Abstract
Honeybees (Apis mellifera) are well known for their communication and orientation skills and for their impressive learning capability1,2. Because the survival of a honeybee colony depends on the exploitation of food sources, forager bees learn and memorize variable flower sites as well as their profitability. Forager bees can be easily trained in natural settings where they forage at a feeding site and learn the related signals such as odor or color. Appetitive associative learning can also be studied under controlled conditions in the laboratory by conditioning the proboscis extension response (PER) of individually harnessed honeybees3,4. This learning paradigm enables the study of the neuronal and molecular mechanisms that underlie learning and memory formation in a simple and highly reliable way5-12. A behavioral pharmacology approach is used to study molecular mechanisms. Drugs are injected systemically to interfere with the function of specific molecules during or after learning and memory formation13-16.
Here we demonstrate how to train harnessed honeybees in PER conditioning and how to apply drugs systemically by injection into the bee flight muscle.
Keywords: Neuroscience, Issue 47, Classical conditioning, behavioural pharmacology, insect, invertebrate, honeybee, learning, memory
Protocol
1. Catching Bees from the Hive
One day before the experiment starts, between 2 and 4 p.m., bees leaving the hive are caught. To do so, a UV light-permeable plexiglass pyramid (height = 30 cm, apex 3,5 x 3, 5 cm, base 18 x 18 cm), which is closable at the apex and the base, is held at a 20-30 cm distance in front of the hive entrance with the base open and the apex closed so that bees leaving the hive enter the base of the pyramid. The base is then closed and the captured bees are brought into the lab for further handling.
2. Transferring Bees from the Pyramid Into Glass Vials
In the lab, the pyramid is placed on its base. The walls of the pyramid are darkened (e.g. with a towel) but the apex is left uncovered. Because of their positive phototaxis, bees will leave the pyramid through the apex when opened. One by one, bees are transferred from the pyramid into glass vials by holding the vials over the open apex. One vial is used per bee. Therefore, the apex is closed when one bee enters the vial.
3. Harnessing Bees in Tubes
Bees are immobilized by cooling them in the glass vials on ice for 2.5-3.5 min. It is advisable to watch the bee and remove it from the ice as soon as it stops moving.
A single immobilized bee is harnessed in a small plastic tube with sticky tape, such that it is able to move its proboscis freely but not its head, thorax or legs. It is important that the neck is not compressed.
Every bee fixed in a plastic tube is put into a numbered borehole on a rack for better handling and identification. After it has been removed for conditioning or memory retrieval the tube is always returned to the exact same borehole.
4. Feeding Bees
On the first evening (4-6 p.m.), after being caught, bees are fed to satiation with sucrose solution (0,88 M, white refined household sugar dissolved in tap water). To feed a bee, its PER is elicited by touching its antennae with sucrose solution and the animal is allowed to consume one droplet (4 μL) of sucrose solution. Bees are fed one after another repeatedly until they no longer show a fast and reliable PER when their antennae are touched with sucrose solution.
On each subsequent evening (4-6 p.m.) of the experiment bees are fed one after another four times with 1 droplet of sucrose solution (4 μL, 0,88 M, white refined sugar solved in tap water). It is important that sucrose solution is not smeared on the bee's antennae or the proboscis during feeding and that the tubes are not contaminated with sucrose solution. Bees should not be fed at or near the conditioning site to exclude the possibility of associating the training context with the sucrose stimulus.
5. Keeping Harnessed Bees Overnight
Bees are kept overnight in a plastic bowl at room temperature. Tap water is filled into the bowl (approximately 0,5-1 cm high). Racks with the harnessed bees are placed in the bowl above the waterline using ELISA plates as a platform. Finally, the bowl is covered with cardboard.
6. Olfactory Conditioning
On the first morning (10 a.m.) of the experiment olfactory conditioning is carried out. The conditioned stimulus (CS) is an odor, the unconditioned stimulus (US) is sucrose solution.
4 μL of the odor (e.g. clove oil) are pipetted onto a round filter paper (1,3 cm diameter) which is then inserted into a 20 mL syringe. The odor is pipetted under a hood and filter tips are used to prevent contamination of the pipette.
The rack with harnessed bees is placed near the conditioning site 30 min before the conditioning procedure starts, but at a distance to the place in front of an exhaust pipe where single bees are conditioned.
Conditioning consists of three pairings of odor (the conditioned stimulus, CS) and sucrose solution (the unconditioned stimulus, US, 1,25 M) with an inter-trial interval (ITI) of 10 min. An acoustic signal delivered to the experimenter via an audio player ensures the precise timing of stimulus onset, stimulus offset, stimulus duration and placing. An acquisition trial starts with a 10 sec placement of the animal in front of an exhaust. Shortly before the end of the 10 sec the syringe containing the odor is placed 3 cm in front of the bee targeted at the bee's antennae. Subsequently, the odor is presented for 5 sec, by pushing 20 mL air through the syringe. After the first 3 sec the distal flagella of both antennae are touched with a sucrose solution-moistened toothpick and animals are allowed to lick the moistend toothpick for 4 sec. 13 sec after the CS stimulation ends, bees are taken out of the training context and placed back in the rack. Altogether one training trial lasts 28 sec. It is important that neither the bee's antennae nor the proboscis are covered with sucrose after conditioning. Therefore it has to be ensured that the toothpick is only moistened with sucrose solution and that no drops of sucrose solution form on the toothpick.
After conditioning the racks are placed back in the bowl.
The animals' behavior during the experiment, i.e. the occurence of the PER during placement and CS and US presentation, is monitored and noted down by the experimenter.
7. Memory Retention
Retention tests can be performed at any interval (min to days). The rack with bees is placed near the conditioning site 30 min before the memory test starts.
A memory test consists of 5 sec CS presentation without US presentation. The test starts with a 10 sec placement of the animal in front of the exhaust. Subsequently, the odor is presented for 5 sec as described above. The behavior of the animal, i.e. the occurence of the PER during placement and the CS presentation is noted down during the experiment.
At the end of the experiment the proboscis extention response is again elicited by touching the antennae with sucrose solution to ensure that the animal is still able to extend its proboscis.
8. Systemic Injection
The time point of systemic injection depends on the experimental design.
A small hole is made in the cuticula of the posterior part of the scutum next to the scutal fissure above the flight muscle17 by using a disposable hypodermic needle (21 G).
Using a glass capillary tube, 1 μL solution is injected through the hole in the cuticula into the flight muscle. Trained experimenters are able to inject one bee every 30 sec, allowing a precise timing of injection and conditioning.
9. Feeding Bees During the Experiment
On the evening (4-6 p.m.) after conditioning, bees are fed one after another four times with 1 droplet of sucrose solution (4 μL, 0,88 M, white refined sugar dissolved in tap water). It is important that sucrose solution is not smeared on the bee's antennae or the proboscis during feeding and that the tubes are not contaminated with sucrose solution. Bees should not be fed at or near the conditioning site to exclude the possibility of associating the training context with the sucrose stimulus.
10. Data Collection and Data Analysis
Occurrence of the proboscis extension response during the experiment is monitored. A bee is scored positive if it extends its proboscis between the onset of the CS and the presentation of the US (during training) or during the CS presentation (during test phase), crossing a virtual line between the open mandibles.
To be included in the analysis animals must fulfill two criteria: they must survive the entire experiment and they must show the unconditioned proboscis extension response (PER) to sucrose solution at the end of the experiment. The percentage of bees showing the PER during acquisition and retention tests is plotted for each CS presentation.
11. Representative Results:
Two experiments are presented here.
In the first experiment we look at the impact of systemic injection of phosphate-buffered saline (PBS; in mM: 137 NaCl, 2,7 KCl, 10,1 Na2HPO4, 1,8 KH2PO4, pH 7,2) on learning and long-term memory formation. Three groups of bees were trained with three CS-US pairings with an inter-trial interval of 10 min: a non-treated group, a group that was injected with 1 μL PBS 30 min before training, and a sham-injected group treated the same way as the PBS-injected group but without injecting PBS. 24 h after training memory was tested with one CS presentation in all three groups. The percentage of animals showing the conditioned response during conditioning increases significantly across the three training trials (Fig.1, ANOVA for repeated measurement for the factor time F2,378=340,456, p < 0,05). No difference in PER could be observed between the three groups during conditioning (ANOVA for repeated measurement for the factor groups F2, 189 = 1,299, p > 0,05). This holds true for a 24 h retention test (F2, 187 = 0,752, p>0,05) when comparing non-treated animals with PBS-injected and sham-injected animals.
In the second experiment we demonstrate the inhibition of memory retention three and four days after training when the protein synthesis inhibitor anisomycin (10 mM) was systemically injected 30 min after three CS-US pairings. Comparison of an anisomycin-injected group with an PBS-injected group reveals a significant difference between groups during the retention test (Fig.2, ANOVA for repeated measurement for the factor group F1, 94=8,86, p < 0,05). A major difference can be observed at the memory test on day 3 and day 4. This resembles the results of Wüstenberg et al.18, who also conditioned bees with three CS-US pairings, albeit with a different protocol.
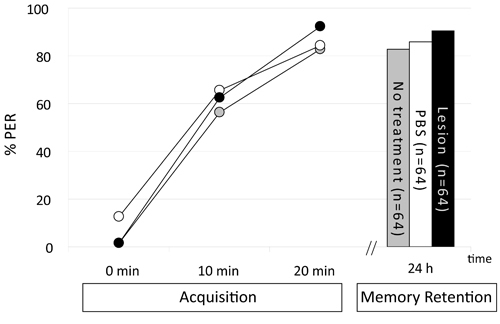 Figure 1. Cuticula lesion or injection of PBS does not alter aquisition and memory retention. Animals were trained with three CS-US pairings (acquisition). After 24 h, memory was tested (Memory Retention). Thirty minutes prior to training, a hole was made in the cuticula of two groups (lesion, PBS) and one of these groups was injected with 1 μL PBS (PBS). A third group was left intact (no treatment). Presented is the percentage of animals for each group responding to odor presentation with a PER. An ANOVA does not reveal any significant differences between groups in the acquisition phase (F2, 189 = 1.299, p > 0.05) or in memory retention (F2, 187 = 0.752, p>0.05).
Figure 1. Cuticula lesion or injection of PBS does not alter aquisition and memory retention. Animals were trained with three CS-US pairings (acquisition). After 24 h, memory was tested (Memory Retention). Thirty minutes prior to training, a hole was made in the cuticula of two groups (lesion, PBS) and one of these groups was injected with 1 μL PBS (PBS). A third group was left intact (no treatment). Presented is the percentage of animals for each group responding to odor presentation with a PER. An ANOVA does not reveal any significant differences between groups in the acquisition phase (F2, 189 = 1.299, p > 0.05) or in memory retention (F2, 187 = 0.752, p>0.05).
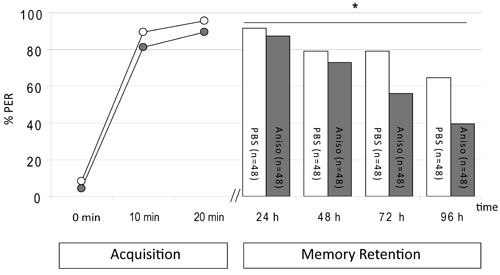 Figure 2. Injection of anisomycin 40 min after the last CS-US pairing reduces memory retention three days and four days after training. Animals were trained with three CS-US pairings (acquisition) and injected with 1 μL 10 mM anisomycin (Aniso) or 1 μL PBS 1 h after the first training trial. Memory was tested (Memory Retention) 24 h, 48 h, 72 h and 96 h later. Presented is the percentage of animals per group responding to odor presentation with a PER. The injection of anisomycin leads to a significant reduction of memory retention 72 h and 96 h after training (F1, 94=8.86, p < 0.05) as revealed by an ANOVA.
Figure 2. Injection of anisomycin 40 min after the last CS-US pairing reduces memory retention three days and four days after training. Animals were trained with three CS-US pairings (acquisition) and injected with 1 μL 10 mM anisomycin (Aniso) or 1 μL PBS 1 h after the first training trial. Memory was tested (Memory Retention) 24 h, 48 h, 72 h and 96 h later. Presented is the percentage of animals per group responding to odor presentation with a PER. The injection of anisomycin leads to a significant reduction of memory retention 72 h and 96 h after training (F1, 94=8.86, p < 0.05) as revealed by an ANOVA.
Discussion
Catching bees: To collect experienced foraging bees, bees are caught either in the morning or in the afternoon, avoiding the time when young bees are on orientation flights19. Foragers collect either pollen or nectar. Differences between pollen and nectar foragers have been observed according to their sugar responsiveness and their learning abilities20. In order to catch only nectar foragers, an artificial food source filled with a sucrose solution can be installed near the hive and animals can be collected there. Pollen foragers returning to the hive can be identified by the pollen baskets on their hind legs20.
Fixing and handling: Extended cooling of bees on ice should be avoided because it might affect their ability to learn and to survive until the end of the experiment. Tarsi have to be fixed carefully within the tube to avoid accidental stimulation with sucrose solution. This is important, because bees have been shown to learn by tarsal stimulation with succrose solution3,21.
Olfactory conditioning: The number of conditioning trials, the inter-trial interval, and the test time point can be varied depending on the focus of the study3,22.
Feeding: The satiation level of bees is known to affect their learning performance and memory retention3,23. This has to be taken into account when planning the experiments.
Injection: Air sacs are located in the honeybee thorax17. These vital parts of the respiratory system are necessary for survival. Thus care should be taken not to damage the air sacs during injection. The release of air bubbles indicates damage to the air sacs.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a grant from the German Federal Ministry of Education and Research (BMBF) within the Bernstein Focus: Neuronal Basis of Learning (BFNL) to D.E. We thank Randolf Menzel for valuable comments on an earlier version of the manuscript.
References
- Menzel R, Giurfa M. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci. 2001;5:62–71. doi: 10.1016/s1364-6613(00)01601-6. [DOI] [PubMed] [Google Scholar]
- Menzel R, Leboulle G, Eisenhardt D. Small brains, bright minds. Cell. 2006;124:237–239. doi: 10.1016/j.cell.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Menzel R. In: Neurobiology of comparative cognition. Kesner RP, Olton DS, editors. Hillsday, New Jersey, Hove, London: Lawrence Erlbaum Associates Publishers; 1990. [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schafer S. Classical-Conditioning of Proboscis Extension in Honeybees (Apis-Mellifera) J. Comp. Psyc. 1983;97:107–119. [PubMed] [Google Scholar]
- Menzel R, Muller U. Learning and memory in honeybees: from behavior to neural substrates. Annu. Rev. Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. Journal of Comparative Physiology A-Neuroethology Sensory Neural and Behavioral Physiology. 1999;185:323–340. [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- Eisenhardt D. Learning and memory formation in the honeybee (Apis mellifera) and its dependency on the cAMP-protein kinase A pathway. Animal Biology. 2006;56:259–278. [Google Scholar]
- Hourcade B, Muenz TS, Sandoz JC, Rossler W, Devaud JM. Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain. J. Neurosci. 2010;30:6461–6465. doi: 10.1523/JNEUROSCI.0841-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker M, Finke R, Schaupp F, Grun S, Menzel R. Neural correlates of odor learning in the honeybee antennal lobe. Eur. J. Neurosci. 2010;31:119–133. doi: 10.1111/j.1460-9568.2009.07046.x. [DOI] [PubMed] [Google Scholar]
- Szyszka P, Galkin A, Menzel R. Associative and non-associative plasticity in kenyon cells of the honeybee mushroom body. Front Syst. Neurosci. 2008;2:3–3. doi: 10.3389/neuro.06.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada R, Rybak J, Manz G, Menzel R. Learning-related plasticity in PE1 and other mushroom body-extrinsic neurons in the honeybee brain. J. Neurosci. 2007;27:11736–11747. doi: 10.1523/JNEUROSCI.2216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Bundrock G, Muller U. Focal and temporal release of glutamate in the mushroom bodies improves olfactory memory in Apis mellifera. J. Neurosci. 2005;25:11614–11618. doi: 10.1523/JNEUROSCI.3180-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollhoff N, Menzel R, Eisenhardt D. Spontaneous recovery from extinction depends on the reconsolidation of the acquisition memory in an appetitive learning paradigm in the honeybee (Apis mellifera. J. Neurosci. 2005;25:4485–4492. doi: 10.1523/JNEUROSCI.0117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollhoff N, Eisenhardt D. Consolidation of an extinction memory depends on the unconditioned stimulus magnitude previously experienced during training. J. Neurosci. 2009;29:9644–9650. doi: 10.1523/JNEUROSCI.0495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass RE. Anatomy of the honeybee. New York: Comstock Publishing Associates; 1956. [Google Scholar]
- Wustenberg D, Gerber B, Menzel R. Short communication: long- but not medium-term retention of olfactory memories in honeybees is impaired by actinomycin D and anisomycin. Eur. J. Neurosci. 1998;10:2742–2745. doi: 10.1046/j.1460-9568.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- Vollbehr J. Zur Orientierung junger Honigbienen bei ihrem 1. Orientierungsflug. Zool. Jb. Physiol. 1975;79:33–69. [Google Scholar]
- Scheiner R, Erber J, Page RE. Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.) J. Comp Physiol [A] 1999;185:1–10. doi: 10.1007/s003590050360. [DOI] [PubMed] [Google Scholar]
- Sanchez Brito, G M. Behavioral studies on tarsal gustation in honeybees: sucrose responsiveness and sucrose-mediated olfactory conditioning. J. Comp Physiol A Neuroethol. Sens. Neural Behav. Physiol. 2008;194:861–869. doi: 10.1007/s00359-008-0357-8. [DOI] [PubMed] [Google Scholar]
- Menzel R, Manz G, Menzel R, Greggers U. Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn. Mem. 2001. pp. 198–208. [DOI] [PMC free article] [PubMed]
- Friedrich A, Thomas U, Muller U. Learning at different satiation levels reveals parallel functions for the cAMP-protein kinase A cascade in formation of long-term memory. J. Neurosci. 2004;24:4460–4468. doi: 10.1523/JNEUROSCI.0669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


