Abstract
Juvenile male rhesus monkeys treated with methylphenidate hydrochloride (MPH) to evaluate genetic and behavioral toxicity were observed after 14 mo of treatment to have delayed pubertal progression with impaired testicular descent and reduced testicular volume. Further evaluation of animals dosed orally twice a day with (i) 0.5 mL/kg of vehicle (n = 10), (ii) 0.15 mg/kg of MPH increased to 2.5 mg/kg (low dose, n = 10), or (iii) 1.5 mg/kg of MPH increased to 12.5 mg/kg (high dose, n = 10) for a total of 40 mo revealed that testicular volume was significantly reduced (P < 0.05) at months 15 to 19 and month 27. Testicular descent was significantly delayed (P < 0.05) in the high-dose group. Significantly lower serum testosterone levels were detected in both the low- (P = 0.0017) and high-dose (P = 0.0011) animals through month 33 of treatment. Although serum inhibin B levels were increased overall in low-dose animals (P = 0.0328), differences between groups disappeared by the end of the study. Our findings indicate that MPH administration, beginning before puberty, and which produced clinically relevant blood levels of the drug, impaired pubertal testicular development until ∼5 y of age. It was not possible to resolve whether MPH delayed the initiation of the onset of puberty or reduced the early tempo of the developmental process. Regardless, deficits in testicular volume and hormone secretion disappeared over the 40-mo observation period, suggesting that the impact of MPH on puberty is not permanent.
Keywords: attention deficit hyperactivity disorder, developmental delay, male puberty
During studies in the rhesus monkey to address the genetic and behavioral toxicity of methylphenidate hydrochloride (MPH) in the nonhuman primate (NHP) (1), it was noted that testicular growth was retarded and testicular descent was delayed in animals treated with a high dose of MPH. As in boys, an acceleration of testicular growth in the rhesus monkey, which generally occurs between 3 and 4 y of age, is a reliable somatic marker of the initiation of puberty (2, 3). Puberty in both man and the monkey is triggered by the reemergence of a robust pattern of pulsatile hypothalamic gonadotropin-releasing hormone (GnRH) release that activates the pituitary-gonadal axis at the end of the juvenile phase of development (2, 3). Interestingly, in higher primates the GnRH drive to pituitary gonadotropin secretion is also observed during infancy, but throughout juvenile development (and during childhood in man) this mode of hypothalamic GnRH release is held in check resulting in a relatively hypogonadotropic state that guarantees the quiescence of the prepubertal primate gonad (2, 3). Thus, the rhesus macaque is a particularly good model for human puberty.
Potential modulators of the pubertal process, such as MPH, may be conceptualized to influence either the timing of the onset of puberty or the tempo at which this developmental event unfolds once initiated. To examine this issue in the monkey, the findings on the effect of MPH on the reproductive axis observed in the study of genetic safety (1), were extended at 14 mo of treatment by initiating monthly measurements of pubertal development (testicular volume and descent, plasma hormone concentrations, and semen parameters) for up to an additional 27 mo.
Results
Body Weight, Crown-Rump Length, and Plasma MPH Levels.
Although body weight increased (P < 0.0001) over the treatment period, no significant effect of MPH was detected (SI Results). Crown-rump length (used as an indicator of linear growth) increased significantly (P < 0.0001), but no effect of dose was detected. Initial MPH doses did not produce expected plasma concentrations, and after 4 mo of treatment, doses were increased in the low-dose groups to achieve mean plasma levels of MPH within the clinical range observed in humans (4.05–14.03 ng/mL) (Fig. S1, Upper). Similarly, the dose of the high-dose group was increased to produce a concentration 5- to 10-fold higher than clinically observed, ranging from 28.75 to 270.85 ng/mL (Fig. S1, Lower). The high-dose group was included to ensure a broad dose-range for the genetic and behavioral toxicity studies (1).
Endocrine Analyses.
Testosterone.
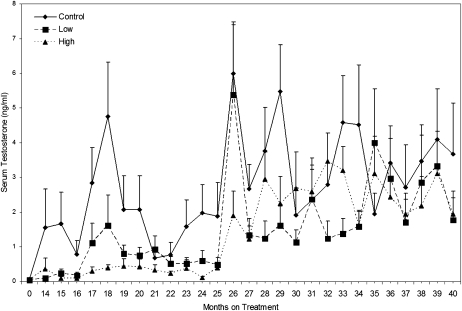
Circulating morning testosterone levels increased during the 40-mo treatment period in all groups, but the pubertal rise in this testicular steroid was delayed in the MPH-treated groups (Fig. 1). Significantly lower testosterone levels were observed in both low-dose (P = 0.0017) and high-dose (P = 0.0011) groups compared with those in the control group. When analyzed on a monthly basis, testosterone levels were significantly reduced (P < 0.05) in the low-dose group at treatment months 29 and 33, and in the high-dose group at months 17, 18, 24, and 29. However, after treatment month 35, morning testosterone levels in the two treatment groups were not significantly different from the control group.
Fig. 1.
Effect of dose and length of treatment on mean serum testosterone concentrations (± SEM) in peripubertal male rhesus monkeys chronically exposed to MPH. Levels increased (P < 0.0001) with age. Relative to controls, testosterone levels were lower in the low-dose, (P = 0.0017) and high-dose (P = 0.0011) groups.
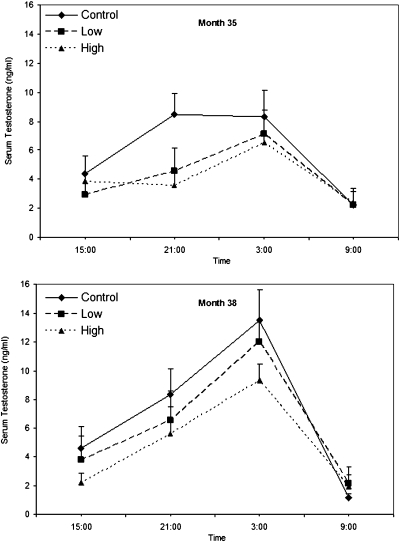
The diurnal pattern of serum testosterone levels, as evidenced by a significant time-of-day effect, was assessed in month 35 (Fig. 2, Upper) (P = 0.0002) and month 38 (Fig. 2, Lower) (P < 0.0001) when the animals were ∼60 to 63 and 66 to 69 mo old, respectively. At month 35, testosterone levels were lower in the high-dose group than in the control group (P = 0.059) at 2100 hours.
Fig. 2.
Effect of dose and time of day on mean serum testosterone concentrations (± SEM) in male rhesus monkeys chronically exposed to MPH. Samples were collected at 1500, 2100, 0300, and 0900 hours. At month 35 (Upper), a time-of-day effect was detected (P = 0.0002), and serum testosterone levels were lower in the high-dose group than in controls at 2100 hours (P = 0.059). No significant differences were found at month 38 (Lower) at any time point.
Inhibin B.
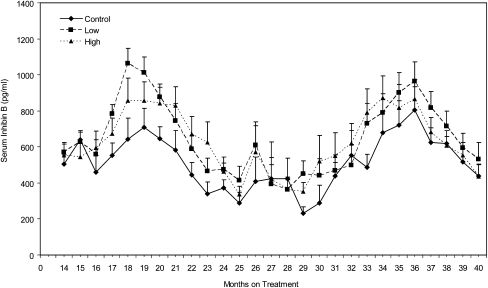
As the animals matured, age differences (P < 0.0001) in inhibin B levels were observed (Fig. 3). Inhibin B levels increased from months 14 to 18, declined to trough levels from months 25 to 29, and then increased and decreased again between months 30 to 40. The inhibin B level in the low-dose group was significantly higher (P = 0.0328) than in the control group. When analyzed on a monthly basis, inhibin B levels were higher (P ≤ 0.05) in the low-dose group than in the control group at months 18 and 29.
Fig. 3.
Effect of dose and length of treatment on mean serum inhibin B concentrations in peripubertal male rhesus monkeys chronically exposed to MPH. Inhibin B levels increased initially (P < 0.0001) as the animals matured. Relative to controls, inhibin B levels were higher in the low-dose animals (P = 0.0328).
Leptin.
Serum leptin concentrations changed erratically during months 14 to 18 of treatment, but then increased significantly (P < 0.0001) during the remainder of the experiment (Fig. S2). ANOVA revealed a significant effect (P = 0.0188) of dose on serum leptin concentrations; exposure to the low-dose was associated with a significantly higher (P = 0.0212) level of leptin as was exposure to the high-dose (P = 0.0316) of MPH (Fig. S2). A significant difference between the control group and the high-dose group was found at month 23 (P = 0.0208) and month 29 (P = 0.0403).
Testicular Volume.
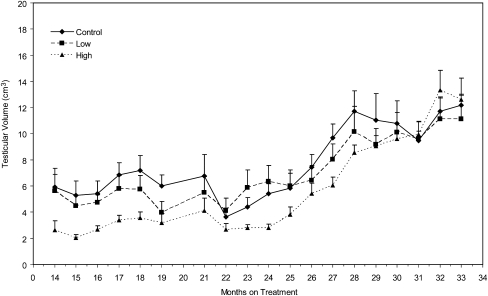
Testicular volume increased (P < 0.0001) as the animals matured (Fig. 4), and was significantly affected by dose (P = 0.038). Testicular volume in the high-dose group was significantly lower (P = 0.0241) than in the control group. When analyzed on a monthly basis, testicular volume was significantly lower (P ≤ 0.05) in the high-dose group than in the control group at months 15 to 19, and 27.
Fig. 4.
Effect of dose and length of treatment on mean testicular volume (± SEM) in peripubertal male rhesus monkeys chronically exposed to MPH. ANOVA indicated that testicular volume increased (P < 0.0001) over the exposure period. Testicular volume varied (P = 0.038) as an inverse function of dose. The Dunnett's test revealed that testicular volume was significantly (P = 0.0241) reduced in the high-dose animals.
Testicular Descent.
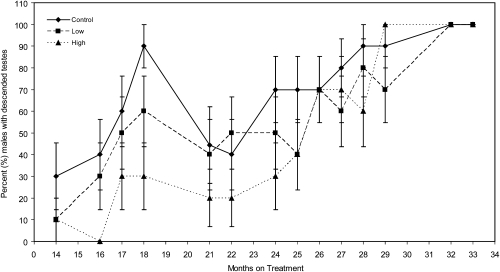
The percentage of animals with descended testes increased (P = 0.0123) with age (Fig. 5). The percentage of animals with undescended testes in the high-dose group was significantly larger (P = 0.0475) than in the control group. Significantly fewer animals had descended testes in the high-dose group at month 16 (P < 0.0001) and month 29 (P < 0.0001). After ∼30 mo of dosing, most males were classified as having descended testes.
Fig. 5.
Effect of dose and length of treatment on the mean number of males with descended testicles (± SEM) in peripubertal rhesus monkeys chronically treated with MPH. The percentage of males with descended testes increased (P = 0. 0123) as the animals aged. At month 16 of dosing, the number of animals with descended testes was lower than in the high-dose group than in the control group (P < 0.0001). All animals had descended testes by month 30 of dosing.
Discussion
Secretion of testicular testosterone is governed by a neuroendocrine axis comprised of the hypothalamus, anterior pituitary, and testis (4). Testosterone secretion by the Leydig cell in the interstitial compartment of the testis is stimulated by circulating luteinizing hormone (LH) released from the anterior pituitary. The secretion of LH is in turn driven by the hypothalamic peptide, GnRH, which is discharged in an intermittent mode into the hypophysial portal circulation (4). Pulsatile GnRH stimulation of the pituitary gonadotrophs leads to a corresponding pattern of LH release; in many species, including the monkey, this produces a dramatic episodic pattern of testicular testosterone release (5). As a result, circulating levels of this androgen in the adult male monkey may swing from values close to those observed in castrated animals (<0.5 ng/mL) to concentrations as high as 20 ng/mL or more in a matter of minutes (5). The intensity of GnRH pulsatility, and therefore the central drive to the male reproductive axis, is regulated by a negative-feedback action of testosterone on the hypothalamus to retard the frequency of pulsatile GnRH release (4). In addition, GnRH pulsatility is diurnally modulated with a decrease in pulse frequency during the light phase of the 24-h cycle, and this leads to the characteristic low morning levels in circulating testosterone observed in the rhesus monkey (5).
In primates, the hypothalamic-pituitary-Leydig cell axis is functional during the first few months of postnatal life and, as a result, blood gonadotropin and testosterone levels in infantile male monkeys and boys are similar to those in adult male monkeys and men (2, 3). In late infancy, pulsatile GnRH release by the primate hypothalamus is arrested and the drive to gonadotropin secretion is therefore lost, guaranteeing the relative quiescence of the testis during subsequent childhood and juvenile development (2, 3). Several years later, the GnRH pulse-generating mechanism in the primate hypothalamus is reactivated and the onset of puberty is initiated, leading again to increased LH secretion and testicular testosterone release (2, 3). The increased secretion of testosterone at the time of puberty, in combination with elevated FSH secretion, leads to growth and maturation of the testis and the initiation of spermatogenesis (6).
In the rhesus monkey, the pubertal acceleration in testicular growth and resurgence in testicular testosterone secretion are generally initiated between 3 and 4 y of age (2, 6). Thus, in the present study, the marked rise in circulating testosterone concentrations in the control group between 44 and 48 mo of age (months 14–18 of treatment) was to be expected. Although MPH induced suppression of the morning testosterone concentrations was most marked during this pubertal phase of development, more frequent analysis of testosterone concentrations during the 24-h period, including nighttime assessments, revealed that the hypoandrogenic effect of MPH was still evident at 60 to 63 mo of age (month 35 of treatment). Although the clearance of testosterone was not evaluated in the present study, the most likely explanation for the reduction in circulating testosterone concentrations was that MPH suppressed testicular secretion of the steroid. The foregoing results in the monkey are consistent with the finding of lowered salivary testosterone content and the abrogation of the diurnal rhythm of salivary testosterone in children treated with MPH (7). Also consistent with our results were those of Adriani et al. (8), in which significantly smaller testes were found in male rats exposed to MPH for 2 wk during puberty.
Although the delay in testicular descent could influence spermatogenesis, it is unlikely to explain the alterations in testicular testosterone secretion, as undescended testis in humans with complete androgen insensitivity produce normal or elevated amounts of testosterone (9). For this reason, species differences in the cremasteric reflex are also unlikely to account for the delay in testicular testosterone production. Instead, a reduction in testosterone secretion could have resulted from either a direct action of MPH on the testis or from an indirect action of MPH to compromise LH secretion at a pituitary or hypothalamic site. The finding that the MPH-induced hypoandrogenic state at month 35 was not inversely associated with elevated LH levels as a result of reduced testosterone negative feedback (Fig. S3 Upper, mo 35; Fig. S3 Lower, mo 38) indicates that the site of action of MPH is probably at the level of the hypothalamus and pituitary. However, a central action above the hypothalamus at sleep and feeding centers for example, is not excluded. A hypothalamic site of action of MPH may also explain the more pronounced suppression of testosterone by MPH in the younger animals. As the transition from the juvenile to the pubertal stage of development unfolds, there is a progressive increase in the pulsatile GnRH signal to the pituitary (10). Intuitively, it might be expected that suppression of the pubertal GnRH signal may be more marked during early pubertal development before the full adult capacity for GnRH pulse generation is acquired. Although the cellular and molecular mechanisms involved in MPH induced testosterone suppression remain to be determined, it is apparent that a level of interference with pubertal testosterone secretion is maintained as late as 5 y of age.
Inhibin B is secreted primarily by the Sertoli cell and is the major testicular feedback signal regulating FSH secretion in sexually mature male primates (11, 12). Sertoli cell number, which is the major determinant of the spermatogenic ceiling of the adult testis, increases dramatically during puberty, after which this cell type undergoes terminal differentiation and the cessation of proliferation (6). In the normal adult monkey, circulating concentrations of inhibin B are correlated with Sertoli cell number and therefore reflect the spermatogenic capacity of the postpubertal testis (13). The secretion of inhibin B by the adult testis, in contrast to that of testosterone, is independent of the light-dark cycle (14) and control by the pituitary is provided by FSH (15, 16). Taking the foregoing considerations together with the finding in the present study that blood levels of inhibin B and FSH (Fig. S4) were similar in the control and treated animals at the end of the study (months 35–40 of treatment), it seems reasonable to conclude that any impact of MPH on either Sertoli cell number or function during the early phase of pubertal development (months 16–21 of treatment) were transitory. This view is consistent with the concomitant observation that the suppression of testicular volume by MPH during early puberty was not maintained into adulthood and that sperm count was similar in the treated and control groups at the end of the study (data summarized in SI Results and Figs. S5 and S6). The reasons for the dramatic variations in serum inhibin B concentrations observed among all groups throughout the duration of the study are unknown, but clearly they were not related to treatment, as the timing of peaks and troughs in concentrations in this circulating hormone were the same in control and treated animals.
Leptin is secreted by adipose tissue in direct proportion to the amount of body fat, and is thought to be an indicator of energy deficiency (17). Although the pubertal reactivation of pulsatile GnRH release is dependent on a permissive action of leptin in monkey and man (3, 18), the impact of MPH to impair the hypothalamic-pituitary-testicular axis during puberty in the present study is unlikely to involve leptin because levels of this adipocyte hormone were increased rather than decreased by treatment (Fig. S2).
It is not clear how MPH acted to increase leptin in this study. Food intake was adjusted throughout the study to assure that body weight was not a variable among groups. Differences in other metabolic signals from either the endocrine or nervous systems may have affected leptin levels (19). MPH treatment did reduce sucrose intake and serum insulin levels in adult rats (20).
There are limitations to this study that must be taken into account in interpreting the findings. The study was initially designed to evaluate the putative genetic toxicity of MPH in NHP. The sample size may be considered as limited in terms of the numbers of subjects included in a human study. However, the sample size was sufficient to detect significant differences on a number of reproductive parameters. The animals were dosed 5 d a week, rather than every day; however, one would expect that this feature would bias against finding treatment effects. Other measurements, which require larger blood sample volumes or training of the NHP before initiation of the study, such as behavioral indices acquired with actographic tools, or analysis of the cardiovascular system by echocardiography, could not be implemented without compromising the genetic safety experiments.
Nevertheless, our findings demonstrate that chronic treatment with MPH may delay the initiation of the onset of puberty or reduce the early tempo of this developmental process in a representative higher primate. These results are consistent with, and may provide an insight into potential neuroendocrine mechanisms that underlie delayed growth and maturation associated with the use of one of the most widely prescribed medications in children (21). The fact that effects were transient and that no permanent deficits were found in the monkey model alleviates concerns about the clinical use of MPH in youth. Finally, we clearly recognize the importance of confirming these findings in additional studies in both animal models and humans.
Materials and Methods
Animals and Animal Husbandry.
All animal procedures were approved by the National Center for Toxicological Research Institutional Animal Care and Use Committee. Thirty, approximately 2-y-old male, Indian-origin, natural habitat-reared rhesus macaques (Macaca mulatta) were obtained from Alpha Genesis. All procedures are described in the SI Materials and Methods.
Test Article, Preparation, and Dosing.
MPH, USP grade, was obtained in 25-g vials from Mallinckrodt Chemicals. All lots of MPH were tested by the Chemistry Support Group, Department of Biochemical Toxicology at the National Center for Toxicological Research for identity and purity. MPH was dissolved in Prang (Bioserv), an oral rehydration solution, commonly used for studies with NHP. Preparation of the test article occurred weekly and each of the dose preparations was analyzed before use by mass spectroscopy for accuracy and homogeneity of dose. Only dose preparations within ±10% of the target dose were considered acceptable for use in this study.
Ten NHP were assigned to each of the three dose groups by randomized weight ranking and each animal completed the 40-mo treatment period. Dosing of NHP occurred twice a day (between 0900 and 1100 hours and 1300 and 1500 hours), 5 d per week, from month 0 (∼2.5 y of age and equivalent to an 8- to 9-y-old boy) through month 40 (∼6 y of age). Test subjects were weighed before the morning dose and the individual doses were calculated at that time. The 10 control animals each received 0.5 mL/kg of the vehicle, Prang, via an oral dosing syringe. The 10 animals in the low-dose group received 0.15 mg/kg of MPH twice a day for approximately 5 mo, at which time the dose was increased over a 4-mo period to 2.5 mg/kg of MPH twice a day. After an 8-wk ramp-up period, the 10 animals in the high-dose group received 1.5 mg/kg twice a day for 4 mo. This dose was increased over a 4-mo period to 12.5 mg/kg twice a day. The increase in dose was necessary because of lower than expected plasma concentrations of MPH (1).
Exposure Assessment.
One-hundred microliters of plasma were obtained by venipuncture 30 min after the morning dose at monthly intervals throughout the study. Plasma samples were stored at −80 °C until processed and analyzed (1). Analysis of plasma concentrations after 4 mo of treatment revealed lower-than-expected plasma levels and the doses were increased until the desired levels were reached (see SI Results).
Endocrine Analyses.
Serum for hormone measurement was collected by venipuncture between 0900 and 1100 hours. The first sample for the measurement of testosterone and leptin levels was collected at the beginning of the study, Time 0. Subsequent samples for the measurement of testosterone, inhibin B, FSH, and leptin levels were collected at monthly intervals beginning at month 14 of treatment and continuing until month 40. In addition, serum was collected at 1500, 2100, 0300, and 0900 hours at months 35 and 38 of treatment to assess the diurnal rhythm of testosterone and LH. Details for these analyses are presented in SI Results.
Testicular Measurements.
Testicular measurements were made at monthly intervals beginning at 39 to 42 mo of age (month 14 of dosing). Measurements were made until 48 to 53 mo of age.
Statistical Methods.
Several variable measurements were transformed to normalize the data. The arcsine-transformation was applied to the proportional variables, the square root transformation was applied to the count variables, and the log-transformation was applied to the sperm motility measures and sperm concentration. The sperm morphology measures were not transformed. The mixed linear model and ANOVA were used to test for significance of effects of the MPH treatment. The mixed linear model was used to test for effects of time, dose, and their interactions, where time was considered as a repeated measurement. When a variable showed a significant overall dose-effect across time periods, the ANOVA test was further used to test for dose-effects at each time period. The numbers of time periods for those significance variables were from 20 to 27. The significance of the dose-effects for each time period and each variable was not adjusted for multiple testing because of the sample size. However, the Dunnett's multiple testing procedure was used to compare each dose group with the control in the mixed model and within each ANOVA. A 5% level of significance was used in the analysis.
Supplementary Material
Acknowledgments
This work was supported by an InterAgency Agreement between the Eunice Kennedy Shriver National Institute for Child Health and Development/National Institutes of Health and the National Center for Toxicological Research/Food and Drug Administration; an Interagency Agreement between the National Center for Toxicological Research and the National Institute for Occupational Safety and Health; an Interagency Agreement between the Oak Ridge Institute for Science and Education and the National Center for Toxicological Research/Food and Drug Administration (H.-M.L. and H.-C.C.); intramural funds from the National Institute for Child Health and Human Development (to D.R.M.); and National Institutes of Health Grant R01 HD 013254 (to T.M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102187108/-/DCSupplemental.
References
- 1.Morris SM, et al. The genetic toxicology of methylphenidate hydrochloride in non-human primates. Mutat Res. 2009;673(1):59–66. doi: 10.1016/j.mrgentox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Plant TM, Witchel S. In: Knobil and Neill's Physiology of Reproduction. 3rd Ed. Challis JRG, et al., editors. San Diego: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- 3.Witchel SF, Plant TM. In: Yen and Jaffe's Reproductive Endocrinology. 6th Ed. StraussIII JF, Barbieri RL, editors. NY: Elsevier; 2009. pp. 395–431. [Google Scholar]
- 4.Plant TM. Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocr Rev. 1986;7(1):75–88. doi: 10.1210/edrv-7-1-75. [DOI] [PubMed] [Google Scholar]
- 5.Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981;25:244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- 6.Plant TM, Ramaswamy S, Simorangkir D, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann N Y Acad Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016. [DOI] [PubMed] [Google Scholar]
- 7.Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary biomarker levels and diurnal variation: Associations with medications prescribed to control children's problem behavior. Child Dev. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 8.Adriani W, et al. Short-term effects of adolescent methylphenidate exposure on brain striatal gene expression and sexual/endocrine parameters in male rats. Ann N Y Acad Sci. 2006;1074:52–73. doi: 10.1196/annals.1369.005. [DOI] [PubMed] [Google Scholar]
- 9.Griffin JE, Wilson JD. In: Williams Textbook of Endocrinology. 10th Ed. Larsen PR, et al., editors. Philadelphia: Sanders; 2003. pp. 709–769. [Google Scholar]
- 10.Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta) Endocrinology. 1998;139:2774–2783. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Plant TM. Operation of the follicle-stimulating hormone (FSH)-inhibin B feedback loop in the control of primate spermatogenesis. Mol Cell Endocrinol. 2001;180(1–2):93–101. doi: 10.1016/s0303-7207(01)00498-1. [DOI] [PubMed] [Google Scholar]
- 12.Hayes FJ, Hall JE, Boepple PA, Crowley WF., Jr Clinical review 96: Differential control of gonadotropin secretion in the human: Endocrine role of inhibin. J Clin Endocrinol Metab. 1998;83:1835–1841. doi: 10.1210/jcem.83.6.4884. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Evidence that in a physiological setting Sertoli cell number is the major determinant of circulating concentrations of inhibin B in the adult male rhesus monkey (Macaca mulatta) J Androl. 1999;20:430–434. [PubMed] [Google Scholar]
- 14.Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79(1):93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- 15.Young J, et al. Effects of human recombinant luteinizing hormone and follicle-stimulating hormone in patients with acquired hypogonadotropic hypogonadism: Study of Sertoli and Leydig cell secretions and interactions. J Clin Endocrinol Metab. 2000;85:3239–3244. doi: 10.1210/jcem.85.9.6811. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta) Endocrinology. 2003;144:1175–1185. doi: 10.1210/en.2002-221078. [DOI] [PubMed] [Google Scholar]
- 17.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann Intern Med. 2010;152(2):93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann DR, Plant TM. In: Leptin and Reproduction. Henson MC, Castracane VD, editors. New York: Kluwer Academic/Plenum Press; 2003. pp. 133–150. [Google Scholar]
- 19.Rayner DV, Trayhurn P. Regulation of leptin production: Sympathetic nervous system interactions. J Mol Med (Berl) 2001;79(1):8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- 20.Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res Bull. 2006;70:422–429. doi: 10.1016/j.brainresbull.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Swanson JM, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.