Abstract
Introduction
We created the first tissue-engineered vascular graft (TEVG) to be successfully used in humans. The TEVG is made by seeding autologous bone marrow-derived mononuclear cells (BM-MNCs) onto a biodegradable tubular scaffold fabricated from polyglycolic-acid mesh coated with a 50:50 copolymer of poly-L-lactide and–ɛ-caprolactone. In the initial clinical study, the BM-MNCs were isolated using a Ficoll density centrifugation method. Use of this cell isolation technique is problematic in that it is performed using an open system and therefore is susceptible to contamination. As a first step toward creating a closed system for assembling a TEVG, we evaluated the use of a filter-based method for isolating BM-MNCs and compared it to density centrifugation in Ficoll.
Methods
BM-MNCs were isolated from human BM using density centrifugation in Ficoll or a filter-based method. BM-MNCs were seeded onto biodegradable tubular scaffold and incubated for 24 h before implantation. The TEVG were implanted as inferior vena cava interposition grafts in SCID/bg mice (n=24) using microsurgical technique. Grafts were followed with ultrasonography and computed tomography–angiography. Ten weeks after implantation the TEVG were explanted and examined using histology and immunohistochemistry.
Results
Both methods isolated similar number of cells (Ficoll: 8.5±6.6×106/mL, Filter: 6.6±3.5×106/mL; p=0.686) with similar viability as assayed using fluorescence-activated cell sorting (FACS) (Ficoll: 97.0%±1.5%, Filter: 95.9%±3.0%; p=0.339). FACS analysis demonstrated that the fraction of lymphocytes and monocytes to total cells was lower in the filter group (CD4 in Ficoll: 8.9%±1.1%, CD4 in Filter: 3.5%±0.8%; p=0.002, CD8 in Ficoll: 9.4%±2.1%, CD8 in Filter: 3.9%±1.4%; p=0.021, Monocyte in Ficoll: 6.9%±1.0%, Monocyte in Filter: 2.7%±1.0%; p=0.008), consistent with granulocyte contamination (Ficoll: 46.6±2.7×106/mL, Filter: 58.1±5.2×106/mL; p<0.001). The ratio of stem cells to BM-MNCs was comparable between groups. There were no statistically significant differences with regard to TEVG patency and morphology between groups. Both methods of cell isolation produced neovessels with similar histology.
Conclusion
Filter-based BM-MNC isolation is comparable to BM-MNC isolation using density centrifugation in Ficoll for TEVG assembly. The filter-based cell isolation technique has the added advantage of the potential to create a closed disposable system.
Introduction
To address the shortcomings of existing vascular grafts, current research is focused on the development of tissue-engineered vascular grafts (TEVG) made by seeding autologous bone marrow-derived mononuclear cells (BM-MNCs) onto a biodegradable, tubular scaffold. This process has previously been shown to result in the creation of a living vascular conduit with properties comparable to a native vessel.1–4 We have used this TEVG in congenital heart surgery applications where the growth potential of this TEVG can be used to its greatest advantage.5,6
However, while there have been many advances in this field, there remain practical and financial limitations that prevent the widespread application of TEVGs. One of the challenges is the need to develop a method for isolating the BM-MNCs in a rapid, sterile, and cost-efficient manner. In previous studies we employed a density centrifugation with Ficoll. This process is less than ideal because it typically uses an open system and thus requires use of an ISO class 7 facility to meet standard of current Good Manufacturing Practice (cGMP). Use of cGMP facilities is costly. In addition, density centrifugation processing is labor intensive and time consuming. To overcome these disadvantages, a novel leukocyte reduction filter-based system (LRF) was developed as a simple, faster, cGMP-compliant, and more cost-efficient alternative to density centrifugation with Ficoll.
In this study, we evaluated the use of this newly developed filtration system to isolate BM-MNCs for use in TEVGs and evaluated its performance in a murine model.
Materials and Methods
MNC enrichment by filtration
Human BM (hBM; Lonza) was transferred to an initial filtration chamber using a syringe, and subsequently filtered using LRF by longitudinal flow due to simple gravitational force. After washing the filter media with phosphate-buffered saline (PBS) twice to reduce contamination by red blood cells, MNCs were recovered by back-flushing the filter, thus reversing the direction of flow with 20 mL of sterile dextran solution included in the packaged filtration system: Pall B1623 prototype cell harvest filter set for the recovery of progenitor cells from BM (Pall Corporation) (Table 1).
Table 1.
Detail Steps of MNC Enrichment by Filtration or Density Centrifugation System with Time Course
| Time | Filtration system | Density-centrifugation system |
|---|---|---|
| Start | Load bone marrow into filtration set | Carefully layer bone marrow onto Ficoll solution |
| 10 min | Filter by gravity flow Recover cells by back-flushing filter with Harvest Solution | |
| 30 min | Centrifuge at 400 g for 30 min | |
| 1 h | Transfer interface mononuclear cell layer into new tube | |
| Centrifuge 100 g for 10 min | ||
| Remove supernant, add fresh PBS | ||
| Centrifuge 100 g for 10 min | ||
| 2.5 h | Remove supernant and resuspend cells in PBS |
Filtration system is faster and simpler compared with density centrifugation system.
MNC, mononuclear cells; PBS, phosphate-buffered saline.
MNC enrichment by density centrifugation
MNCs were isolated from hBM by density-gradient centrifugation using histopaque-1077 (Sigma) according to the manufacturer's instructions. In brief, hBM was diluted in a 1:1 ratio with PBS, and centrifuged at 400 g for 30 min. The MNC layer was then removed and washed twice with PBS (Table 2).
Table 2.
Results of Fluorescence-Activated Cell Sorting Analysis Showed a Lower Ratio of Lymphocytes and Monocytes to Total Cell Number in the Filtered Group
| % of total cell | FACS marker | Ficoll | Filter | p-Value |
|---|---|---|---|---|
| Cell viability | 7AAD− | 97.0±1.5 | 95.9±3.0 | 0.339 |
| CD4 | CD3+/CD4+ | 8.9±1.1 | 3.5±0.8 | 0.002 |
| CD8 | CD3+/CD8+ | 9.4±2.1 | 3.9±1.4 | 0.021 |
| B cell | CD19+ | 4.4±2.5 | 2.0±0.5 | 0.180 |
| NK | CD56+ | 4.5±2.7 | 1.3±1.4 | 0.146 |
| Monocyte | CD14+ | 6.9±1.0 | 2.7±1.0 | 0.008 |
| CEC | CD45−/CD146+/CD31+/CD133− | 0.02±0.01 | 0.04±0.05 | 0.588 |
| EPC | CD45−/CD14−/VEGFR2−/CD133+ | 0.0004±0.0002 | 0.0007±0.0002 | 0.237 |
| MSC | CD73+/CD90+/CD105+/CD34− | 0.001±0.0007 | 0.002±0.003 | 0.541 |
| HSC | CD45+/CD34+/CD133+/CD14− | 0.05±0.034 | 0.01±0.007 | 0.103 |
| MAPC | CD90+/VEGFR2+/CD133+/HLA-DR−/CD45− | 0.0015±0.0021 | 0.0005±0.0007 | 0.591 |
The percentage of viable cells and ratio of stem cells to MNCs were comparable.
NK, natural killer cells; CEC, circulating endothelial cells; EPC, endothelial progenitor cells; FACS, fluorescence-activated cell sorting; MSC, mesenchymal stem cells; HSC, hematopoietic stem cells; MAPC, multipotent adult progenitor cells.
Characterization of MNCs
MNC number was counted using a hemocytometer. The mixed cell population, consisting of T cells (CD3+), B cells (CD19+), monocytes (CD14+), natural killer cells (CD56+), circulating endothelial cells (CEC) (CD45−/CD146+/CD31+/CD133−), endothelial progenitor cells (EPCs) (CD45−/CD14−/VEGFR2−/CD133+), mesenchymal stem cells (CD73+/CD90+/CD105+/CD34−), hematopoietic stem cells (CD45+/CD34+/CD133+/CD14−), and multipotent adult progenitor cells (CD90+/VEGFR2+/CD133+/HLA-DR−/CD45−) were characterized by fluorescence-activated cell sorting (FACS). Dead cells were denoted by positive staining for 7-aminoactiomycin D.
Construction of biodegradable PGA-P(CL/LA) scaffolds
About 0.8-mm-diameter scaffolds were constructed from a nonwoven polyglycolic-acid (PGA) mesh (ConcordiaFibers,), and a 50:50 copolymer sealant solution of poly-L-lactide and ɛ-caprolactone (P(CL/LA)) (263,800 Da; Absorbable Polymers International).7
Preparation of TEVGs for implantation into mouse model
MNCs isolated from hBM using either the density centrifugation or filtration method were statically seeded onto biodegradable scaffolds, and cultured overnight in Roswell Park Memorial Institute-1640 (RPMI-1640) media with 10% fetal bovine serum and 1% penicillin–streptomycin.
Inferior vena cava interposition surgery
Female C.B-17 SCID/bg mice (Taconic Farms), 3–4 months of age, received TEVGs inserted as inferior vena cava (IVC) interposition grafts using a running 10-0 nylon suture for the end-to-end proximal and distal anastamoses (n=12 for each MNC isolation group). The grafts were assigned in random manner. Animals recovered from surgery, and were maintained without the use of anticoagulation or antiplatelet therapy. All animal experiments were done in accordance with institutional guidelines.
In vivo evaluation of TEVGs
TEVGs were evaluated with ultrasonography (Vevo 770; Visualsonics) and 3D computed tomography–angiography (CTA). TEVGs were explanted at 10 weeks and examined by histological analysis and immunohistochemical staining. The data were analyzed in a blinded fashion.
Immunohistochemistry
TEVGs were stained with mouse anti-human α-smooth muscle actin (α-SMA) (Dako), rabbit-anti-human vonWillebrand factor (vWF) (Dako), and rabbit-anti-mouse calponin (Abcam) antibodies. Antibody binding was detected using appropriate biotinylated secondary antibodies, followed by the binding of streptavidin–horseradish peroxidase and color development with 3,3-diaminobenzidine (Vector). Nuclei were counterstained with hematoxylin.
Results
Relative comparison of number of MNCs isolated
No significant difference in the number of MNCs isolated by the filtration and density centrifugation methods was observed (Ficoll: 8.5±6.6×106/mL, Filter: 6.6±3.5×106/mL; p=0.686).
Time for MNC isolation
Time for separation procedure was significantly shorter in the Filter group than in the Ficoll group (Ficoll: 106±11 min, Filter: 10±12 min; p<0.001).
Characterization of isolated MNCs by FACS
FACS analysis demonstrated a lower ratio of lymphocytes and monocytes to total cell number in the filtration group. These results are consistent with increased granulocyte contamination in this filtration group (Ficoll: 46.6%±2.7%, Filter: 58.1%±5.2%; p<0.001). However, both the percentage of viable cells, and ratio of stem cells to MNCs were comparable (Table 2).
TEVG histology
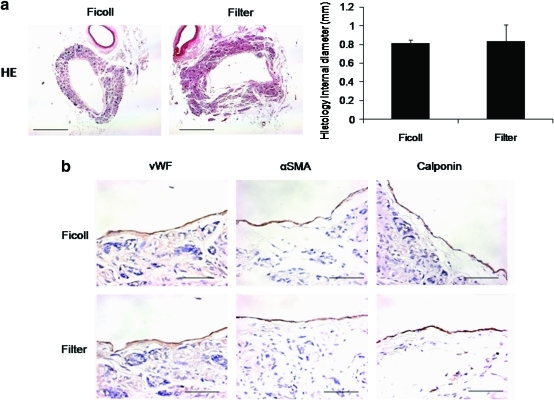
There was no histological evidence of aneurysm formation, graft rupture, or ectopic calcification. At 10 weeks postimplantation, the internal diameter of the TEVGs in the filtration group (0.81±0.03 mm) was comparable to that of the internal diameter of scaffolds seeded with cells isolated by density centrifugation (0.83±0.17 mm); p=0.694 (Fig. 1a). Endothelialization in both grafts at 10 weeks was confirmed by a monolayer of cells staining positive for vWF. Cells positive for α-SMA and calponin demonstrated the formation of a smooth muscle cell layer in the TEVG neotissue in both groups (Fig. 1b).
FIG. 1.
(a) Histology of tissue-engineered vascular graft (TEVG) at 10 weeks. The internal diameters of TEVGs seeded with MNCs isolated by filtration were comparable to those of TEVGs seeded with cells isolated by density centrifugation. (b) Immunohistochemistory of TEVG at 10 weeks. Endothelialization in both groups was confirmed by a monolayer of von Willebrand factor (vWF)–positive cells. α-Smooth muscle actin (α-SMA)- and calponin-positive cells evinced the formation of a smooth muscle cell layer in both groups. Color images available online at www.liebertonline.com/tec
Monitoring the remodeling of TEVGs by ultrasonography
Internal diameters of both the filter-isolated and density-centrifugation-isolated MNC-seeded grafts increased to a size comparable to that of native IVC in the postimplantation period. The internal diameter of the TEVG at 10 weeks in the filtered group (1.05±0.10 mm) was comparable to that of the density centrifugation group (0.91±0.15 mm) at the same time-point; p=0.193 (Fig. 2).
FIG. 2.
Ultrasonography showed the internal diameters of both groups to have increased to a size comparable to that of native mouse inferior vena cava during the postimplantation period. The internal diameter of the TEVG at 10 weeks in the filtered group (1.05±0.10 mm); p=0.193 was comparable to that of the density centrifugation group (0.91±0.15 mm). White lines demarcate the position of the TEVGs.
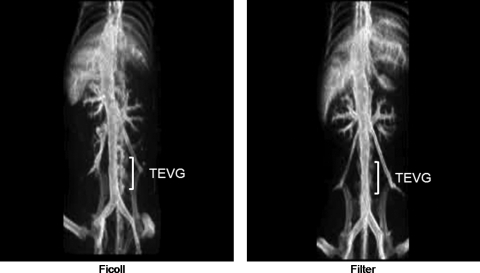
CTA of TEVG
CTA revealed that vessels in both groups were more than 75% of its original diameter at 10 weeks. There was no aneurysm formation, graft rupture, or ectopic calcification (Fig. 3).
FIG. 3.
Computed tomography–angiography revealed no aneurysm formation, graft rupture, or ectopic calcification. Vessels remained widely patent in both experimental groups at 10 weeks.
Discussion
While much progress has been made concerning the development of TEVGs, practical and technical limitations still exist, posing significant obstacles limiting their widespread clinical application for treating congenital cardiac defects. Included among these is the timely derivation of an autologous cell source, such as BM-MNCs—a process that in the past has proven both costly and time consuming. In this study, we tested and demonstrated the successful application of a novel filtration system that possesses numerous advantages, including the minimization of variability between operators such as careful layering of BM in the Ficoll solution without contamination while pipetting the MNC layer the potential capability of being performed in a closed system. Importantly, in terms of TEVG neotissue formation, this filtration method achieves results identical to those obtained by traditional Ficoll MNC isolation methods.
Traditional methods of isolating MNCs from hBM rely on density centrifugation, which consequently necessitates a time-consuming, labor-intensive process that does not always ensure reproducibility of results. The novel filtration system examined in this study proffers a viable alternative to current cell isolation techniques.8 LRF was initially developed to address the need of blood banks for advanced technologies to expedite the isolation of normal white blood cells (WBC) to minimize human leukocyte antigen (HLA) alloimmunization, and to more effectively remove WBC-borne viruses from blood components.9 However, the applicability of this filtration system to tissue engineering vascular graft has been made evident in reports showing that physiologically functional monocytes,10 EPCs,11 and CD34+ cells12 can be successfully isolated from these leuko-reduction filters.
The MNC separation protocol, including the type of separation and washing solutions, or the conditions of storage, can have an impact on the functional activity of isolated cells, and can thus affect clinical outcomes. As an example, two large, randomized, controlled clinical trials, REPAIR-AMI13 and ASTAMI,14 which examined the use of BM stem/progenitor cells in cardiac repair after acute myocardial infarction, reported conflicting outcomes despite similar trial designs and patient profiles. Because the exact solutions used and protocols followed for MNC isolation were different in these two trials—Ficoll versus Lymphoprep—it is possible that the conflicting outcomes are due to these different MNC isolation methods.15,16 From such results, it is thus evident that consistency in cell isolation methodology and processing is necessary to achieve reproducible and reliable data.
While in our study, the filtration system was not as efficient as the traditional separation method with regard to the removal of granulocytes, this latter goal can be achieved. Contaminating granulocytes can be removed by density gradient centrifugation, immunomagnetic depletion, or FACS, thus yielding a purified population of MNCs although these procedures negate the closed system benefit of the filter method.17 Nevertheless, in spite of the increased presence of granulocytes, this novel filtration system achieved the same results as the density centrifugation procedure in terms of TEVG neotissue formation. Moreover, the filtration system has the added advantage of the potential to create a closed disposable system developed as a simple, faster, cGMP-compliant, and more cost-efficient alternative to density centrifugation with Ficoll.
In addition, our thorough characterization of stem cell marker expression of the processed samples showed that the filtration system yields similar results to cells isolated by density centrifugation. In sum, these data suggest that MNCs separated by filtration are phenotypically and functionally similar to cells isolated by density centrifugation, the previously established gold-standard approach to MNC isolation.
As a limitation of this study, while data obtained using the SCID-bg IVC interposition graft model provide data supporting functional equivalence in neovessels formed from BM-MNC isolated using either technique, validation studies using additional animal models will be essential before translation. In addition, this study is a part of long-term goal of complete closed seeding system. As a next step of this study, cell seeding step beside the purification of BM-MNCs will be important to create complete closed system.
In conclusion, this novel approach to MNC isolation is favorable for numerous reasons. First, it offers an easier, faster, cGMP-compatible, and more inexpensive method than traditional MNC isolation procedures. Further, it can be performed in a closed system, and does not involve nonhuman material, which has the potential for use as a cGMP technique for enriching MNCs for human TEVG. For these reasons, this filtration system may prove a useful tool for expediting and simplifying the preparation of TEVGs to be used in vivo and in clinical trials.
Disclosure Statement
Edward Snyder and Christopher K. Breuer have grant support from Pall Corp.
References
- 1.Goyal A., et al. Development of a model system for preliminary evaluation of tissue-engineered vascular conduits. J Pediatr Surg. 2006;41:787. doi: 10.1016/j.jpedsurg.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura G., et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12:3075. doi: 10.1089/ten.2006.12.3075. [DOI] [PubMed] [Google Scholar]
- 3.Hibino N., et al. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005;129:1064. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Brennan M.P., et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248:370. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin'oka T., et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Shin'oka T. Imai Y. Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 7.Roh J.D., et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29:1454. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro M.J. To filter blood or universal leukoreduction: what is the answer? Crit Care. 2004;8(Suppl 2):S27. doi: 10.1186/cc2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fergusson D., et al. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51:417. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- 10.Ebner S., et al. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001;252:93. doi: 10.1016/s0022-1759(01)00337-4. [DOI] [PubMed] [Google Scholar]
- 11.Teleron A.A. Carlson B. Young P.P. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion. 2005;45:21. doi: 10.1111/j.1537-2995.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 12.Ivanovic Z., et al. Whole-blood leuko-depletion filters as a source of CD 34+ progenitors potentially usable in cell therapy. Transfusion. 2006;46:118. doi: 10.1111/j.1537-2995.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V., et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 14.Lunde K., et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 15.Seeger F.H., et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 16.Yeo C., et al. Ficoll-Paque versus Lymphoprep: a comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen Med. 2009;4:689. doi: 10.2217/rme.09.44. [DOI] [PubMed] [Google Scholar]
- 17.Meyer T.P., et al. Filter buffy coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005;307:150. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]