Abstract
Morphine is a highly potent analgesic with high addictive potential in specific contexts. Although dopamine neurons of the ventral tegmental area (VTA) are widely believed to play an essential role in the development of drug addiction, neuronal circuits underlying morphine action on dopamine neurons have not been fully elucidated. Here we combined in vivo electrophysiology, tract-tracing experiments, and targeted neuronal inactivation to dissect a neural circuit for acute morphine action on dopamine neurons in rats. We found that in vivo, morphine targets the GABAergic tail of the VTA, also called the rostromedial tegmental nucleus, to increase the firing of dopamine neurons through the activation of VTA μ opioid receptors expressed on tail of the VTA/rostromedial tegmental nucleus efferents. Our data also reveal that in the absence of VTA glutamatergic tone, there is no morphine-induced activation of dopamine neurons. These results define the anatomical organization and functional role of a neural circuit for acute morphine action on dopamine neurons.
Keywords: glutamate, NMDA, AMPA, GABAA, in vivo recordings
Dopamine (DA) neurons within the ventral tegmental area (VTA) play a central role in motivated behaviors (1, 2), natural reward processing (3, 4), and development of drug addiction (5, 6). Addictive properties of opiates (e.g., morphine, heroin) rely on μ opioid receptor (μOR) activation (7) and are probably associated with their ability to activate VTA-DA neurons (8). γ-Aminobutyric acid (GABA) transmission in the VTA is critical for morphine-induced activation of DA neurons (9). Indeed, a broadly accepted circuit model for morphine action on DA neurons suggests that morphine excites VTA-DA neurons by a disinhibitory mechanism involving neighboring GABA neurons (10).
A newly defined structure with dense μOR immunoreactivity (11), the tail of the VTA (tVTA), also called the rostromedial tegmental nucleus (RMTg), has recently been proposed to be a potential inhibitory control center for dopaminergic activity (12–14). However, it remains unclear whether μOR activation on tVTA/RMTg GABA neurons can account for morphine-induced activation of VTA-DA neurons.
In addition to potent tonic GABAergic modulation (15), VTA-DA neurons receive glutamatergic inputs from diverse brain nuclei (16) and from VTA glutamatergic neurons (17). One role of this glutamatergic influence is to mediate a switch from pacemaker to high-frequency bursts of activity, leading to a large increase in DA release in target areas (18, 19). Glutamate transmission is a key component of the regulation of dopamine cells and is known to play an important role in the actions of many drugs of abuse (1, 5). In the VTA, the persistent potentiation of DA neuron excitatory synapses contributes to pathological drug-seeking behavior (5). Furthermore, several lines of evidence also indicate a critical role for VTA glutamate receptors in morphine rewarding properties (20–23), but the crucial role of VTA glutamatergic transmission for in vivo effects of morphine on DA neurons has not been demonstrated.
In the present study, we used tract-tracing approaches and in vivo electrophysiological recordings to demonstrate that morphine-induced excitation of VTA-DA neurons results from an inhibition of the tVTA/RMTg GABAergic tone and is dependent on NMDA/AMPA receptor activation within the VTA.
Results
Morphine-Induced Activation of VTA-DA Neurons Is Mediated by μ Opioid Receptors Expressed on tVTA/RMTg Efferents.
The majority of the afferents to DA neurons are GABAergic and inhibitory (24), and it has been demonstrated in ex vivo preparations that GABAergic inputs control the firing pattern of DA neurons (25). We recorded extracellular activity from single VTA-DA neurons identified according to their electrophysiological properties (SI Materials and Methods) and their neurochemical identity through single-cell labeling and immunohistochemistry (Fig. S1 and Fig. 1 D–F). Here we show that picrotoxin (GABAA receptor antagonist) microinfusion within the VTA produced an increase in firing rate (+59.3%) and bursting activity (+123.4%) of VTA-DA neurons (Fig. S2 A–C). These results confirmed in vivo that the firing pattern of VTA-DA neurons is tonically controlled by GABAergic inputs (25, 26). Previous work demonstrated that the tVTA/RMTg, a GABAergic structure, projects mainly onto VTA-DA neurons (12, 13). A recent in vivo electrophysiological study showed that tVTA/RMTg stimulation was able to phasically silence VTA-DA neurons (14). To examine the impact of tVTA/RMTg activity on tonic VTA-DA neurons in vivo, we selectively inhibited tVTA/RMTg neurons using microinjection of a labeled GABAA receptor agonist (muscimol-bodipy). Like picrotoxin infusion within VTA, tVTA/RMTg inactivation produced a marked increase in firing rate (+34%) and bursting rate (+67%), but also in the number of spikes per burst (+16%) of VTA-DA neurons (Fig. S2 D–F). In addition to a phasic influence of the tVTA/RMTg onto VTA-DA neurons (14), our results show that VTA-DA neurons are under tonic inhibitory control originating in the tVTA/RMTg.
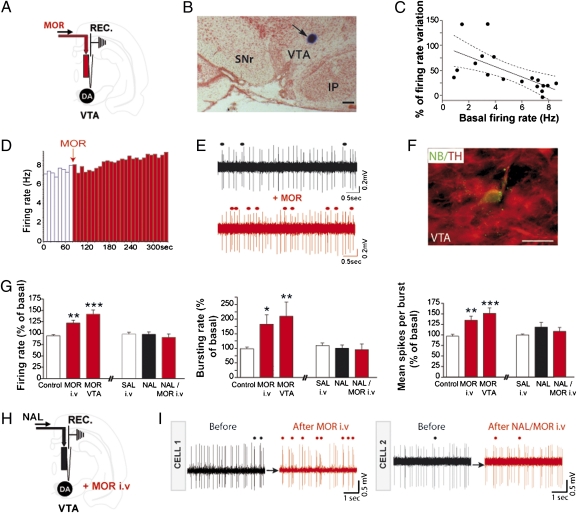
Fig. 1.
Morphine-induced excitatory effects on VTA-DA neurons involve intra-VTA μORs. (A) Experimental protocol. (B) Microphotograph of a coronal section through the VTA showing the recording site (blue spot at arrow). IP, interpeduncular nucleus; SNr, substantia nigra pars reticulata. (Scale bar, 250 μm.) (C) Firing rate in response to intra-VTA morphine as a function of the basal activity of VTA-DA neurons. There is a correlation between the variation of VTA-DA neuron-firing activity by morphine and the basal firing rate of VTA-DA neurons. Pearson's correlation calculation; Pearson's r = −0.65, P < 0.01. Solid line, regression curve; area between dotted lines, 95% confidence intervals; n = 18. (D–F) Example of VTA-DA neuron activity following an intra-VTA infusion of morphine (1 mg/mL, 60 nL). This neuron, juxtacellularly injected with neurobiotin (NB), was immunohistochemically identified as dopaminergic (TH; tyrosine hydroxylase). Individual channels for TH and NB are shown in Fig. S1. (Scale bar, 40 μm.) (E) Representative traces before and after intra-VTA morphine infusion. Dots indicate burst occurrences. (G) Analysis of firing rate, bursting activity, and mean spikes per burst (i) after control conditions (saline i.v. condition pooled with intra-VTA artificial cerebrospinal fluid (aCSF) condition because of the lack of significant difference), after i.v. injection of morphine, and after intra-VTA morphine ejection, and (ii) after i.v. injection of saline, after intra-VTA naltrexone injection, and after i.v. morphine injection following naltrexone injection within the VTA. One-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.005. Statistical analysis is shown in SI Materials and Methods. (H) Experimental protocol. (I) Representative traces of two VTA-DA neurons before and after i.v. morphine injection (cell 1) or after i.v. morphine injection following an intra-VTA injection of naltrexone (cell 2). Circles above traces represent burst occurrence.
Opiates have powerful reinforcing actions within the VTA (27) and activate VTA-DA neurons through disinhibition processes (10). We examined in vivo the role of the VTA μOR in morphine-induced activation of VTA-DA neurons. We confirmed that systemic morphine increased the impulse activity, bursting rate, and number of spikes per burst of VTA-DA neurons (Fig. 1G) (28), and we observed similar effects with morphine delivery directly into the VTA (+47% for firing rate, +111% for bursting activity, and +54% for the number of spikes per burst) (Fig. 1). In line with the disinhibition model (10), we show that the magnitude of the morphine-induced activation of VTA-DA neurons was inversely correlated to their basal firing activity (Fig. 1C). A typical example of an individually recorded DA neuron labeled with neurobiotin and showing an increase in firing rate and busting activity after intra-VTA morphine is reported in Fig. 1 D–F. Furthermore, systemic morphine-induced activation of VTA-DA neurons was blocked by naltrexone infusion, an OR antagonist, within the VTA (Fig. 1 H and I). Altogether, these experiments indicate that targeting VTA μORs is necessary for an in vivo excitatory effect of morphine on VTA-DA neurons.
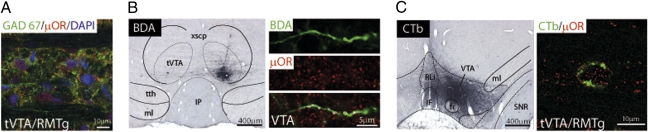
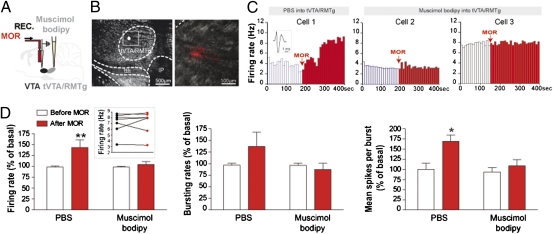
Previous studies showed that tVTA/RMTg heavily projects to the VTA (11) and that it is a highly immunoreactive structure for μORs (11). Thus, we investigated whether tVTA/RMTg GABA neurons projecting to the VTA contain μORs. μOR immunoreactivity was detected in the majority of GABAergic neurons of the tVTA/RMTg (Fig. 2A and Fig. S3). We traced tVTA/RMTg-VTA projection by injecting the anterograde tracer biotinylated dextran amine into the tVTA/RMTg (Fig. 2B) or the retrograde tracer cholera toxin B subunit into the VTA (Fig. 2C). μOR immunoreactivity was detected within tVTA/RMTg anterogradely labeled axons in the VTA (Fig. 2B) and within 76% of retrogradely labeled cell bodies in the tVTA/RMTg (712/930 cells analyzed, n = 5 rats) (Fig. 2C). Supporting these anatomical data and consistent with a previous study (14), local infusion of morphine into the tVTA/RMTg decreased the impulse activity of tVTA/RMTg neurons (−61%; Fig. S4 D and E). Morphine was applied onto 6 of the 32 tVTA/RMTg recorded neurons. As previously shown (14), excitatory response at a short latency (<8 ms) evoked by lateral habenula (LHb) stimulation was used to identify tVTA/RMTg neurons (Fig. S4 A–C). VTA-DA and tVTA/RMTg neurons thus display an opposite response to morphine (Fig. S4F), consistent with the hypothesis that an inhibition of tVTA/RMTg neurons by morphine may contribute to VTA-DA neuron excitation. During the recording of tVTA/RMTg neurons (Fig. S5), we established their electrophysiological signatures. The mean firing rate of recorded tVTA/RMTg neurons was 18.2 ± 3 Hz, the mean of interspike intervals was 73 ± 26 ms, and the mean duration of their action potential measured from peak to trough was 1 ± 0.03 ms, which is consistent with previous studies (12, 14). The basal firing rate of tVTA/RMTg neurons ranged from 1 to 60 Hz. Furthermore, histological controls confirmed the tVTA/RMTg location of all recorded neurons (Fig. S5 C and D). Finally, to directly address the contribution of tVTA/RMTg neurons in morphine-induced activation of VTA-DA neurons, we locally infused morphine onto VTA-DA neurons after selective inactivation of the tVTA/RMTg (Fig. 3 A and B). In this case, morphine failed to increase VTA-DA neuron activity (Fig. 3 C and D). Thus, morphine excitation of VTA-DA neurons in vivo requires the activity of GABAergic tVTA/RMTg neurons.
Fig. 2.
tVTA/RMTg efferents in the VTA express μORs. (A) Microphotographs illustrating the GAD67 and μOR double labeling in the tVTA/RMTg at a cellular level. GAD67, glutamic acid decarboxylase 67 kDa. (B) Following BDA injection into tVTA/RMTg (Left), μORs were detected within anterogradely labeled axons in the VTA (Right). (C) Following CTb injection into VTA (Left), μORs were observed in retrogradely labeled cell bodies in the tVTA/RMTg (Right). fr, fasciculus retroflexus; IF, interfascicular nucleus; ml, medial lemniscus; RLi, rostral linear nucleus of the raphe; tth, trigeminothalamic tract; xscp, superior cerebellar peduncle decussation.
Fig. 3.
tVTA/RMTg inactivation prevents morphine-induced excitatory effect on VTA-DA neurons. (A) Experimental protocol. (B) Dark-field images showing a muscimol-bodipy injection within tVTA/RMTg (white spot). (C) Examples of VTA-DA neuron activities following an intra-VTA infusion of morphine, after PBS (cell 1) or muscimol-bodipy infusion (cells 2 and 3) into the tVTA/RMTg. (Inset) A spike of a representative VTA-DA neuron. (D) After tVTA/RMTg inactivation, morphine failed to increase VTA-DA neuron activity. Graphs represent mean ± SEM. *P < 0.05, **P < 0.01. (Inset) The firing rate of VTA-DA neurons before (black dots) and after morphine injection (red dots) following the inactivation of the tVTA/RMTg. Statistical analysis is shown in SI Materials and Methods.
Mesencephalic Glutamatergic Transmission Is Required for the in Vivo Excitatory Effect of Morphine.
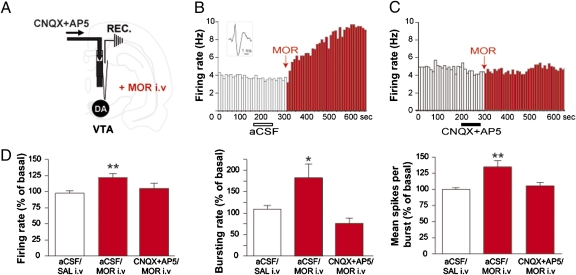
tVTA/RMTg tonic activity appears important for morphine-induced responses of VTA-DA neurons. Does this result preclude a role for VTA glutamatergic transmission in the morphine effect? To address the implication of glutamatergic transmission in morphine-induced activation of VTA-DA neurons, we explored whether blocking ionotropic glutamate receptors in the VTA (Fig. 4A) modulates the DA excitatory properties of morphine. In the VTA, NMDA and AMPA receptor activation was found to be necessary for morphine-conditioned place preference (21). In our study, blocking VTA NMDA and AMPA receptors produced a small but nonsignificant decrease in bursting activity (bursting rate, −21%; number of spikes per burst, −7%; n = 12). Whereas a systemic injection of morphine increased the firing and bursting activity of VTA-DA neurons (Fig. 4 B and D), an intra-VTA infusion of both AMPA and NMDA receptor antagonists prevented morphine-induced excitation of VTA-DA neurons (Fig. 4 C and D). These results suggest that VTA glutamatergic transmission is crucial for the in vivo excitatory effect of morphine.
Fig. 4.
Morphine activation of VTA-DA neurons depends on VTA glutamatergic tone. (A) Experimental protocol. (B and C) Example of systemic morphine effect on VTA-DA neurons following intra-VTA infusion of CNQX (6-cyano-7-nitroquinoxaline-2,3-dione)+AP5 [(2R)-amino-5-phosphonovaleric acid] or aCSF. (B) (Inset) A spike of a representative VTA-DA neuron. (D) Blockade of intra-VTA ionotropic glutamate receptors prevented systemic morphine-induced activation of VTA-DA neurons. Graphs represent mean ± SEM. *P < 0.05, **P < 0.01. Statistical analysis is shown in SI Materials and Methods.
Discussion
In line with the disinhibition model (10), our work reveals a neural circuitry for morphine action on VTA-DA neurons. We provide in vivo electrophysiological evidence showing that acute morphine targets the GABAergic tVTA/RMTg, disrupting the balance between inhibition and excitation on VTA-DA neurons. We show that the inhibition of tVTA/RMTg GABAergic transmission cannot be responsible alone for morphine excitatory effects on VTA-DA neurons, and that another component, VTA glutamatergic transmission, is required.
In this study, we confirmed anatomical data showing that VTA is under an important GABAergic input coming from the tVTA/RMTg (12, 13). We propose that the role of tVTA/RMTg may not be simply to suppress the spiking of single VTA-DA neurons (14) but also to change the pattern of activity of a large assembly of VTA-DA neurons leading to a new steady-state level of tonic activity (Fig. S2 D–F). Thus, inhibition of the tVTA/RMTg GABAergic tone by morphine could play a fundamental role in the synchrony of dopamine neurons to efficiently reset their tonic properties and thus facilitate the processing of learning signals (27). Our work shows that (i) intra-VTA infusion of morphine activates VTA-DA neurons, (ii) systemic morphine-induced activation of VTA-DA neurons is prevented by intra-VTA naltrexone, and (iii) inhibiting the tVTA/RMTg blocks the excitation of VTA-DA neurons by morphine. On this basis, we propose that morphine increases firing of VTA-DA neurons through the activation of VTA μORs expressed on afferents from the tVTA/RMTg. Another interpretation of the failure of morphine to excite VTA-DA neurons following tVTA/RMTg inactivation could be that VTA-DA neurons have reached their limits of discharge. However, this occlusion of response seems unlikely because (i) high-firing VTA-DA neurons (7–8 Hz) still show an increase in their activity after intra-VTA morphine (Fig. 1 C and D and Fig. S4F) and (ii) morphine also fails to increase the activity of slow-firing VTA-DA neurons after tVTA/RMTg inactivation (cell 2 in Fig. 3C).
The unique inhibitory neuronal pathway proposed for acute morphine's action on VTA-DA neurons is fully consistent with the disinhibition model proposed by Johnson and North (10). In fact, the in vitro recordings of this pioneer study have been performed in midbrain horizontal slices that most probably contain a significant amount of tVTA/RMTg neurons and the majority of the tVTA/RMTg terminal field. Moreover, the rostral-most third of the tVTA/RMTg is in fact embedded within the posterior VTA (29). So, an alternate interpretation of the Johnson and North study would be that synaptic mechanisms previously attributed to VTA GABA-containing neurons may be mediated by efferents from tVTA/RMTg. VTA non-DA neurons are clearly hyperpolarized and inhibited by morphine (Fig. S6) (10). However, despite a moderate density of intrinsic GABAergic synaptic connections onto VTA-DA neurons (30), the physiological influence of local VTA-GABA neurons onto VTA-DA neurons is not evident. Three strong anatomical observations suggest a weak influence of VTA-GABA neurons on VTA-DA neuron activity. First, VTA-GABA neurons preferentially make synapses onto dendritic shafts of VTA-DA neurons (30), suggesting a preferential role in fine-tuning excitability rather than a drastic influence on spiking. Second, the close contacts of tVTA/RMTg terminals are on soma and proximal dendrites of VTA-DA neurons, confirming the strong influence of the tVTA/RMTg on VTA-DA neuron activity (Fig. S2 D–F) (14). Finally, in comparison with the other GABAergic sources of the VTA, GABA efferents from the tVTA/RMTg target VTA-DA neurons with a higher specificity (31). Moreover, a recent study using highly specific optogenetic stimulation demonstrated that nucleus accumbens GABA neurons, which are inhibited by a μOR agonist, target VTA GABA-containing neurons but not VTA-DA neurons (32). This evidence supports our hypothesis that morphine increases the firing of VTA-DA neurons through the activation of VTA μORs expressed on tVTA/RMTg efferents and not on VTA GABA-containing neurons or nucleus accumbens efferents.
An important and somewhat unexpected finding from the present study is that blocking glutamatergic signaling in the VTA suppresses VTA-DA neuron excitation by morphine. This is consistent with studies reporting a critical role for VTA glutamate receptors in morphine rewarding properties (20–23). For instance, applying CNQX and AP5 within the VTA blocks the ability of morphine to establish conditioned place preference (21). In summary, morphine acts by disrupting the inhibition/excitation balance onto VTA-DA neurons. Indeed, our findings strongly indicate that morphine-induced excitation of VTA-DA resulted from simultaneous cessation of GABA receptor activation and NMDA/AMPA receptor activation. These inputs may thus work in series rather than in parallel, both inputs being necessary for morphine activation of VTA-DA neurons.
A common feature of all addictive drugs as well as stress is that they trigger adaptive synaptic plasticity in the VTA (33, 34). A single cocaine exposure, known to trigger synaptic plasticity in excitatory afferents onto VTA-DA neurons (34), also enhances opioid-induced affective responses through a circuit involving the VTA (35). Thus, in light of the present findings, it is tempting to propose that the amplitude of morphine's response on VTA-DA neurons may depend on the internal excitatory state of VTA-DA neurons. It will be important in future studies to test this idea by examining whether the excitatory context of dopamine neurons tunes the addictive potency of morphine.
Materials and Methods
Drugs.
Systemic drug injection.
Morphine hydrochloride (1 mg/kg) was administered intravenously.
Local drug microinfusion.
Double-barrel pipettes were used to infuse drugs during recordings (36). The microejected drugs were morphine hydrochloride (1 mg/mL), naltrexone (100 μM), picrotoxin (1 mM), and a mixture of 100 μM AP5 and 50 μM CNQX. See SI Materials and Methods for details.
VTA and tVTA/RMTg Recordings.
Stereotaxic surgeries were performed under halothane anesthesia. A glass micropipette was lowered into the VTA or tVTA/RMTg. Extracellular single-unit recordings were performed as described previously (36). See SI Materials and Methods for details.
tVTA/RMTg Inactivation.
An injection pipette was filled with GABAA agonist ejected in the tVTA/RMTg. See SI Materials and Methods for details.
Electrical Stimulation of the LHb.
As previously shown (14), excitatory response evoked by LHb stimulation was used to identify tVTA/RMTg neurons. See SI Materials and Methods for details.
Tract Tracing.
The anterograde tracer biotinylated dextran amine (BDA) and the retrograde tracer cholera toxin B subunit (CTb) were iontophoretically delivered as previously described (29). See SI Materials and Methods for details.
Histochemistry.
Coronal sections were incubated using appropriate antibodies and processed for immunocytochemistry as previously described (29). See SI Materials and Methods for details.
Supplementary Material
Acknowledgments
We thank Dr. C. Herry for helpful discussions and comments and Lesley Graham for editorial assistance. This work was supported by Institut National de la Santé et de la Recherche Médicale, Région Aquitaine, Centre National de la Recherche Scientifique, and Université de Strasbourg.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105418108/-/DCSupplemental.
References
- 1.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 2.Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen BT, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak GW, editor. The Opiate Receptors. Totowa, NJ: Humana; 2011. [Google Scholar]
- 8.Wise RA. Opiate reward: Sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- 9.Madhavan A, Bonci A, Whistler JL. Opioid-induced GABA potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci. 2010;30:14029–14035. doi: 10.1523/JNEUROSCI.3366-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. γ-Aminobutyric acid cells with cocaine-induced ΔFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiatry. 2010;67:88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Lecca S, et al. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 19.Zweifel LS, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlezon WA, Jr, et al. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- 21.Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Popik P, Kolasiewicz W. Mesolimbic NMDA receptors are implicated in the expression of conditioned morphine reward. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:288–294. doi: 10.1007/pl00005354. [DOI] [PubMed] [Google Scholar]
- 23.Xi ZX, Stein EA. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 2002;164:144–150. doi: 10.1007/s00213-002-1190-3. [DOI] [PubMed] [Google Scholar]
- 24.Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: Modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- 25.Lobb CJ, Wilson CJ, Paladini CA. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. J Neurophysiol. 2010;104:403–413. doi: 10.1152/jn.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- 27.Rompré PP, Wise RA. Opioid-neuroleptic interaction in brainstem self-stimulation. Brain Res. 1989;477:144–151. doi: 10.1016/0006-8993(89)91401-7. [DOI] [PubMed] [Google Scholar]
- 28.Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 30.Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Y, et al. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 34.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 35.Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci USA. 2004;101:5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: A novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.