Abstract
Prostate cancer (CaP) is the most common cancer among adult men in the Western world. Better insight into its tumor-activating pathways may facilitate the development of targeted therapies. In this study, we show that patients who develop prostate tumors with low levels of PTEN and high levels of HER2/3 have a poor prognosis. This is functionally relevant, as targeting Her2 activation to the murine prostate cooperates with Pten loss and drives CaP progression. Mechanistically, this is associated with activation of the MAPK pathway and abrogation of the Pten loss-induced cellular senescence program. Importantly, inhibition of MEK function strongly suppressed proliferation within these tumors by restoring the Pten loss-induced cellular senescence program. Taken together, these data suggest that stratification of CaP patients for HER2/3 and PTEN status could identify patients with aggressive CaP who may respond favorably to MEK inhibition.
Keywords: ErbB2, PI3K, mouse models
Prostate cancer (CaP) is the most common cancer diagnosed in men in developed countries, and its incidence continues to rise worldwide due to prostate-specific antigen testing as well as an aging population (1–3). As CaP is a heterogeneous condition and its behavior is highly variable, it is important to delineate key signaling pathways that confer poor prognosis and predict a favorable response to targeted therapy. Prostate carcinogenesis represents a multistep process thought to arise from accumulation of mutations in tumor-related genes that lead to the transformation of benign prostatic epithelium to locally invasive disease and ultimately metastasis (4).
PTEN is one of the most frequently altered tumor suppressors in cancer (5). Up to 70% of primary prostate tumors show loss or alterations in at least one PTEN allele, whereas complete inactivation of PTEN appears to be associated with metastatic disease (6–8). PTEN acts as a tumor suppressor by negatively regulating PI3K/AKT signaling (7). Previous in vivo studies have established PTEN as a haploinsufficient tumor suppressor gene, as heterozygous deletion of PTEN can promote tumor initiation (9). Although the complete loss of PTEN in mice can lead to invasive CaP, a strong p53-dependent senescence response is evoked that can oppose tumor progression (8). This may explain why human primary CaP does not initially select for complete loss of PTEN. Hence, further mutations in PI3K/AKT and RAS/RAF/MAPK cascades, which are frequently activated in CaP, may be required in addition to PTEN mutations for tumor progression (5). Recent work by the DePinho group has identified a four-gene signature (PTEN, SMAD4, CyclinD1, and SPP1) that prognosticates in terms of biochemical recurrence and lethal metastasis in human CaP (10). They found that loss of Smad4 in their Pten null mouse model overcame senescence, leading to aggressive metastatic CaP. This is similar to previously published CaP murine models where inactivating mutations of Nkx3.1 and p53, as well as those overexpressing Erg, overcame Pten-loss induced cellular senescence (PICS) (8, 11, 12).
Receptor tyrosine kinase (RTK)-mediated signaling is activated in almost 40% of primary CaPs and 90% of metastatic cancers (5). Oncogenic alterations in the ErbB family of RTKs, especially HER2, are common in breast and prostate cancer (13). HER2 is overexpressed in up to 20% of advanced CaP. Saal et al. demonstrated that there was a significant correlation between HER2 amplification and low PTEN expression in breast cancer (14). We hypothesized that HER2 activation may also cooperate with PTEN loss in prostate carcinogenesis. To investigate whether activation of HER2/3 can cooperate with PTEN loss to promote prostate tumorigenesis, we tested for evidence of synergism between the PTEN/AKT and HER2/3 signaling pathways in both clinical and in vivo (murine) systems. We show that HER2/3 overexpression conferred a poor prognosis in patients with CaP tumors that have low PTEN expression. Importantly, activation of HER2 was sufficient to overcome PICS and drive tumor progression in a MEK/ERK-dependent manner.
Results
HER2/3 Expression Correlates with PTEN Loss in Human Prostate Cancer and Confers a Poor Prognosis.
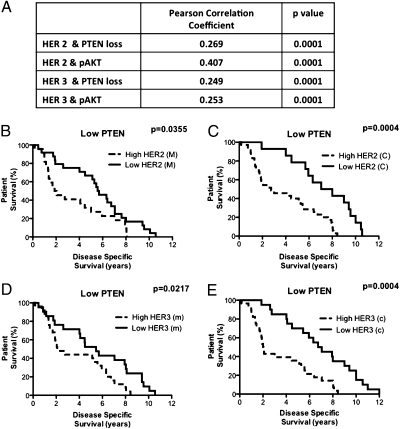
We examined a cohort of 226 patients with CaP and found that the expression of HER2 and HER3 (both cytoplasmic and membranous) was up-regulated in CaP (all Gleason grades) compared with the benign prostatic hypertrophic (BPH) control cohort (Figs. S1 and S2). We also observed reduced PTEN expression and up-regulation of pAKTSer473 levels in CaP cases relative to BPH controls (Figs. S1 and S2). In terms of coexpression, we were able to demonstrate significant correlations between up-regulated expression of either membranous HER2 or HER3 with low PTEN or elevated pAKT expression, respectively (Fig. 1A).
Fig. 1.
HER2/HER3 up-regulation correlates with reduced PTEN and up-regulation of pAKTSer473 and Kaplan–Meier survival curves of patients with HER2 and HER3 up-regulation who have low levels of PTEN. Pearson's correlation coefficients and respective P values revealed significant correlations between up-regulation of HER2 and loss of PTEN, up-regulation of both HER2 and pAKT, up-regulation of HER3 and loss of PTEN, and up-regulation of both HER3 and pAKT (A). Survival curves of cytoplasmic (C) and membranous (M) HER2 (B and C) and HER3 (D and E) in patients with reduced PTEN. These revealed that patients with high levels of HER2 or HER3 in conjunction with low PTEN expression had a reduced overall survival compared with those with low levels of HER2 or HER on a low PTEN background.
Analysis of disease-specific survival in our patient cohort showed no association with HER2, HER3, pAKT, or PTEN status in isolation. However, among the patient cohort with low-PTEN-expressing tumors (n = 59), up-regulated HER2 expression conferred a marked reduction in disease-specific survival: cytoplasmic HER2 [median of 7.53 vs. 2.75 y, P = 0.0004, hazard ratio 3.08, 95% confidence interval (CI) 1.65–5.72] and membranous HER2 (median of 5.63 vs. 1.96 y, P = 0.0355, hazard ratio 1.97, 95% CI 1.05–3.72) (Fig. 1 B and C). Similarly, HER3 up-regulation in conjunction with low PTEN expression was also associated with shorter disease-specific survival: cytoplasmic HER3 (median of 6.90 vs. 2.03 y, P = 0.0004, hazard ratio 3.22, 95% CI 1.71–6.08) and membranous HER3 (median of 5.45 vs. 2.10 y, P = 0.0217, hazard ratio 2.10, 95% CI 1.11–3.95) (Fig. 1 D and E).
Her2KI and Pten Loss Cooperate to Drive Aggressive CaP in the Mouse.
To validate the functional significance of our findings in vivo, we targeted Her2 activation and Pten deletion to the murine prostate. To achieve this, we used the PB-Cre4 transgenic line crossed with a Her2KI line, which resulted in minimal levels of Her2 up-regulation in the prostate epithelium (15). This contrasts with the high levels of Her2 seen in a transgenic mouse line overexpressing mammary tumors (MMTV-NIC Ptenfl/fl) (Fig. S3) (16). The PB-Cre4: Her2KI mice (n = 14) were aged for up to 18 mo without any demonstrable phenotype compared with wild-type animals (Fig. S3). The Pten signal remained intact within these prostates and there was minimal up-regulation of Her2 and Her3 as assessed by immunohistochemistry (IHC) (Fig. S3).
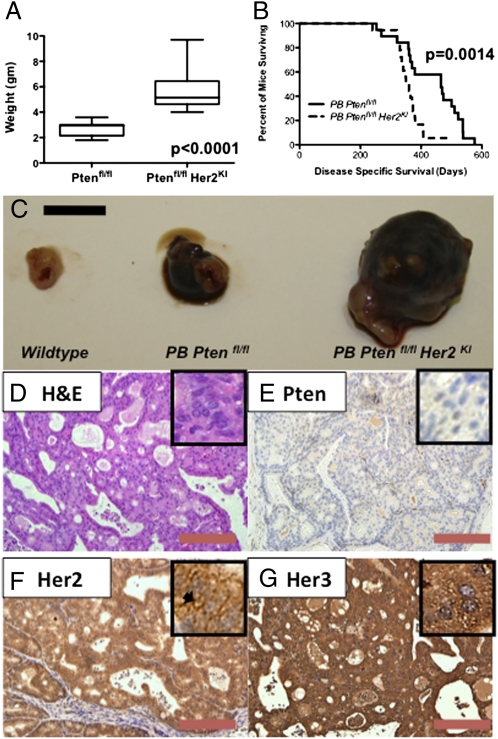
We next crossed the PB-Cre4 with a conditional knockout Pten allele (where exon 5 is flanked by lox p sites) and the Her2KI allele (17). The control PB-Cre4: Ptenfl/fl mice (n = 39) demonstrated a phenotype similar to that previously published, namely high-grade prostatic intraepithelial neoplasia (HG-PIN) at 12 wk with a protracted progression to invasive CaP (>10 mo) and without evidence of metastasis even in mice aged up to 18 mo (Fig. S3) (8). In all cases, tumors demonstrated epithelial loss of Pten (while stromal Pten expression was retained), with no Her2 expression and minimal Her3 expression (Fig. S3). However, the double mutant PB-Cre4: Ptenfl/fl Her2KI (n = 32) sibling cohort of mice developed prostate tumors much faster than the PB-Cre4: Ptenfl/fl mice (median 465 vs. 355 d, P = 0.0014, hazard ratio 3.65, 95% CI 1.65–8.08), and on autopsy the Ptenfl/fl Her2KI tumors were significantly larger in size compared with tumors from the Ptenfl/fl mice (5.2 vs. 2.9 g, P < 0.0001) (Fig. 2 A–C). All tumor samples were subsequently assessed and verified to be prostatic adenocarcinoma by two different consultant uropathologists (M.S. and R.J.B.). Histologically, the double mutant tumors demonstrated a reduction in androgen receptor (AR) expression, with minimal Pten expression and significant up-regulation of both Her2 and Her3 immunoreactivity (Fig. 2 D–G compared with Figs. S3 and S4). We observed similar levels of Her2 up-regulation in these prostate tumors and the mammary tumors developed in the MMTV-NIC Ptenfl/fl mice (Fig. S3N). We also observed discontinuity of a basal cell marker, p63, as well as smooth muscle actin at the circumferences of the malignant glands in the PB-Cre4: Ptenfl/fl Her2KI mice compared with the single mutant (Fig. S4).
Fig. 2.
Histology of PB-Cre4: Ptenfl/fl Her2KI prostate tumors and comparison of tumor weight and overall survival between PB-Cre4: Ptenfl/fl and PB-Cre4: Ptenfl/fl Her2KI cohorts. The boxplot revealed that PB-Cre4: Ptenfl/fl Her2KI prostate tumors are significantly larger than those of PB-Cre4: Ptenfl/fl mice (P < 0.0001) (A). Kaplan–Meier survival curves revealed a significant reduction in survival of PB-Cre4: Ptenfl/fl Her2KI mice compared with PB-Cre4: Ptenfl/fl mice (P < 0.001) (B). Photographs demonstrating that PB-Cre4: Ptenfl/fl Her2KI prostates are significantly larger than those of PB-Cre4: Ptenfl/fl and wild-type (C). H&E (D) and IHC revealing minimal staining of Pten (E), with up-regulation of both Her2 (F) and Her3 (G) in prostate tumors from PB-Cre4: Ptenfl/fl Her2KI. [Scale bars, 1 cm (C), 100 μm (D–G).] Mice were aged-matched at 12 mo of age. Insets represent 40× magnification.
To investigate whether tumor onset was altered in the mice, we intercrossed the PB-Cre4: Ptenfl/fl and PB-Cre4: Ptenfl/fl Her2KI mice to mice carrying the Z/EGFP reporter transgene (18). The Z/EGFP reporter mouse expresses lacZ throughout embryonic development and adult stages. When PB-Cre4 is switched on at puberty it excises the lacZ gene, which allows expression of a second reporter, EGFP. The expression of EGFP occurs in all lobes of the prostate, mirroring the PB-Cre4 expression pattern. IHC for GFP showed EGFP expression in the prostate tumors of these mice (Fig. S5). Using serial whole-body fluorescence imaging (with OV100), we were able to detect tumors in the double mutants over 100 d earlier than in Ptenfl/fl mice (n = 5, P = 0.002) (Fig. S5).
Her2 Activation Overcomes Senescence Induced by Pten Loss in a MAPK-Dependent Manner in Vivo.
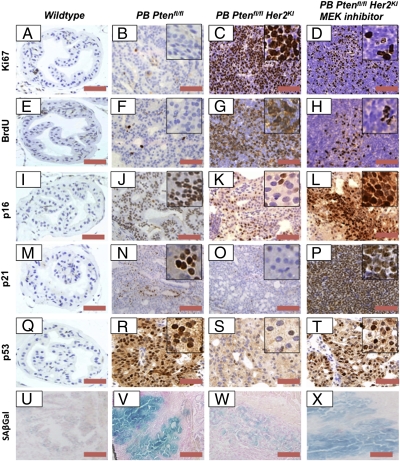
It has previously been demonstrated that senescence plays a key tumor-suppressive role in Pten-deficient prostates, explaining the long tumor latency in this model (8). As we observed acceleration of tumor onset in the double mutant mice, we hypothesized that Her2 activation may modify this pathway. Therefore, we examined markers of proliferation and senescence in prostate tumors derived from PB-Cre4: Ptenfl/fl and PB-Cre4: Ptenfl/fl Her2KI mice. The PB-Cre4: Ptenfl/fl Her2KI tumors had significantly higher levels of proliferation than the PB-Cre4: Ptenfl/fl tumors, as assessed by Ki67 and BrdU (P = 0.0007 and 0.0004, respectively) (Fig. 3 B, C, F, and G and Fig. S6). Furthermore, there was a marked down-regulation of the growth arrest and senescence markers p16 and p21 in the PB-Cre4: Ptenfl/fl Her2KI compared with the PB-Cre4: Ptenfl/fl mice (Fig. 3 J, K, N, and O and Fig. S6). Moreover, there was a robust down-regulation of senescence-associated β-galactosidase (SAβGal) signals in the PB-Cre4: Ptenfl/fl Her2KI prostates compared with PB-Cre4: Ptenfl/fl mice (Fig. 3 V and W). As the double mutant Ptenfl/fl Her2KI prostate tumors showed enhanced HER2 and HER3 levels, we hypothesized that HER2/HER3 heterodimer-mediated signaling promotes prostate carcinogenesis by overcoming PICS. To test this hypothesis, prostate tumor cells from PB-Cre4: Ptenfl/fl mice were treated with heregulin, a Her3 ligand that induces Her2/Her3 dimerization and heterodimer activation. Supporting the notion that HER2/HER3-mediated signaling evades PICS, heregulin treatment significantly decreased senescence in primary cells cultured from PB-Cre4: Ptenfl/fl tumors (Fig. S6F).
Fig. 3.
PB-Cre4: Ptenfl/fl Her2KI prostate tumors demonstrate increased proliferation and loss of their senescent phenotype, and the MEK1/2 inhibitor returns PB-Cre4: Ptenfl/fl Her2KI prostate tumors to a senescent phenotype. We observed increased levels of proliferative markers Ki67 (A–C) and BrdU (E–G) as well as reduced levels of markers of senescence p16 (I–K), p21 (M–O), p53 (Q–S), and SAβGal (U–W) in PB-Cre4: Ptenfl/fl Her2KI prostate tumors relative to both wild-type and PB-Cre4: Ptenfl/fl prostates. When PB-Cre4: Ptenfl/fl Her2KI mice were treated with a MEK1/2 inhibitor for 7 d, we observed a decrease in proliferation as identified by a reduction in Ki67 (D) and BrdU (H) as well as increased levels of senescence markers such as p16 (L), p21 (P), p53 (T), and SAβGal (X). (Scale bars, 100 μm.) Mice were aged-matched at 12 mo of age.
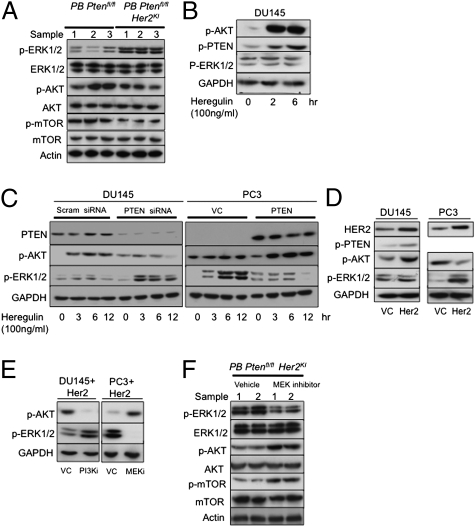
Because PICS is thought to be mediated by mTOR-dependent activation of p53 (19), we studied the activation of AKT, mTOR, S6K, and p53 in double mutant mice. Interestingly, decreased pAKT, p-mTOR, p-S6K, and p53 activation were observed in the Ptenfl/fl Her2KI mice compared with the Ptenfl/fl mice (Figs. 3 R and S and 4A and Figs. S7 and S8). Total levels of AKT and mTOR were unaffected (Fig. 4A and Fig. S8). We also investigated the levels of activated MAPK within PB-Cre4: Ptenfl/fl Her2KI and control PB-Cre4: Ptenfl/fl tumors. Tumors from PB-Cre4: Ptenfl/fl Her2KI mice showed elevated pERK1/2 activation (with total ERK1/2 levels unchanged), whereas PB-Cre4: Ptenfl/fl tumors had very low levels of pERK1/2 (Fig. 4A and Figs. S7 and S8).
Fig. 4.
Reversion to senescent phenotype is associated with mTOR activation. Immunoblot analysis of indicated proteins in lysates extracted from prostate tumors (n = 3) demonstrated increased pERK1/2 and reduction in pAKT and p-mTOR in Ptenfl/fl Her2KI compared with Ptenfl/fl mice, with no differences in levels of total ERK1/2, AKT, and mTOR. (A) DU145 cells treated with heregulin showed increased pAKT and pPTEN. (B) Heregulin-treated DU145 cells transfected with scrambled (control) siRNA and PTEN siRNA and heregulin-treated PC3 cells transfected with vector control or PTEN-expressing plasmid showed reciprocal increases in pERK with respect to PTEN. (C) Her2- and vector control (VC)-transfected DU145 and PC3 cells. (D) LY294002 (10 μM for 12 h)- and PD184352 (10 μM for 12 h)-treated Her2-transfected DU145 and PC3 cells, respectively, showed reciprocal feedback inhibition between the PI3K/AKT and MEK/ERK pathways (E), and prostate tumors of Ptenfl/fl Her2KI mice treated with MEK inhibitor demonstrated an increase in pAKT and p-mTOR levels with a reduction in pERK1/2 expression (n = 2) with no differences in levels of total ERK1/2, AKT, and mTOR (F). Actin and GAPDH were used as loading controls. Tumor lysates were from mice aged-matched at 12 mo of age.
To address the plausible mechanism by which HER2KI selectively activated the MEK/ERK pathway in PB-Cre4: Ptenfl/fl Her2KI mice, DU145 prostate cancer cells were stimulated with heregulin to activate HER2/HER3-mediated signaling. Consistent with previous reports, heregulin treatment in PTEN-proficient DU145 cells enhanced AKT activation, whereas ERK activation was unaltered (Fig. 4B). Heregulin treatment also increased PTEN phosphorylation, which can in turn decrease the ability of PTEN to inhibit PI3K-mediated AKT activation (Fig. 4B) (20). Hence, we hypothesized that HER2/HER3 activation induced sustained activation of PI3K/AKT signaling by inactivating PTEN via phosphorylation. However, in the absence of PTEN, where AKT activation is already high, heregulin would then be able to induce the MAPK pathway. To test this hypothesis, the following cell models were challenged with heregulin: (i) PTEN-proficient DU145 cells in which PTEN had been knocked down, and (ii) PTEN-deficient PC3 cells in which the PTEN wild type was stably reexpressed alongside appropriate vector controls. In contrast to DU145 cells transfected with scrambled siRNA that showed a marked up-regulation of AKT following treatment with heregulin, PTEN knockdown cells showed a sustained increase in ERK activation with minimal AKT activation (Fig. 4C). Consistent with this, vector control PTEN-deficient PC3 cells showed enhanced ERK (but not AKT) activation, whereas PTEN-expressing PC3 cells showed a potent AKT (and not ERK) activation in response to heregulin (Fig. 4C). Overexpression of Her2 in PTEN-proficient DU145 cells increased AKT activation, whereas enhanced ERK activation was observed in Her2-overexpressing PTEN-deficient PC3 cells (Fig. 4D). Moreover, PI3K inhibition by LY294002 increased ERK activation in Her2-overexpressing DU145 cells, whereas treatment with the MEK inhibitor PD184352 in PC3 cells with Her2 overexpression resulted in increased AKT activation. This suggests a strong reciprocal feedback regulation of the PI3K/AKT and MEK/ERK signaling cascades (Fig. 4E).
To confirm the reciprocal feedback regulation in vivo and test whether the observed Her2-mediated ERK activation was suppressing PICS, we treated 12-mo-old PB-Cre4: Ptenfl/fl Her2KI mice that had detectable tumors with either PD184352, a MEK1/2 inhibitor (n = 3), or vehicle (n = 3) (Fig. S7). Treatments were administered for 7 d at 200 mg⋅kg−1⋅d−1 i.p. twice daily. After 7 d, we observed a significant difference in tumor bulk between the two treatment groups (median difference in tumor weight of 1.1 g, P = 0.04) (Fig. S8). Immunohistochemistry for Ki67 and BrdU suggested a reduction in proliferation in PD184352-treated tumors relative to those in vehicle-treated mice (Fig. 3 C and F and Fig. S8; P < 0.002, Mann–Whitney test). Consistent with earlier data from the Lowe laboratory, we observed significant intratumoral neutrophil infiltration, signifying an inflammatory response as a contributing factor in the reduction of tumor size by clearing the senescent cells present (Fig. S9) (21). Interestingly, increased apoptosis was also observed in the MEK1/2 inhibitor-treated cohort (as demonstrated by an increase in cleaved caspase 3 immunoreactivity), which may have also led to decreased tumor burden (Fig. S8). It is worth noting that the staining pattern of vehicle-treated PB-Cre4: Ptenfl/fl Her2KI tumors recapitulated that of PB-Cre4: Ptenfl/fl Her2KI tumors (Fig. 3 and Fig. S9). Moreover, the MEK1/2 inhibitor-treated mice showed up-regulation of SAβGal expression (Fig. 3R) and reactivated expression of p16 and p21 (P < 0.001, Mann–Whitney test; Fig. 3 I and L) compared with vehicle treatment (Fig. S8). This observed restoration of PICS upon MEK1/2 inhibitor treatment may be via reactivation of p-AKT and p-mTOR (Fig. 4F). Interestingly, MEK1/2 inhibitor treatment also decreased elevated levels of HER3 in PB-Cre4: Ptenfl/fl Her2KI tumors (Fig. S7Q). Because AKT activation has been previously linked to down-regulation of HER3, the observed decrease may be due to reactivation of AKT following MEK1/2 inhibition (22).
Taken together, our data suggest that when Her2 is up-regulated it activates the ERK pathway overcoming the PICS-like phenotype (Fig. S10), whereas MEK inhibition restored this PICS-like phenotype to PB-Cre4: Ptenfl/fl Her2KI tumors. Consistent with this, we also observed increased p53 and PI3K signaling within tumors treated with the MEK1/2 inhibitor (Figs. 3L and 4F and Fig. S8). To assess whether we could observe this correlation between HER2 and pERK1/2 in human samples, we stained the same human tissue microarray for pERK1/2 and found a strong correlation between HER2 and pERK1/2 up-regulation independent of PTEN expression (Pearson's correlation coefficient = 0.412, P < 0.0001) (Fig. S9).
Discussion
Prostate cancer is a significant health problem worldwide, being the most common solid organ malignancy among men in North America and second only to lung cancer as a cause of cancer-related death in men. In this study, patients with CaP showing simultaneously reduced PTEN and enhanced HER2/3 expression had a relatively poor survival outcome. Neither factor in isolation altered survival in human CaP. In the mouse, presence of both mutations cooperated to drive prostate carcinogenesis by overcoming growth arrest/senescence induced by PTEN loss. Importantly, treatment with a MEK1/2 inhibitor appeared to negate the effects of activated HER2, restoring the PICS phenotype (Fig. S10).
PTEN ris a tumor suppressor gene traditionally thought to be mutated in ∼30% of primary prostate cancer and 63% of metastatic CaP (6, 7). Recent studies have confirmed the important role of the PI3K pathway in CaP (5). A large-scale sequencing study of 218 prostate cancers found PTEN-inactivating mutations in 4% of primary and 42% of metastatic tumors. When we examined the entire PI3K pathway (including loss of the tumor suppressors PHLPP and INPP4B as well as activation of the PI3KCA gene itself), we found that the PI3K pathway was deregulated in 42% of all primary tumors and 100% of metastases (5). Therefore, within human CaP, deregulation of PI3K signaling appears essential for prostate cancer progression. Murine studies have shown that Pten heterozygosity can act to promote tumor initiation and progression (9). However, complete Pten loss can provoke a p53-dependent cellular senescence program, and thus tumorigenesis is protracted in PB-Cre4: Ptenfl/fl animals, a finding that we confirm in this study (8). Hence, although PTEN haploinsufficiency can initiate CaP, selection for the loss of the remaining PTEN allele will not occur until later in CaP progression, when other signaling pathways are deregulated to bypass PICS (8). From our studies, we postulate that activation of HER2 signaling is one such pathway that overcomes PICS in human cancer, leading to an aggressive CaP phenotype.
HER2 is a transmembrane RTK belonging to the ErbB family that is involved in regulation of cell proliferation and differentiation (23). ErbB receptors form signaling complexes by homo- and heterodimerization of members within this kinase family (23). HER2 is a ligandless receptor that signals via association with other ErbB RTKs, including the receptor HER3. HER3 has no kinase activity, and is thus dependent upon formation of heterodimers with other members of the HER family. The HER2/3 heterodimer potentiates the strongest downstream signal response (24). Up to 15–20% of breast cancers overexpress HER2 (secondary to gene amplification), providing a useful therapeutic target (13, 25). Studies of HER2 in prostate cancer have yielded widely varying rates of immunohistochemical expression (0–100%) and gene amplification (0–53%) (26–40). This diversity is presumably due to differences in immunostaining protocols, cutoffs for fluorescence in situ hybridization, and varying methods of tissue procurement. The current consensus is that HER2 overexpression occurs in up to 20% of CaP in the absence of gene amplification (26). Thus, we used a knock-in of the Her2 allele that has a low level of expression rather than a transgene that conferred high levels of up-regulation (15). The single published study that looked at Her2 activation (using a transgene approach) in the murine prostate demonstrated development of CaP after a long latency with an incomplete penetrance, suggesting the requirement for additional mutations (41). In our clinical cohort, patients with low PTEN expression as well as elevated (membranous or cytoplasmic) levels of HER2/3 expression demonstrated a poor prognosis, indicating that both membranous and cytoplasmic HER2/3 can contribute to disease progression. In agreement with this, we also observed increased membranous and cytoplasmic HER2/3 in our murine Her2 knock-in model. There has been less investigation of the role of Her3 in CaP; however, there is the suggestion that it may be involved in disease progression (24, 38, 42). Our data would again suggest this to be the case, with HER3 overexpression also conferring a poor prognosis in a background of low PTEN expression.
Mechanistically in our study, we suggest that Her2-mediated activation of the MAPK pathways overcomes PICS to drive tumor progression. This interaction appears to be borne out in human CaP, where HER2 overexpression positively correlates with levels of pERK1/2. One interesting observation is that, in the murine tumors following Her2 activation, we also observed a MEK-dependent down-regulation of PI3K signaling. It has been previously demonstrated that PICS is p53-dependent and mediated by AKT-induced activation of mTOR (8). Hence, the decrease in PICS by HER2 activation in our model may be a result of decreased AKT and mTOR activation. Further studies are warranted to elucidate how HER2-mediated activation of ERK decreases PI3K-mediated activation of AKT and mTOR and subsequent p53 activation.
Currently, 11 MEK inhibitors are in clinical trials against a variety of different advanced cancers (43). It is still uncertain which tumors are more likely to respond to MEK1/2 inhibition. Preclinical studies suggest that patients harboring activating mutations in RAS or BRAF genes are better candidates for this treatment (44). Thus, selection of appropriate patient populations based on genetic lesions or validated biochemical markers will be critical for future clinical trial evaluation. Indeed, the complex nature of advanced cancers suggests that MEK1/2 inhibitors will be required in combination with other targeted agents/cytotoxic drugs. An example from preclinical studies has shown that PI3K pathway activation significantly decreases the response of KRAS mutant cancer cells to MEK1/2 inhibitors, and thus simultaneous inhibition of the ERK1/2 and PI3K pathways was required to exert a synergistic effect on tumor regression (45, 46). These observations have provided a strong rationale for the combination of MEK1/2 and PI3K inhibitors in cancers that harbor concurrent activating mutations in these signaling pathways.
It is interesting to note that unlike with breast cancer, Herceptin has not yet yielded clinical benefit in Her2-expressing prostate cancer. Potential reasons for this include (i) the patient cohorts studied were not appropriately chosen for high expression of HER2, (ii) lower levels of HER2 in prostate (compared with breast) tumors may not yield sufficient response to the HER2-targeted therapies, or (iii) one of the key mechanisms of resistance to Herceptin may be PTEN loss (47). Thus, as the aggressive prostate cancers that have Her2/3 overexpression already have low levels of PTEN, this might explain why prostate cancer may be inherently resistant to Herceptin. Our data suggest that stratifying patients for PTEN and HER2 levels may predict responsiveness to MEK/ERK inhibition. Therefore, given that high HER2 levels and low levels of PTEN confer a poor prognosis, MEK inhibitors could be clinically efficacious in this patient group.
Materials and Methods
Mice.
ARR2Probasin-Cre (PB-Cre4) mice (48) were intercrossed with mice harboring Her2KI (15), Ptenfl/fl (17), and Z/EGFP (18) in combinations as described below. Further information is given in SI Materials and Methods.
Mice were genotyped by PCR by Transnetyx (15, 17, 18, 48). Mice of a mixed background and littermates were used as control mice. Prostates were placed in formalin for overnight fixation before being embedded in paraffin. All prostates were processed according to our protocol (49), except for SAβGal staining, for which tissues were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek) and stored at −80 °C.
MEK Inhibitor Treatment.
PD184352 (200 mg/kg i.p. twice daily; Selleck Chemicals) or vehicle treatment (10% DMSO, 10% Cremophor (Sigma; 80% water) was given for 7 d.
Immunohistochemistry.
IHC was performed on formalin-fixed, paraffin-embedded (FFPE) samples as previously described (details in SI Materials and Methods).
Microscopy.
Light microscopy was carried out using the Olympus BX51. For GFP in vivo imaging, we used the Olympus OV100 whole-body imaging system as we have previously published (49).
Ultrasound Analysis.
Ultrasound analysis was performed on live mice using a Vevo 770 system (VisualSonics) (49).
Human Tissue Microarray.
We studied FFPE sections from 239 CaP patients. These consisted of 209 primary CaP and 30 BPH samples. Further information is given in SI Materials and Methods.
Immunoblotting.
Immunoblotting was performed as previously described. Further information is given in SI Materials and Methods.
Statistics.
All statistics (Mann–Whitney, Pearson's correlation coefficient, Cox regression analysis, and Kaplan–Meier survival analysis) were performed using Minitab version 15.
Supplementary Material
Acknowledgments
We thank Beatson Institute for Cancer Research services, biological services unit, the Beatson histology department, and the ‘Think Pink’ charity for the purchase of the Aperio slide scanner and the Slidepath software. This work was funded by Cancer Research UK and a Medical Research Council fellowship to I.A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101263108/-/DCSupplemental.
References
- 1.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, et al. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, et al. PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- 7.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 10.Ding Z, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abate-Shen C, et al. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- 12.Carver BS, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saal LH, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrechek ER, et al. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci USA. 2000;97:3444–3449. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schade B, et al. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J Biol Chem. 2009;284:19018–19026. doi: 10.1074/jbc.M109.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 18.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 19.Alimonti A, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross AH, Gericke A. Phosphorylation keeps PTEN phosphatase closed for business. Proc Natl Acad Sci USA. 2009;106:1297–1298. doi: 10.1073/pnas.0812473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandarlapaty S, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 24.Koumakpayi IH, et al. Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res. 2006;12:2730–2737. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]
- 25.Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26:6577–6592. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minner S, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez KM, et al. Evaluation of HER-2/neu expression in prostatic adenocarcinoma: A requested for a standardized, organ specific methodology. Cancer. 2002;95:1650–1655. doi: 10.1002/cncr.10839. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim GK, et al. Differential immunoreactivity of her-2/neu oncoprotein in prostatic tissues. Surg Oncol. 1992;1:151–155. doi: 10.1016/0960-7404(92)90028-j. [DOI] [PubMed] [Google Scholar]
- 29.Ware JL, Maygarden SJ, Koontz WW, Jr, Strom SC. Immunohistochemical detection of c-erbB-2 protein in human benign and neoplastic prostate. Hum Pathol. 1991;22:254–258. doi: 10.1016/0046-8177(91)90159-m. [DOI] [PubMed] [Google Scholar]
- 30.Mellon K, et al. p53, c-erbB-2 and the epidermal growth factor receptor in the benign and malignant prostate. J Urol. 1992;147:496–499. doi: 10.1016/s0022-5347(17)37287-7. [DOI] [PubMed] [Google Scholar]
- 31.Visakorpi T, Kallioniemi OP, Koivula T, Harvey J, Isola J. Expression of epidermal growth factor receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas. Mod Pathol. 1992;5:643–648. [PubMed] [Google Scholar]
- 32.Kuhn EJ, Kurnot RA, Sesterhenn IA, Chang EH, Moul JW. Expression of the c-erbB-2 (HER-2/neu) oncoprotein in human prostatic carcinoma. J Urol. 1993;150:1427–1433. doi: 10.1016/s0022-5347(17)35799-3. [DOI] [PubMed] [Google Scholar]
- 33.Ross JS, et al. Contribution of HER-2/neu oncogene expression to tumor grade and DNA content analysis in the prediction of prostatic carcinoma metastasis. Cancer. 1993;72:3020–3028. doi: 10.1002/1097-0142(19931115)72:10<3020::aid-cncr2820721026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Gu K, Mes-Masson AM, Gauthier J, Saad F. Overexpression of her-2/neu in human prostate cancer and benign hyperplasia. Cancer Lett. 1996;99:185–189. doi: 10.1016/0304-3835(95)04061-7. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, et al. Prognostic significance of HER-2/neu gene amplification status by fluorescence in situ hybridization of prostate carcinoma. Cancer. 1997;79:2162–2170. doi: 10.1002/(sici)1097-0142(19970601)79:11<2162::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Reese DM, et al. HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am J Clin Pathol. 2001;116:234–239. doi: 10.1309/VXKK-YVRH-9B11-YDPT. [DOI] [PubMed] [Google Scholar]
- 37.Jorda M, et al. Her2 expression in prostatic cancer: A comparison with mammary carcinoma. J Urol. 2002;168:1412–1414. doi: 10.1097/01.ju.0000030159.27410.38. [DOI] [PubMed] [Google Scholar]
- 38.Edwards J, et al. The role of HER1–HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin Cancer Res. 2006;12:123–130. doi: 10.1158/1078-0432.CCR-05-1445. [DOI] [PubMed] [Google Scholar]
- 39.Edwards J, et al. HER2 and COX2 expression in human prostate cancer. Eur J Cancer. 2004;40:50–55. doi: 10.1016/j.ejca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Montironi R, et al. HER2 expression and gene amplification in pT2a Gleason score 6 prostate cancer incidentally detected in cystoprostatectomies: Comparison with clinically detected androgen-dependent and androgen-independent cancer. Hum Pathol. 2006;37:1137–1144. doi: 10.1016/j.humpath.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Szabolcs M, Terwilliger JD, Efstratiadis A. Prostatic intraepithelial neoplasia and adenocarcinoma in mice expressing a probasin-Neu oncogenic transgene. Carcinogenesis. 2006;27:1054–1067. doi: 10.1093/carcin/bgi324. [DOI] [PubMed] [Google Scholar]
- 42.Mellinghoff IK, et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 43.Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji H, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–4939. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 45.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wee S, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- 47.Nagata Y, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad I, et al. β-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene. 2011;30:178–189. doi: 10.1038/onc.2010.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.