Abstract
Microscopic computed tomography (microCT) offers high-resolution volumetric imaging of the anatomy of living small animals. However, the contrast between different soft tissues and body fluids is inherently poor in micro-CT images 1. Under these circumstances, visualization of blood vessels becomes a nearly impossible task. To overcome this and to improve the visualization of blood vessels exogenous contrast agents can be used. Herein, we present a methodology for visualizing the vascular network in a rodent model. By using a long-acting aqueous colloidal polydisperse iodinated blood-pool contrast agent, eXIA 160XL, we optimized image acquisition parameters and volume-rendering techniques for finding blood vessels in live animals. Our findings suggest that, to achieve a superior contrast between bone and soft tissue from vessel, multiple-frames (at least 5-8/ frames per view), and 360-720 views (for a full 360° rotation) acquisitions were mandatory. We have also demonstrated the use of a two-dimensional transfer function (where voxel color and opacity was assigned in proportion to CT value and gradient magnitude), in visualizing the anatomy and highlighting the structure of interest, the blood vessel network. This promising work lays a foundation for the qualitative and quantitative assessment of anti-angiogenesis preclinical studies using transgenic or xenograft tumor-bearing mice.
Protocol
1. Animal Injections
Prior to intravenous tail vein injection, the tail skin should be cleaned with isopropyl alcohol scrubs for sterilization purposes. Mice should then be injected with 0.2 mL/20 g of a 20-mg iodine/mL iodinated bloodpool contrast agent (eXIA 160XL; Binito Biomedical, Inc. Ottawa, ON, Canada) via the distal tail vein using low dead space ½ CC U-100 28G½ Insulin Syringes (Product #329461, Becton Dickinson and company, NJ, USA).
2. Animal Preparation
After 10 minutes of injection, the animals should be prepared for microCT imaging. First, the animal should be anesthetized in a box using 2% isoflurane in 100% oxygen at rate of 2.5 liters per minute. An ocular lubricating ointment should be applied to prevent desiccation of the corneas during anesthesia. Body temperature should be maintained at 37°C by a heat pads.
3. MicroCT Imaging
Mice then should be scanned on a microCT unit capable to perform live animal scans. To minimize movement artifacts and stability, mice can be placed in a commercially available multi-modality chamber (Numira Biosciences, Salt Lake City, UT) with provision for air and exhaust. To avoid a hypothermic episode on the animal during microCT scans, Gel Pads (Hot Cold Therapy Brace, CVS Pharmacy Woonsocket, RI), Toe Warmer (Heat Factory, Vista, CA) or Thermipaq Clay Pads (Thermionics, Springfield, IL) can be used. Place these heating pads under the foam bed to maintain the mouse at 37°C while avoiding direct contact with the animal. Vital signs of animal such as body temperature, respiration and heart rate should be monitored using the Small Animal Monitoring and Gating Systems such as SA Instruments (Stony Brook, NY).
The following imaging protocol can be used as a guideline to help determine the appropriate scan parameters for any microCT units. The animals used in this experiment were scanned at 93 μm resolution on a volumetric CT scanner GE eXplore Locus (GE Healthcare, London, Ontario). This volumetric scanner uses a 3500 x 1750 CCD detector for Feldkamp cone-beam reconstruction. The platform independent parameters of current, voltage and exposure time were kept constant at 450 μA, 80 kVP and 100 ms, respectively. The standard parameters of exposure time, frames per view and number of views can be varied and could be 100 ms, 5-8, and 360-720, respectively with total scan time of approximately 20 minutes. Images were reconstructed with the manufacturer's proprietary EVSBeam software.
4. Image Rendering
The reconstructed microCT data can be used for advanced isosurface, one-dimensional, or two-dimensional transfer function rendering Images using Seg3D image processing software (Seg3D, http://www.sci.utah.edu/cibc/software).
5. Representative Results
Please see the attached figure of a wildtype mouse scanned with eXIA160XL with the settings mentioned in methods section.
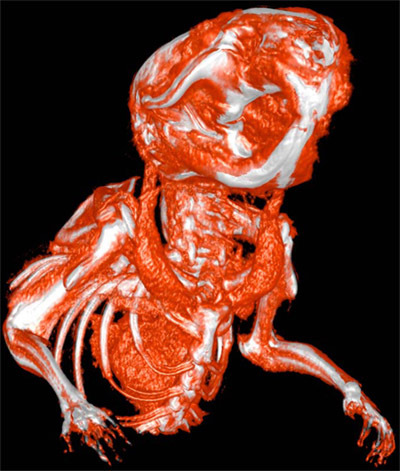 Figure 1. Two-Dimensional Transfer Function (2DTF) rendering image of a wildtype mouse created using Seg3D image processing software (Seg3D, http://www.sci.utah.edu/cibc/software). The animal was injected with eXIA160XL and 10 minutes later microCT scanned at 93 μm isometric resolution with the settings mentioned in methods section.
Figure 1. Two-Dimensional Transfer Function (2DTF) rendering image of a wildtype mouse created using Seg3D image processing software (Seg3D, http://www.sci.utah.edu/cibc/software). The animal was injected with eXIA160XL and 10 minutes later microCT scanned at 93 μm isometric resolution with the settings mentioned in methods section.
Video 1. Two-Dimensional Transfer Function (2DTF) rendering based 360° rotation movie of a wildtype mouse created using Imagevis3D image processing software (Imagevis3D, http://www.sci.utah.edu/cibc/software). The animal was injected with eXIA160XL and 10 minutes later microCT scanned at 93 μm isometric resolution with the settings mentioned in methods section.Click here to watch video
Discussion
The foremost goal of this technique of contrast enhanced vessel imaging using microCT is to provide optimal method for generating high quality datasets for analysis of vascular networks in live mice. For microCT imaging, better signal-to-noise ratio of soft tissue is achieved by longer scan times with increased numbers of views and number of frames per view 2.
Vessel identification is critically dependent upon accurate soft tissue identification and differentiation from bone. eXIA 160XL is iodine-based which attribute the property of being highly radio-opaque and a density which is different form soft tissue and bone. This makes it convenient to focus on the blood vessels in a microCT data.
A 2-dimensional transfer function even further improves the distinction between vessel contrast and bone during rendering. In case of two-dimensional transfer function image renderings, colors and opacities are assigned to the ray sample based on the transfer function, which in turn relies on CT value and gradient magnitude 2. Transfer functions can be manually adjusted based on guidance provided by CT value histograms to highlight areas/objects of interest.
This technique could very well be used for qualitative and quantitative assessment of anti-angiogenesis preclinical studies using transgenic or xenograft mice. In future, advances in CT technology can easily make vessel imaging in transgenic or xenograft mouse models easier, faster, and more accurate over time. Better resolutions, will also allow visualization of fine structures like capillaries.
Disclosures
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Health Science Center at San Antonio.
Acknowledgments
This work was supported in part by NCI award 5R01CA133229-03.
References
- Holdsworth DW, Thornton MM. Micro-CT in small animal and specimen imaging. Trends in Biotechnology. 2002;20:417–424. [Google Scholar]
- Kindlmann GL, Weinstein DM, Jones GM, Johnson CR, Capecchi MR, Keller C. Practical Vessel Imaging by Computed Tomography in Live Transgenic Mouse Models for Human Tumors. Molecular Imaging. 2005;4:417–424. doi: 10.2310/7290.2005.05166. [DOI] [PubMed] [Google Scholar]


