626.1580. [DOI] [PubMed] [Google Scholar]
- Landau I, Boulard Y. Life cycles and Morphology. London/New York/San Franciso: Academic Press; 1978. [Google Scholar]
- Vanderbergh JPY, M Effects of temperature on sporogonic development of Plasmodium berghei. J Parasitol. 1966;52:559–564. [PubMed] [Google Scholar]
- Gadsby N, Lawrence R, Carter R. A study on pathogenicity and mosquito transmission success in the rodent malaria parasite Plasmodium chabaudi adami. Int J Parasitol. 2009;39:347–354. doi: 10.1016/j.ijpara.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Pattaradilokrat S, Culleton RL, Cheesman SJ, Carter RGene. encoding erythrocyte binding ligand linked to blood stage multiplication rate phenotype in Plasmodium yoelii yoelii. Proc Natl Acad Sci U S A. 2009;106:7161–7166. doi: 10.1073/pnas.0811430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattaradilokrat S, Cheesman SJ, Carter R. Linkage group selection: towards identifying genes controlling strain specific protective immunity in malaria. PLoS One. 2007;2:e857–e857. doi: 10.1371/journal.pone.0000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon MJ, Read AF. Genetic Relationships between Parasite Virulence and Transmission in the Rodent Malaria Plasmodium chabaudi. Evolution. 1999;53:689–703. doi: 10.1111/j.1558-5646.1999.tb05364.x. [DOI] [PubMed] [Google Scholar]


