Abstract
Background
To determine the impact at a single center of the United Network for Organ Sharing-mandated sharing program for human leukocyte antigen (HLA)-A/-B/-DR 0-mismatched (0MM) kidneys, we analyzed the results of 264 kidney transplants from 0MM distant donors between 1993 and 2006, with a follow-up through January 31, 2007. We compared these results with that of concurrent kidneys transplanted from HLA more than 0MM local donors and with shipped more than 0MM kidneys from “payback” donors.
Results
Despite a significantly longer preservation time, we found an 11% increase in 8-year graft survival (63% vs. 52% P<0.003) of 0MM shipped versus locally procured, >0MM donor kidneys. Graft survival of 0MM shipped kidneys at 8 years was significantly better in nonsensitized (<20% panel reactive antibodies; 68% vs. 55% P<0.0005) but not in sensitized (≥20% panel reactive antibodies) recipients, who showed an early (2 years) but short-lived benefit. The benefit of receiving a HLA-A, -B, and -DR 0MM shipped kidney remained strong and statistically significant (0.71 relative risk of graft loss vs. local; P<0.02) when adjusted for 22 potentially confounding variables in a Cox proportional hazards analysis.
Conclusions
The recent change in United Network for Organ Sharing policy restricting mandated sharing of 0MM kidneys to sensitized and pediatric recipients will give greater flexibility to the local organ procurement organization in allocating organs. However, the survival benefit to nonsensitized patients is real and long lasting and will be lost.
Keywords: HLA 0 mismatch, Kidney transplantation, Graft survival
In the first 20 years of clinical kidney transplantation (1962–1982), immunosuppression and organ preservation options were limited, and the field was dominated by transplants from living-related donors. During this time, it was discovered that similar to animal models of kidney transplantation, major histocompatibility complex human leukocyte antigen (HLA) haplotype matching mattered. Transplants between HLA-identical siblings, mismatched for minor H (nonmajor histocompatibility complex) antigens only, fared much better than a one haplotype or full (two haplotypes) HLA-mismatched donor-recipient combination and were more likely to survive even after cessation of all immunosuppression (1). The origin of the HLA-0 mismatched (HLA-0MM) national kidney sharing program in the United States dates from the mid- 1980s, a time of a great expansion of kidney transplants from deceased donors. In rare cases, the accident victim or other brain-injured donor happened to be matched with the recipient for both alleles of HLA-A, -B, and -DR based on serologic typing available at that time. Studies of such transplants showed outcomes superior to that of any other kind of kidney transplant from a deceased donor (2, 3). Nonetheless, at the time of the introduction of the United Network for Organ Sharing (UNOS) program of mandated sharing between transplant centers of six HLA antigen-matched kidneys in 1987, there was a concern that the increase in preservation time for kidneys being transported out of one organ procurement organization (OPO) and into another would negate the advantages gained by having an HLA-matched kidney donor (4). In addition to the uncertainties about cold storage, there were also uncertainties about the accuracy of typing procedures used to establish HLA match between two unrelated individuals. For example, before the introduction of DNA-based typing in 1992 (5), serologic methods used for typing HLA class II alleles HLA-DQ and -DR were only 75% reliable, as was the accuracy of assigning homozygosity for any HLA-A or -B allele based on the appearance of a “blank” in a class I HLA typing (6). Despite these technical drawbacks, however, the mandated sharing of six HLA antigen-“matched” kidneys produced encouraging results (7, 8). In 1995, UNOS mandated the sharing of all HLA-0MM kidneys, a decision which allowed a “blank” (e.g., A2-, B7-, and DR4-) to be considered as evidence of homozygosity for the purposes of the sharing program. This new confidence was based on significant advances in accuracy of HLA typing made possible by the polymerase chain reaction technique with sequence-specific primer (5) and other DNA-based approaches (9).
Between 1995 and 1998, the UNOS registry showed 36% of all kidneys transplanted in the United States being shared between OPOs, with 15.6% being shared as HLA-0MM kidneys and 20.4% as paybacks (10). Stegall et al. (10) compared the survival of mandatorily shared, 0MM cadaver kidneys during this period with that of payback kidney transplants (>0MM). They found a consistent 5% to 7% increase in graft survival beginning at 12 months and extending to 4 years posttransplant. This difference in graft survival was largely due to a 10% lower incidence of transplant rejection, particularly in patients with low (0%–9%) or intermediate (10%–79%) panel reactive antibodies (PRA). These results showing a graft survival benefit of 0MM shipped kidneys were confirmed in a separate study of paired donor kidney transplants (11). It should be noted that the most highly sensitized patients, those with antibodies reactive to 80% or more of a random panel of blood donors, did not enjoy a significant graft survival benefit from the 0MM sharing program (10). Nonetheless, in June 2008, the UNOS board, citing their desire to “increase efficiency in kidney matching by focusing mandatory regional and national sharing of zero-antigen mismatched kidneys for adult candidates who are harder to match because of their highly sensitized immune response,” approved a proposal to limit mandatory sharing of 0MM kidneys to pediatric patients and those with a PRA more than or equal to 20% (12).
The purpose of this study was to evaluate the experience of a single center with the 0MM shipped kidney program in the era of molecular HLA typing. Single-center studies of graft survival have been of great value, because many variables such as organ preservation method, induction and maintenance immunosuppression regimen, and recipient demographics are similar for all transplants. Because the national program of mandated 0MM kidney sharing has now been terminated, except for pediatric and highly sensitized patients, it is particularly opportune at this juncture to determine what its impact has been at individual transplant centers.
Materials and Methods
All cadaveric kidney transplants performed at University of Wisconsin, Madison (UW-Madison) between April 22, 1993, and December 31, 2006 were considered; the last follow-up date was January 31, 2007. Transplanted organs were classified with respect to origin (local vs. shipped in) and mismatch score (0MM vs. >0MM). Wilcoxon rank sum tests were used to compare cold ischemia times. Kaplan-Meier estimates were used to estimate survival within groups and the log-rank test to compare them. Cox proportional hazards models were used to model survival as a function of the following risk factors: organ type (the four combinations resulting from considering origin and mismatch score), donor age, donation after brain death (DBD) versus donation after cardiac death (DCD), transplant number, recipient age at transplant, diabetes, peak PRA, and hours of cold ischemia time. Proportional hazards were tested with the Grambsch-Therneau test. P less than 0.05 (two sides) was used as the criterion for statistical significance. All graphics and computations were obtained in R version 2.5.1 (13). Data matching was done with the pairmatch function in the R package optmatch using the method of Hansen and Klopfer (14).
Results
Table 1 shows a detailed breakdown by transplant type of 1739 kidney transplants from deceased donors performed at the UW-Madison from 1993 to 2007. HLA-A, -B, and -DR 0MM kidney transplants comprised 16% of the total, mainly due to 264 shipped-in kidneys; only 29 (1.8% of total) HLA 0MM kidneys were procured locally. In addition to 1310 locally procured kidneys with a more than 0MM, there were also 181 more than 0MM kidneys accepted as paybacks by our center for a total of 1491 such kidneys transplanted. Donor, but not recipient, age was significantly lower in shipped versus local kidneys, reflecting the practice of the UW-Madison OPO to accept organs from older donors if locally procured and a reluctance to accept older shipped-in kidneys. In addition, the number of transplants, duration of end-stage renal disease at time of transplant, and incidence of HLA sensitization (peak PRA) were higher in the recipients of 0MM shipped kidneys (Table 1). Not surprisingly, locally procured kidneys were cold stored for an average of 10 hr less than shipped-in kidneys (3–6.0 hr vs. 12–14.0 hr; P<0.001); however, the differences in total preservation time (cold storage plus pump time), while still highly significant, were considerably less (21–22 hr vs. 23–26 hr; P<0.001), indicating a limitation on the rapidity of transplanting both locally procured and shipped-in kidneys. As expected, when an HLA 0MM transplant was performed, both recipient and donor tended to be white (>94%); this was not equally true for other transplants, as reflected in the significant (P<0.001) difference among all four groups in ethnic composition. Diabetics were also highly represented among recipients of 0MM when compared with more than 0MM kidneys. Finally, the use of calcineurin inhibitors (CNIs) cyclosporine versus tacrolimus, and the proportion of patients on CNI-free immunosuppression, tended to differ significantly (P<0.001) among the groups.
TABLE 1. UW-Madison renal transplants from deceased donors, 1993–2007.
| Transplant type | |||||
|---|---|---|---|---|---|
| Local >0MM | Local 0MM | Shipped >0MM | Shipped 0MM | P | |
| n | 1310 | 29 | 181 | 264 | — |
| Recipient age (yr) | 48.7±13.4 | 51.4±14.3 | 49.8±13.8 | 48.0±12.2 | 0.3 |
| Donor age (yr) | 42.5±17.5 | 36.5±16.9 | 38.75±18.7 | 34.7±14.9 | <0.001 |
| Tx No. | 1.25±0.5 | 1.28±0.53 | 1.15±0.44 | 1.48±0.77 | <0.001 |
| Recipient BMI (kg/m2) | 26.6±6.6 + 5.3 | 32.74±18.7 | 27.2±5.9 | 26.8±5.4 | 0.06 |
| Donor BMI (kg/m2) | 28.5±7.9 | 27.74±5.65 | 27.93±7.2 | 27.1±6.8 | 0.62 |
| ESRD (mo) | 14.8±11.6 | 19.5±12.3 | 13.4±10.8 | 17.3 ±11.9 | <0.001 |
| Peak PRA | 12.75±23.3 | 17.6±29.6 | 7.6±17.4 | 25.4±34.0 | <0.001 |
| Cold storage time (hr) | 2.98±6.4 | 5.6±9.1 | 14.4±8.4 | 12.2±7.4 | <0.001 |
| Total preservation time (hr) | 22.5±6.9 | 21.4±5.9 | 25.1 ±5.9 | 23.2±5.9 | <0.001 |
| Race (% white) | |||||
| Recipients | 79.8 | 93.1 | 77.3 | 94.3 | <0.001 |
| Donors | 94.7 | 96.6 | 80.4 | 94.7 | <0.001 |
| Gender (% male) | |||||
| Recipients | 58.7 | 62.1 | 61.3 | 61.7 | 0.75 |
| Donors | 60.3 | 72.4 | 54.7 | 59.5 | 0.27 |
| High PRA (% ≥20) | 17.6 | 27.6 | 8.8 | 35.2 | <0.001 |
| Diabetes (%) | 24.7 | 44.8 | 24.9 | 32.6 | 0.006 |
| Donor type (% SCD) | 45.9 | 51.7 | 53.6 | 52.7 | 0.07 |
| Induction immunosuppression | |||||
| Steroids (%) | 99.9 | 100 | 100 | 99.6 | 0.46 |
| Calcineurin inhibitors (%) | <0.001 | ||||
| Cyclosporine | 55.9 | 55.2 | 63.0 | 56.4 | |
| Tacrolimus | 25.0 | 31.0 | 21.0 | 36.0 | |
| None | 19.1 | 13.8 | 16.0 | 7.6 | |
| Antiproliferatives (%) | 0.6 | ||||
| Azathioprine | 14.7 | 6.9 | 17.7 | 11.7 | |
| Mycophenolate | 82.7 | 89.7 | 81.8 | 85.6 | |
UW-Madison, University of Wisconsin, Madison; 0MM, 0-mismatch; Tx, transplant; BMI, body mass index; ESRD, end-stage renal disease; PRA, panel reactive antibodies.
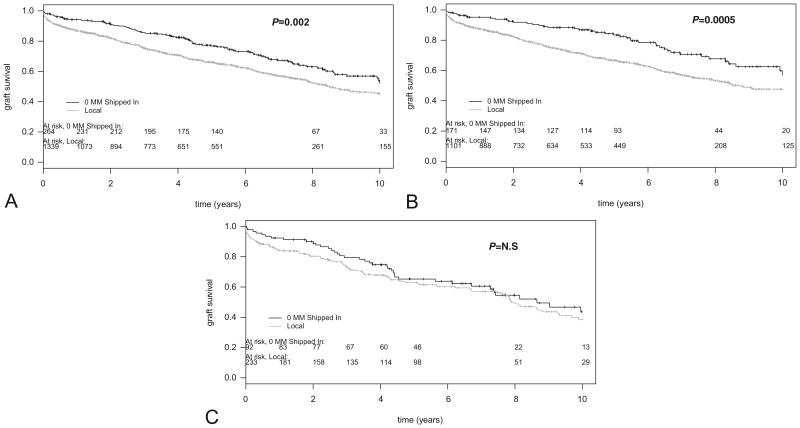
Figure 1 compares the graft survival of local more than 0MM (n= 1310) versus 0MM shipped-in kidneys (n=264) at the UW-Madison between 1993 and 2007. Figure 1(A) shows a significant (P=0.002) graft survival advantage of 0MM shipped-in kidneys over locally procured, HLA-mismatched kidneys. The benefit of receiving a 0MM shipped kidney was most pronounced in patients with a low (<20%) PRA level at the time of transplantation, who enjoyed a 13% graft survival benefit at 8 years over recipients of a locally procured HLA-mismatched kidney (P<0.0004; Fig. 1B). In contrast, despite an early benefit, overall graft survival of 0MM shipped-in kidneys in HLA-sensitized (≥20% PRA) patients was not significantly different from the survival of a locally procured, HLA-mismatched kidney (P>0.5; Fig. 1C).
Figure 1.
Graft survival of 0-mismatched (0MM) shipped versus a local more than 0MM kidneys at the University of Wisconsin, Madison, from 1993 to 2006. (A) All patients. Patients received a 0MM shipped (n=264; black line) or a local >0MM (n= 1339; gray line) kidney transplant from a deceased donor. Individual graft survival time points are indicated by vertical marks on survival curves. Log-rank P=0.002; number of patients at risk for rejection over time are indicated on this and subsequent figures. (B) Nonsensitized patients only. Patients with less than 20% panel reactive antibodies (PRA) at the time of transplant received a 0MM shipped (n=171; black line) or a local >0MM (n= 1101; gray line) kidney transplant from a deceased donor. Log-rank P value=0.0005. (C) Sensitized patients only. Patients (≥20% PRA) received a 0MM shipped (n=92; black line) or a local more than 0MM (n= 233; gray line) kidney transplant from a deceased donor. Log-rank P=0.583 (not significant).
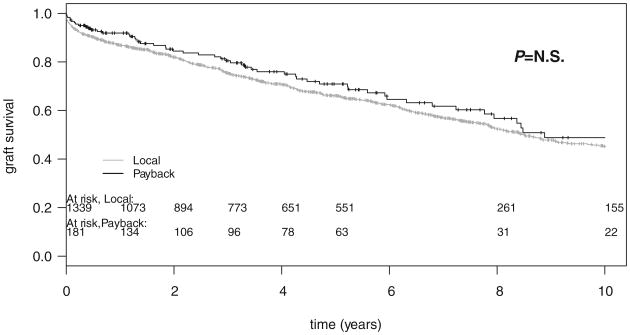
The potential damage to the shipped kidney because of prolonged cold storage and increased total preservation time was also investigated. Graft survival of “payback” kidneys was similar to that of locally procured kidneys, indicating no significant disadvantage incurred by the slightly longer preservation time of the former (Fig. 2).
Figure 2.
Graft survival of local versus shipped in human leukocyte antigen-mismatched kidney transplants at the University of Wisconsin, Madison, from 1993 to 2006—all patients. Patients received a >0-mismatched (>0MM) shipped “payback” (n=92; black line) or a local >0MM (n=233; gray line) kidney transplant from a deceased donor. Log-rank P=0.235 (not significant).
One difficulty in interpreting univariate analysis of kidney transplant survival data is the variability in donor characteristics over this time frame, for example, the increased use of donation after cardiac death (DCD) and donor age differences in local versus shipped kidney transplants. To adjust for these variables, we took two separate approaches. First, we used a Cox proportional hazards model to determine the relative impact of 0MM shipped-in kidney status on graft survival by adjusting for the potential confounding variables listed in Table 1 plus an additional variable, “transplant year,” to adjust for changes in transplant protocols over time. Table 2 summarizes the key findings of this analysis. Donor age, DCD versus donation after brain death, duration of end-stage renal disease, transplant number, and cold storage time were not found to contribute significantly to graft survival duration, although both the impact of transplant number (P=0.17) and the cold storage time (P=0.16) approached statistical significance. We confirmed the well-known influence of recipient age (lower risk of graft loss with increasing age), race (greater risk in non-white recipients), type 1 or 2 diabetes (greater risk vs. nondiabetic), use of CNI (lower graft loss risk vs. no CNI), and peak PRA (greater risk with increasing PRA) on graft survival at our center (Table 2; all P<0.05). In addition to these, only the variable of having, or not having, a 0MM shipped-in kidney from a distant donor significantly impacted graft survival. The hazard ratio (0.71; P<0.02) indicated that recipients of local >0MM kidneys had a approximately 1.5-fold greater risk of graft loss when compared with recipients of a 0MM shipped-in kidney transplant. The significant negative impact of increase in peak PRA in the model was consistent with the univariate finding that a PRA of >20% tended to diminish the graft survival benefit of receiving a HLA 0MM shipped-in kidney transplant.
TABLE 2. Cox multivariable analysis of risk factors for graft loss.
| Variablea | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Payback vs. local >0MM | 0.888 | 0.64–1.24 | 0.48 |
| Shipped 0MM vs. local >0MM | 0.718 | 0.55–0.95 | 0.019 |
| DCD vs. DBD | 0.926 | 0.77–1.10 | 0.45 |
| Donor age (yr) | 1.007 | 0.99–1.03 | 0.52 |
| Recipient age (yr) | 0.951 | 0.92–0.98 | 0.002 |
| Transplant No. | 1.128 | 0.95–1.34 | 0.17 |
| Peak PRA (CDC) | 1.005 | 1.001–1.008 | 0.005 |
| Cold storage time (hr) | 1.008 | 0.99–1.02 | 0.16 |
| Total preservation time(hr) | 0.996 | 0.98–1.009 | 0.53 |
| Nondiabetic vs. diabetes mellitusb | 0.661 | 0.54–0.805 | <0.001 |
| Recipient race: other vs. white | 1.261 | 1.012–1.570 | 0.041 |
| Donor race: other vs. white | 0.841 | 0.590–1.198 | 0.34 |
| Recipient BMI (kg/m2) | 1.012 | 0.997–1.028 | 0.12 |
| ESRD duration (mo) | 0.995 | 0.987–1.003 | 0.21 |
| CNI, cyclosporine vs. none | 0.323 | 0.26–0.402 | <0.001 |
| CNI, tacrolimus vs. none | 0.358 | 0.279–0.459 | <0.001 |
| Transplant year | 1.001 | 0.966–1.039 | 0.92 |
“A vs. B” should be interpreted as hazard ratio of A relative to B.
Includes both types 1 and 2 diabetes.
CI, confidence interval; 0MM, 0-mismatch; DCD, donation after cardiac death; DBD, donation after brain death; PRA, panel reactive antibodies; CDC, complement-dependent cytotoxicty; BMI, body mass index; CNI, calcineurin inhibitor; ESRD, end-stage renal disease.
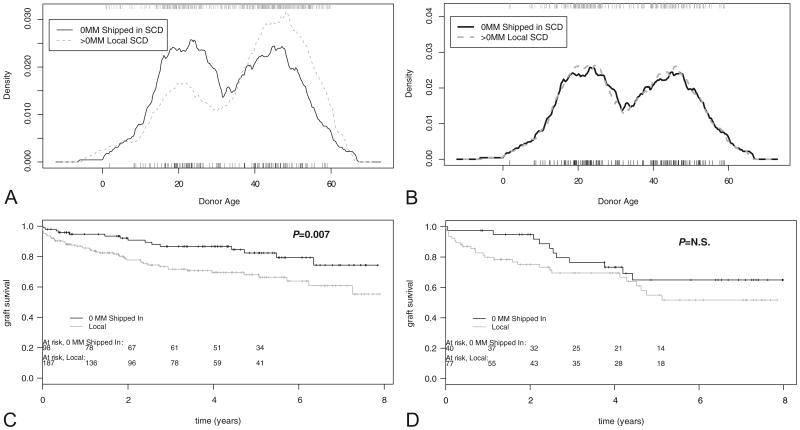
Second, we used a statistical device to compare kidney transplants with similar donor characteristics. To adjust for donor age and type, we used a 2:1 donor age-matching procedure, restricting our analysis to only standard criteria donor (SCD) transplants. We first extracted the subgroups from the (full, unmatched) SCD set and then performed 2:1 donor age-matching within each subset. Figure 3(A) confirms the analysis shown in Table 1—there were indeed significant differences in the age distribution of local versus shipped-in kidney donors, with a much higher proportion of local donors in the 40 to 50 years age range. The 2:1 pairwise matching procedure abolished this difference in age distribution (Fig. 3B), allowing for analysis of the impact of the 0MM shipped-in kidney independent of donor age variability, all within the SCD kidney donor subgroup. As shown in Figure 3(C), the survival advantage of the 0MM shipped-in kidney in nonsensitized recipients was seen to develop gradually (<7% difference at 1 yr) and tended to increase over time, resulting in a 20% graft survival difference over age-matched, locally procured more than 0MM organs at 6 to 8 years follow-up (Fig. 3C; P=0.007). The impact of a 0MM donor in HLA-sensitized patients was also dramatized, and as shown in Figure 3(D), a 20% difference in graft survival of 0MM shipped-in kidneys versus local more than 0MM kidneys was evident at year 1 and persisted over the first 24 months. However, the benefit of a 0MM kidney in the more than 20% PRA group did not reach statistical significance (P=0.17) primarily because of graft losses between years 2 and 3.
Figure 3.
Analysis of donor age-matched, standard criteria donor (SCD) kidney transplants: impact of 0-mismatched (0MM) shipped versus local more than 0MM kidneys. The impact of shipped 0MM versus local more than 0MM is early but short-lived in high PRA and delayed but long lasting in low panel reactive antibodies (PRA) recipients. (A) Donor age distribution—local versus shipped-in (SCD) kidneys. Wilcoxon P =0.0005. (B) Donor Age distribution of 2:1 age-matched (local: shipped-in) kidneys. Wilcoxon P=0.9426 (not significant). (C) Graft survival plot of 0MM shipped versus more than 0MM local donors (2:1 age matched; n=187 local, 98 shipped) transplanted in nonsensitized (<20% PRA) recipients. Log-rank P=0.007. (D) Graft survival plot of 0MM shipped versus more than 0MM local donor kidneys (2:1 age matched; n=77 local, 40 shipped) transplanted in highly sensitized (≥20% PRA) recipients. Log-rank P=0.169 (not significant).
Discussion
The results of this single-center study confirm the superiority of the 0MM shipped-in kidney transplant over a locally procured, more than 0MM organ as reported previously (7, 8, 10, 11). Although kidney transplant recipients at our center benefitted overall from the mandated sharing of HLA 0MM kidneys, a short-term follow-up (<3 years) might lead to the false conclusion that the main benefit lies in the HLA-sensitized patient population because clear differences were observed early on in that subgroup when kidneys of comparable age and donor quality were compared (Fig. 3D). However, with longer term follow-up, a lasting benefit from the 0 HLA-MM shipped-in kidney transplant program was clearly strongest in high PRA rather than low PRA recipients. This raises important questions regarding the recent UNOS policy change from mandated sharing of all 0MM kidneys to sharing for HLA-sensitized and pediatric recipients only.
Two mechanistic questions that arise from this retrospective analysis are as follows: (1) what factors limit the long-term acceptance of the HLA-A,-B, and -DR 0MM kidney in the highly sensitized patient?; and (2) to what can we attribute the lasting benefit of the 0MM shipped-in kidney transplants in the non-HLA-sensitized patient?
The first question is perhaps the easiest to address. It is now well established that the graft survival of the HLA-identical sibling kidney transplant may be limited in HLA-sensitized recipients by sensitization to non-HLA (e.g., MIC-A, MIC-B, minor H) antigens (15, 16). Sensitization to minor H antigens may have occurred at the T-cell level and, therefore, might be expected to take its toll in late graft losses in an immunosuppressed recipient. In addition, some HLA-specific B cells may play a role in the late demise of the 0MM transplant in the HLA-sensitized recipient. For example, because the typing of DP or DQ was never considered as a part of the UNOS “0MM” criteria, antibodies to these HLA antigens, if present, might still be able to find a target in HLA-A, -B, and -DR 0MM donors, compromising graft survival.
The second question is perhaps the most intriguing one. The simplest explanation is that, with no HLA-A, -B, or -DR mismatches, de novo exposure to mismatched HLA epitopes is reduced or eliminated, and with it the problem of HLA antibody production and humoral rejection. A second possibility is that T-regulatory (Treg) cells are more readily induced and function much more effectively in a setting of 0 HLA MM. Naturally arising CD4+ CD25+ Foxp3+ Tregs, trained to recognize tissue antigens in the context of “self” HLA-DR/-DQ/-DP alleles and suppress T-effector responses to tissue antigens, would be able to recognize the same HLA class II/peptide complexes on both recipient and donor-derived antigen-presenting cell. Thus, HLA class II matching would be expected to more effectively activate the host CD4+ Treg cell response to both donor and tissue or self antigens exposed during the transplant procedure, thereby promoting allotolerance and preventing autoimmunity (17-19). Additional matching for HLA class I could be expected to promote CD8 Treg cells specific for minor H antigens (20). These predictions are consistent with the more than 50% incidence of T-cell–mediated, donor-specific regulation (DSR) in recipients of HLA 0 DR-mismatched transplants from a deceased donor, in contrast to the low (<15%) incidence of DSR in recipients of HLA 2 DR-mismatched kidney allografts (21). Preliminary analysis of regulation in 11 recipients of 0MM kidney transplants using the transvivo delayed type hypersensitivity assay confirms a high incidence (55% 6 of 11) of DSR in these patients (Jankowska-Gan E and Burlingham W, unpublished). Thus, in addition to limiting the number of alloreactive T- or B-cell clones capable of recognizing the donor, a HLA-0MM promotes “dual recognition”—T-cell clones capable of recognizing ligands “directly” on donor cells and “indirectly” as processed peptides on recipient antigen-presenting cell (22). Treg cells genetically engineered for such dual allorecognition have recently been shown to have superior capacity to control allorejection and induce tolerance in a mouse heart transplantation model (23). However, it should be emphasized that regulation and tolerance can also occur in the absence of HLA matching (24); matching simply increases its incidence.
The distant donor organ must be shipped before transplantation, but in this study, as has been reported previously for multicenter analysis (11, 25), the increase in total preservation time over local organs is in the order of only 1 to 2 hr. There are a variety of tests that must be performed before the transplant, which also retard the speed with which the surgeon can transplant the locally procurred kidney; it should also be noted that surgical teams and OPOs are more confident in their organ preservation methods because of major advancements in preservation research during the past 23 years (26). These considerations may help to explain why there was no difference in graft survival attributable to increased total preservation time, in univariate (Fig. 2) or in multivariate (Table 2) analysis. The results of multivariate analysis, showing a benefit of increased recipient age but no significant negative impact of donor age (a factor that was highly significant (P<0.001) in univariate analysis; Munoz del Rio A and Burlingham W, unpublished), replicates earlier findings on locally procured primary renal transplants at the UW-Madison (27).
One of the main sources of resistance against sharing of kidneys is derived from the belief that shared kidneys are selected for sharing because they are of inferior quality. As shown by the comparison of donor age between locally procured and shipped kidneys (Fig. 3), our OPO has exercised the option of selecting only the “best” kidneys offered from other OPOs. Indeed, any center had the option of turning down offers of shipped-in kidneys that did not meet their standards.
In summary, the findings of this single-center study uphold previous multicenter studies showing a significant graft survival benefit of shipped 0MM kidney transplants, an effect that was strongest in nonsensitized individuals. Because the program of mandated sharing of 0MM kidneys has been discontinued in all but the HLA-sensitized and pediatric cases, what can individual transplant centers do to maximize graft survival? Locally procured kidney transplants benefit from HLA-DR matching (or a DR-0MM) (21, 27, 28), an option much more readily achieved than a full HLA-A, -B, and -DR-0MM within a given OPO.
Acknowledgments
This work was supported by grant R21-DK077354-02 from the NIH.
Footnotes
W.J.B. participated in the research design and the writing of the manuscript; A.M.d.R. participated in statistical analysis; D.L. participated in data acquisition and manuscript critique; H.W.S. participated in research design; J.D.P. participated in research design; E.J.-G. participated in data acquisition; and A.D. participated in research design and manuscript critique.
References
- 1.Uehling DT, Hussey JL, Weinstein AB, et al. Cessation of immunosuppression after renal transplantation. Surgery. 1976;79:278. [PubMed] [Google Scholar]
- 2.Opelz G. The benefit of exchanging donor kidneys among transplant centers. N Engl J Med. 1988;318:1289. doi: 10.1056/NEJM198805193182001. [DOI] [PubMed] [Google Scholar]
- 3.Takiff H, Cook DJ, Himaya NS, et al. Dominant effect of histocompatibility on ten-year kidney transplant survival. Transplantation. 1988;45:410. doi: 10.1097/00007890-198802000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 5.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: An alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation [see comments] Tissue Antigens. 1992;39:225. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 6.Lorentzen DF, Iwanaga KK, Meuer KJ, et al. A 25% error rate in serologic typing of HLA-B homozygotes. Tissue Antigens. 1997;50:359. doi: 10.1111/j.1399-0039.1997.tb02888.x. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto S, Carnahan E, Terasaki PI. Beneficial effect of sharing six-antigen-matched cadaver kidneys in the United States. Transplant Proc. 1992;24:1310. [PubMed] [Google Scholar]
- 8.Takemoto SK, Terasaki PI, Gjertson DW, et al. Twelve years' experience with national sharing of HLA-matched cadaveric kidneys for transplantation. N Engl J Med. 2000;343:1078. doi: 10.1056/NEJM200010123431504. [DOI] [PubMed] [Google Scholar]
- 9.Smith AG, Matsubara K, Mickelson E, et al. A comparative study of HLA-DRB typing by transcription-mediated amplification with the hybridization protection assay (TMA/HPA) versus PCR/SSOP. Hum Immunol. 1997;55:74. doi: 10.1016/s0198-8859(97)00085-2. [DOI] [PubMed] [Google Scholar]
- 10.Stegall MD, Dean PG, McBride MA, et al. Survival of mandatorily shared cadaveric kidneys and their paybacks in the zero mismatch era. Transplantation. 2002;74:670. doi: 10.1097/00007890-200209150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Bresnahan BA, Johnson CP, McIntosh MJ, et al. A comparison between recipients receiving matched kidney and those receiving mismatched kidney from the same cadaver donor. Am J Transplant. 2002;2:366. doi: 10.1034/j.1600-6143.2002.20413.x. [DOI] [PubMed] [Google Scholar]
- 12.OPTN/UNOS board approves measures to broaden access for living donation, pediatric transplantation, highly sensitized kidney transplant candidates. [Accessed June 6, 2010];UNOS Website. 2008 Available at: http://www.unos.org/
- 13.Johnson R. R Foundation for Statistical Computing. Vienna, Austria: 2006. [Accessed June 6, 2010]. A language and environment for statistical computing. Available at: http://www.R-project.org. [Google Scholar]
- 14.Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat. 2006;15:609. [Google Scholar]
- 15.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y, Stastny P, Susal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 17.Mezrich JD, Benjamin LC, Sachs JA, et al. Role of the thymus and kidney graft in the maintenance of tolerance to heart grafts in miniature swine. Transplantation. 2005;79:1663. doi: 10.1097/01.tp.0000160679.04441.b7. [DOI] [PubMed] [Google Scholar]
- 18.Torrealba JR, Katayama M, Fechner JH, Jr, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1 + CD4+ T regulatory cell infiltrates. J Immunol. 2004;172:5753. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Lee J, Jankowska-Gan E, et al. Human CD4+ CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178:3983. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 20.Burlingham WJ, Goulmy E. Human CD8+ T-regulatory cells with low-avidity T-cell receptor specific for minor histocompatibility antigens. Hum Immunol. 2008;69:728. doi: 10.1016/j.humimm.2008.08.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez DS, Jankowska-Gan E, Haynes LD, et al. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am J Transplant. 2004;4:537. doi: 10.1111/j.1600-6143.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Lee J, Keller M, et al. Analysis of indirect pathway CD4+ T cells in a patient with metastable tolerance to a kidney allograft: Possible relevance to superior graft survival of HLA class II closely matched renal allografts. Transpl Immunol. 2009;20:203. doi: 10.1016/j.trim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecka JM, Takemoto SK, Gjertson DW. Putting one objection to HLA matching on ice. Am J Transplant. 2002;2:295. doi: 10.1034/j.1600-6143.2002.20401.x. [DOI] [PubMed] [Google Scholar]
- 26.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. Current status of organ preservation with University of Wisconsin solution. Arch Pathol Lab Med. 1991;115:306. [PubMed] [Google Scholar]
- 27.Pirsch JD, D'Alessandro AM, Sollinger HW, et al. The effect of donor age, recipient age, and HLA match on immunologic graft survival in cadaver renal transplant recipients. Transplantation. 1992;53:55. doi: 10.1097/00007890-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Ayoub G, Terasaki P. HLA-DR matching in multicenter, single-typing laboratory data. Transplantation. 1982;33:515. doi: 10.1097/00007890-198205000-00010. [DOI] [PubMed] [Google Scholar]