Abstract
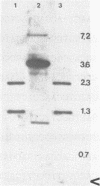
We have cloned and sequenced a polyubiquitin gene from Neurospora crassa that is organized in a four repeat-tandem array. The first repeat contains a small intron and the last is fused to an extra glutamine codon. In Northern blots, two RNA species of 1.3 kb and 0.7 kb hybridize to the isolated clone. The larger ubiquitin (UBI) transcript accumulates after partial inhibition of protein synthesis with cycloheximide, and the smaller one preferentially accumulates in conidia after germination. Unexpectedly, constitutive expression of UBI transcripts in exponentially grown mycelia is not altered by heat-shock or exposure to arsenite.
Full text
PDF
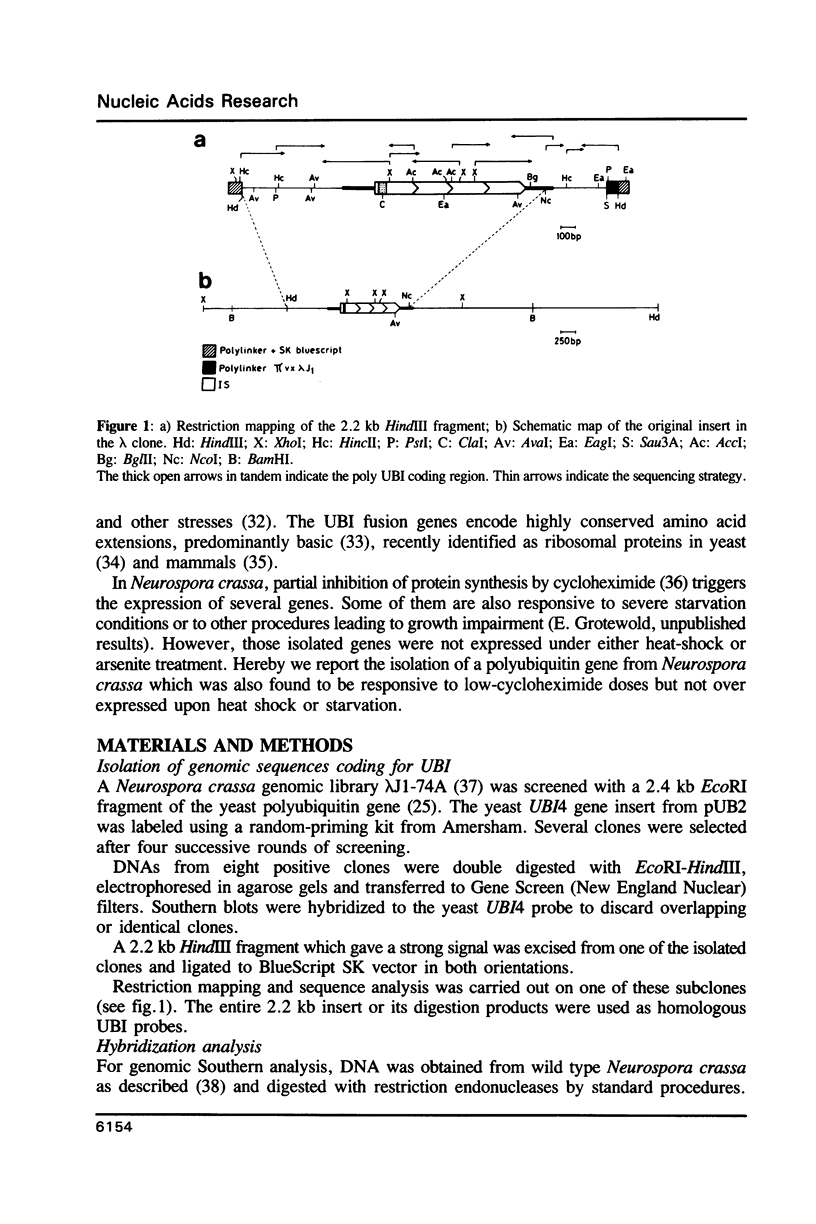


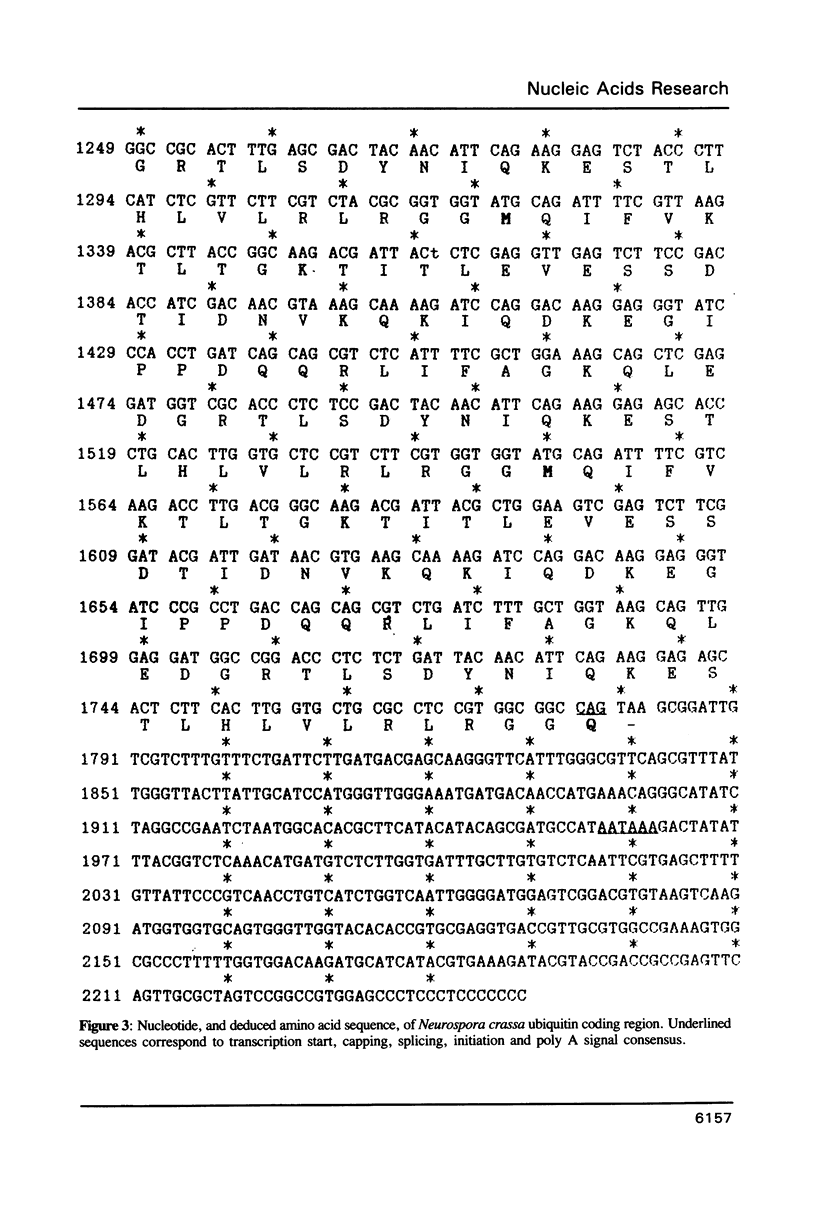

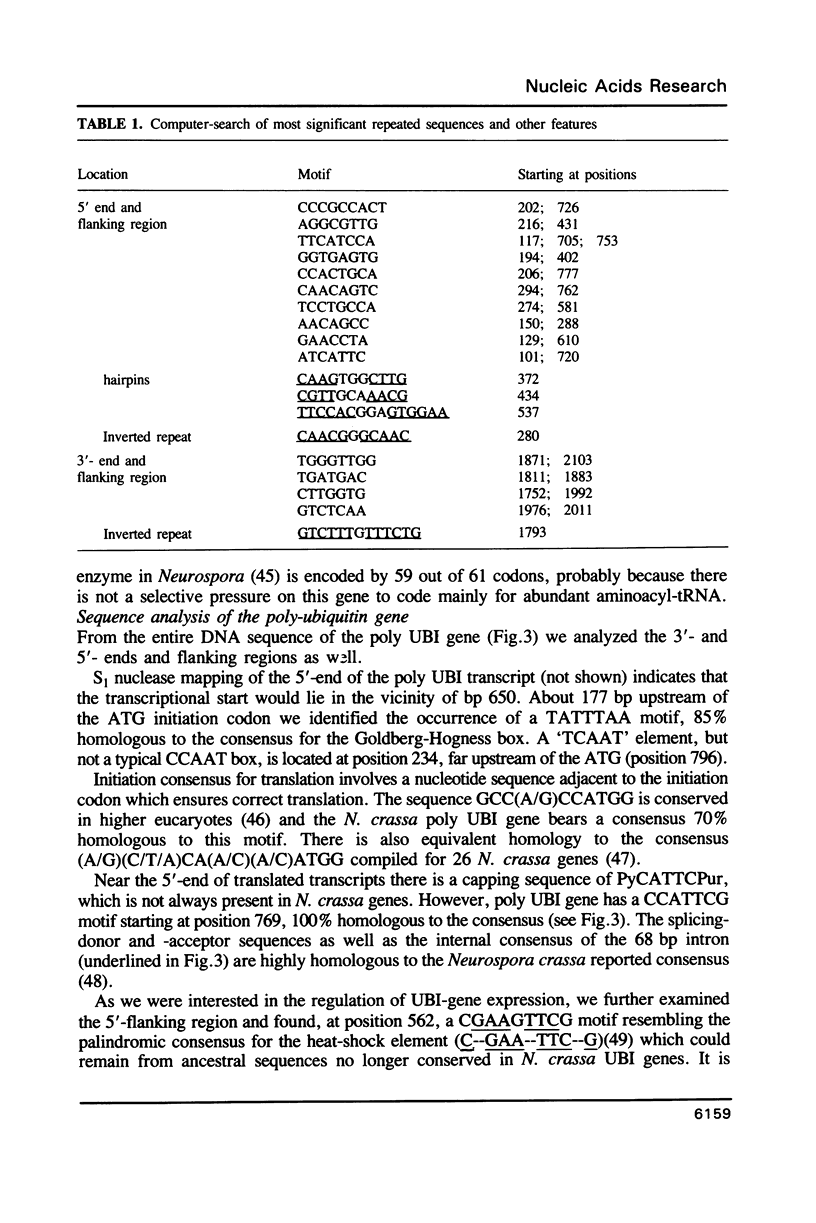






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. T., Board P. G. The human ubiquitin gene family: structure of a gene and pseudogenes from the Ub B subfamily. Nucleic Acids Res. 1987 Jan 26;15(2):443–463. doi: 10.1093/nar/15.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. Mol Cell Biol. 1986 Dec;6(12):4602–4610. doi: 10.1128/mcb.6.12.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. R., Rush S. J. Induction of a 'stress' protein in intact mammalian organs after the intravenous administration of sodium arsenite. Biochem Biophys Res Commun. 1984 Apr 16;120(1):150–155. doi: 10.1016/0006-291x(84)91426-8. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Yanofsky C. Nucleotide sequence of the Neurospora crassa trp-3 gene encoding tryptophan synthetase and comparison of the trp-3 polypeptide with its homologs in Saccharomyces cerevisiae and Escherichia coli. J Biol Chem. 1989 Mar 5;264(7):3840–3848. [PubMed] [Google Scholar]
- Busch H. Ubiquitination of proteins. Methods Enzymol. 1984;106:238–262. doi: 10.1016/0076-6879(84)06025-0. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Khan M. I., Marsh J., Ecker D. J., Crooke S. T. Ubiquitin-metallothionein fusion protein expression in yeast. A genetic approach for analysis of ubiquitin functions. J Biol Chem. 1988 Nov 5;263(31):16364–16371. [PubMed] [Google Scholar]
- Carrazana E. J., Pasieka K. B., Majzoub J. A. The vasopressin mRNA poly(A) tract is unusually long and increases during stimulation of vasopressin gene expression in vivo. Mol Cell Biol. 1988 Jun;8(6):2267–2274. doi: 10.1128/mcb.8.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Finley D., Bartel B., Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989 Mar 30;338(6214):394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Finley D., Ozkaynak E., Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987 Mar 27;48(6):1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Fried V. A., Smith H. T., Hildebrandt E., Weiner K. Ubiquitin has intrinsic proteolytic activity: implications for cellular regulation. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3685–3689. doi: 10.1073/pnas.84.11.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Giorda R., Ennis H. L. Structure of two developmentally regulated Dictyostelium discoideum ubiquitin genes. Mol Cell Biol. 1987 Jun;7(6):2097–2103. doi: 10.1128/mcb.7.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Graham R. W., Jones D., Candido E. P. UbiA, the major polyubiquitin locus in Caenorhabditis elegans, has unusual structural features and is constitutively expressed. Mol Cell Biol. 1989 Jan;9(1):268–277. doi: 10.1128/mcb.9.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Hershko A., Heller H., Elias S., Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983 Jul 10;258(13):8206–8214. [PubMed] [Google Scholar]
- Jentsch S., McGrath J. P., Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987 Sep 10;329(6135):131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Kim K. S., Engman D. M., Donelson J. E. Ubiquitin genes in trypanosomatidae. J Biol Chem. 1988 Sep 5;263(25):12698–12704. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreader C. A., Heckman J. E. Isolation and characterization of a Neurospora crassa ribosomal protein gene homologous to CYH2 of yeast. Nucleic Acids Res. 1987 Nov 11;15(21):9027–9042. doi: 10.1093/nar/15.21.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka R. G., Raboy B., Schuster R., Parag H. A., Diamond G., Ciechanover A., Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988 Oct 25;263(30):15726–15731. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Selective arrangement of ubiquitinated and D1 protein-containing nucleosomes within the Drosophila genome. Cell. 1982 Feb;28(2):375–385. doi: 10.1016/0092-8674(82)90355-5. [DOI] [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. D., Yasuda H., Hatch C. L., Bonner W. M., Bradbury E. M. Identification of ubiquitinated histones 2A and 2B in Physarum polycephalum. Disappearance of these proteins at metaphase and reappearance at anaphase. J Biol Chem. 1985 Apr 25;260(8):5147–5153. [PubMed] [Google Scholar]
- Munro S., Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Smith H. T., Fried V. A. Ubiquitin is a component of the microtubule network. Proc Natl Acad Sci U S A. 1988 May;85(9):3019–3023. doi: 10.1073/pnas.85.9.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Hagmann J., Noegel A., Gerisch G. Ubiquitin gene expression in Dictyostelium is induced by heat and cold shock, cadmium, and inhibitors of protein synthesis. J Cell Sci. 1988 May;90(Pt 1):51–58. doi: 10.1242/jcs.90.1.51. [DOI] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Westphal M., Jaeger E., Noegel A., Gerisch G. Complete cDNA sequence of a Dictyostelium ubiquitin with a carboxy-terminal tail and identification of the protein using an anti-peptide antibody. FEBS Lett. 1988 Mar 14;229(2):273–278. doi: 10.1016/0014-5793(88)81139-6. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Solomon M. J., Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987 May;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Varshavsky A. The yeast ubiquitin gene: head-to-tail repeats encoding a polyubiquitin precursor protein. Nature. 1984 Dec 13;312(5995):663–666. doi: 10.1038/312663a0. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Redman K. L., Rechsteiner M. Extended reading frame of a ubiquitin gene encodes a stable, conserved, basic protein. J Biol Chem. 1988 Apr 5;263(10):4926–4931. [PubMed] [Google Scholar]
- Redman K. L., Rechsteiner M. Identification of the long ubiquitin extension as ribosomal protein S27a. Nature. 1989 Mar 30;338(6214):438–440. doi: 10.1038/338438a0. [DOI] [PubMed] [Google Scholar]
- Reinert W. R., Patel V. B., Giles N. H. Genetic regulation of the qa gene cluster of Neurospora crassa: induction of qa messenger ribonucleic acid and dependency on qa-1 function. Mol Cell Biol. 1981 Sep;1(9):829–835. doi: 10.1128/mcb.1.9.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Berlin V., Hager K. M., Yanofsky C. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol Cell Biol. 1988 Jun;8(6):2411–2418. doi: 10.1128/mcb.8.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Matsumoto K., Toh-e A. Dual regulation of the expression of the polyubiquitin gene by cyclic AMP and heat shock in yeast. EMBO J. 1988 Feb;7(2):495–502. doi: 10.1002/j.1460-2075.1988.tb02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi H. F., Flawiá M. M., Torres H. N. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):990–996. doi: 10.1016/s0006-291x(74)80241-x. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Persico M., Alcalay M. A "housekeeping" gene on the X chromosome encodes a protein similar to ubiquitin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):851–855. doi: 10.1073/pnas.85.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Different intracellular distributions of heat-shock and arsenite-induced proteins in Drosophila Kc cells. Possible relation with the phosphorylation and translocation of a major cytoskeletal protein. J Mol Biol. 1982 Dec 5;162(2):365–378. doi: 10.1016/0022-2836(82)90532-0. [DOI] [PubMed] [Google Scholar]
- Warner J. R. Ubiquitin. A marriage of convenience or necessity? Nature. 1989 Mar 30;338(6214):379–379. doi: 10.1038/338379a0. [DOI] [PubMed] [Google Scholar]
- Wiborg O., Pedersen M. S., Wind A., Berglund L. E., Marcker K. A., Vuust J. The human ubiquitin multigene family: some genes contain multiple directly repeated ubiquitin coding sequences. EMBO J. 1985 Mar;4(3):755–759. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K. D., Cox M. J., O'Connor L. B., Shapira R. Structure and activities of a variant ubiquitin sequence from bakers' yeast. Biochemistry. 1986 Sep 9;25(18):4999–5004. doi: 10.1021/bi00366a005. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]