Abstract
Clostridium difficile infection (CDI) causes antibiotic-associated diarrhea and pseudomembranous colitis. Hypervirulent strains of the pathogen, which are responsible for increased morbidity and mortality of CDI, produce the binary actin-ADP ribosylating toxin Clostridium difficile transferase (CDT) in addition to the Rho-glucosylating toxins A and B. CDT depolymerizes the actin cytoskeleton, increases adherence and colonization of Clostridia by induction of microtubule-based cell protrusions and, eventually, causes death of target cells. Using a haploid genetic screen, we identified the lipolysis-stimulated lipoprotein receptor as the membrane receptor for CDT uptake by target cells. Moreover, we show that Clostridium perfringens iota toxin, which is a related binary actin-ADP ribosylating toxin, enters target cells via the lipolysis-stimulated lipoprotein receptor. Identification of the toxin receptors is essential for understanding of the toxin uptake and provides a most valuable basis for antitoxin strategies.
Keywords: bacterial ADP-ribosyltransferases, receptor binding
Nosocomial Clostridium difficile infection (CDI) is a major health concern. During recent years, the number and severity of C. difficile outbreaks have dramatically increased (1–3). Hypervirulent strains, responsible for high morbidity and mortality of CDI, have emerged in almost all industrial countries. Whereas the main virulence factors of the pathogen are the Rho-protein glucosylating C. difficile toxins A (TcdA) and B (TcdB), recently identified hypervirulent C. difficile strains (e.g., PCR ribotype 027) additionally produce C. difficile transferase (CDT) (4, 5). CDT is also produced by C. difficile ribotype 078, which is an increasing cause of CDI in Europe, with disease severity similar to ribotype 027 and, moreover, is most often found in pigs, cattle, and chickens (6–8).
C. difficile transferase (4, 9, 10), which is not related to cytolethal distending toxins produced by multiple pathogenic Gram-negative bacteria (also abbreviated as CDTs), belongs to the family of binary actin-ADP ribosylating toxins that also includes Clostridium perfringens iota toxin (11), Clostridium spiroforme toxin (12), Clostridium botulinum C2 toxin (13), and the Bacillus cereus vegetative insecticidal proteins (14). All these toxins consist of a biologically active enzyme component and a separated binding component (15). The binding component is activated by proteolytic cleavage and forms heptamers that interact with a so far unknown cell-membrane receptor. After binding of the enzyme component, the receptor-toxin complex is endocytosed. At low pH of endosomes, a conformational change of the heptamers forces membrane insertion, pore formation, and subsequent translocation of the enzyme component into the cytosol (16). In the cytosol, the enzymatic component of the toxins ADP ribosylates G-actin at arginine-177 (17–19), resulting in actin depolymerization and, at high toxin concentrations, death of target cells (20, 21). At low toxin concentrations, the restructuring of the actin cytoskeleton induces the formation of microtubule-based protrusions on the surface of epithelial cells, resulting in increased adherence and colonization of Clostridia (22).
The aim of the present study was to identify the target-cell receptor of CDT. To this end, we used a recently developed genome-wide haploid genetic screen, resulting in the identification of the lipolysis-stimulated lipoprotein receptor (LSR) as the target molecule for cell binding and internalization of CDT. In addition, we present evidence that C. perfringens iota toxin, a related binary actin-ADP ribosylating toxin, shares the LSR for cell entry.
Results
Haploid Genetic Screen Yields the LSR as a Receptor Candidate for CDT.
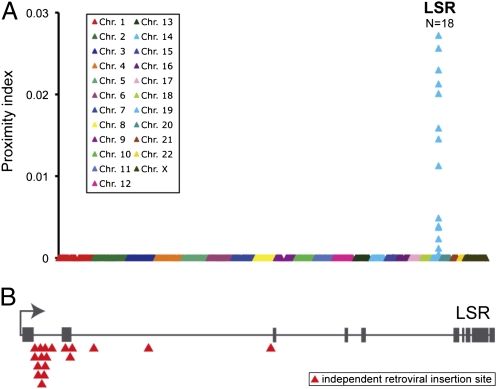
To identify the target-cell receptor of CDT, we used a recently developed genome-wide haploid genetic screen based on the human, near-haploid leukemia cell line KBM7 (23). Because KBM7 cells were insensitive toward CDT, we used a recently described derivative cell line of KBM7 cells named HAP1 (24). HAP1 cells were obtained in an attempt to generate haploid induced pluripotent cells by overexpression of the four reprogramming factors OCT4, SOX-2, c-MYC, and KLF4 in KBM7. Although HAP1 cells failed to reach a stage of pluripotency, expression of the reprogramming factors significantly altered the differentiation state of HAP1 cells compared with the parental KBM7 cells. These adherent haploid cells were susceptible to CDT and rounded up in the presence of the toxin, indicating the presence of the toxin receptor and functional endocytosis machinery. To find genes that are essential for CDT intoxication, HAP1 cells were mutagenized with a retroviral gene-trap vector, resulting in gene mutations in nonessential genes (23), leading to a heterogenous cell population with knockouts (HAP1GT cells). Subsequently, ∼1 × 108 HAP1GT cells were treated with 1 nM CDT and selected for growth of toxin-resistant clones by frequently removing detached and dead cells. This process yielded ∼103 CDT-resistant clones. To identify mutagenized genes that gave rise to toxin resistance, inverse PCR was applied on DNA isolated from the entire pool of CDT-resistant HAP1GT clones followed by parallel sequencing (Solexa) of genomic DNA directly flanking the retroviral integration sites. We plotted the proximity index for each of the retroviral integration sites to identify regions of the genome enriched for independent gene-trap insertions, which revealed a cluster of 18 independent insertion sites in one gene that coded for the lipolysis-stimulated lipoprotein receptor (LSR, LISCH7, ILDR3) (Fig. 1). Importantly, only the LSR gene was significantly enriched for gene-trap insertions (P value = 4 × 10−33), compared with a large dataset of gene-trap insertion sites present in unselected HAP1 cells (Fig. S1). In line with the differential susceptibility of HAP1 and KBM7 cells toward CDT, microarray analysis revealed much higher expression levels of LSR mRNA in HAP1 cells than in CDT-insensitive KBM7 cells (Fig. S2).
Fig. 1.
Clustering of independent gene-trap insertions in the LSR gene. (A) CDT-insensitive cell clones were expanded and gene-trap insertion sites identified by parallel sequencing. A proximity index was determined for each insertion site that corresponds to the calculated inverse of the average distance between a specific insertion and its immediate upstream and downstream insertions. Insertion sites were positioned on the x-axis based on their chromosomal location and calculated proximity values for each gene-trap insertion were plotted. N indicates the number of gene-trap insertion sites found in LSR. (B) Mapping of the independent gene-trap insertions sites in the LSR gene. Gray boxes indicate exons present in the LSR gene.
Ectopic Expression of LSR Restores Sensitivity in an LSR-Deficient Hap1 Clone Against CDT.
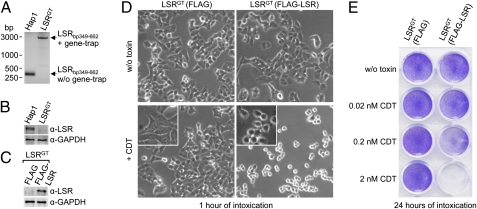
To prove that the LSR is critical for cell intoxication by CDT, a single CDT-insensitive HAP1 cell clone was isolated that carried a defined gene-trap insertion within the first intron of the LSR gene (henceforth named LSRGT cells) (Fig. 2A). Immunoblot analysis of LSRGT cell lysates with anti-LSR antibody confirmed lack of LSR expression (Fig. 2B). Retroviral expression of FLAG-tagged LSR but not of the FLAG-only construct (Fig. 2C) restored sensitivity against CDT in LSRGT cells (Fig. 2 D and E and Movie S1).
Fig. 2.
LSR is essential for intoxication of HAP1 cells with CDT. (A) PCR amplification of a region of the first intron of the LSR gene (bp 349–662) with genomic DNA isolated from HAP1 and from subcloned LSRGT cells. PCR products [with (+) and without (w/o) gene-trap insert] are shown. (B) Whole-cell lysates of HAP1 and LSRGT cells were subjected to SDS/PAGE and Western blotting, followed by detection of LSR with a specific anti-LSR antibody (α-LSR). Equal loading of proteins was verified by detection of GAPDH with a specific antibody (α-GAPDH). (C) SDS/PAGE and Western blotting with whole-cell lysates from LSRGT cells transduced with FLAG-only or FLAG-LSR retroviruses followed detection of LSR with a specific anti-LSR antibody (α-LSR). (D) FLAG- and FLAG-LSR–transduced LSRGT cells were intoxicated with 20 nM CDT or were left untreated. Cell morphology was analyzed microscopically (using the 10× objective). (E) Intoxication of FLAG- and FLAG-LSR–transduced LSRGT cells was performed with increasing concentrations of CDT (0, 0.02, 0.2, and 2 nM) as indicated, before the removal of detached cells by washing and staining of attached cells by Crystal violet dye.
Ectopic Expression of LSR in HeLa Cells Induces Sensitivity Toward CDT.
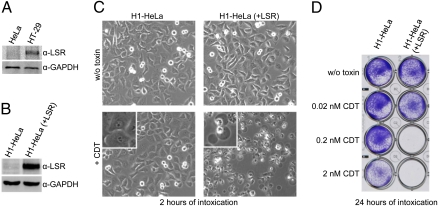
Whereas human colon adenocarcinoma HT-29 cells are sensitive toward CDT, we observed that HeLa cells are highly resistant against the toxin. In contrast, both HT-29 and HeLa cells are sensitive toward the related C. botulinum C2 toxin. Accordingly, immunoblot analysis with anti-LSR antibody revealed expression of LSR in HT-29 but not in HeLa cells (Fig. 3A), suggesting that the receptor deficiency in HeLa cells causes resistance toward CDT. Strikingly, HeLa cells that were transduced with the FLAG-LSR retrovirus (Fig. 3B), exhibited the typical cytotoxic effects of CDT, including cell rounding (Fig. 3C), detachment from culture plates (Fig. 3D), and formation of cell protrusions (Movie S2). The intoxication of FLAG-LSR–transduced HeLa cells by CDT was additionally confirmed by directly probing the ADP ribosylation state of actin in lysates of CDT-intoxicated cells using an in vitro actin-ADP ribosylation assay. The amount of actin amendable to in vitro actin-ADP ribosylation strongly decreased over time in CDT-treated, FLAG-LSR-transduced HeLa cells but not in nontransduced HeLa cells (Fig. S3).
Fig. 3.
Ectopic expression of LSR in H1-HeLa cells increases sensitivity toward CDT. (A) Immunoblot against LSR with whole-cell lysates from CDT-insensitive HeLa cells and CDT-sensitive HT-29 cells. Equal loading of proteins was verified by detection of GAPDH with a specific antibody. (B) Immunoblot as shown in A, but with whole-cell lysates from nontransduced (H1-HeLa) and FLAG-LSR–transduced H1-HeLa cells [H1-HeLa (+LSR)]. (C) Non (H1-HeLa)- and FLAG-LSR–transduced H1-HeLa [H1-HeLa (+LSR)] cells were intoxicated with 2 nM CDT (+CDT) or were left untreated (w/o toxin) and cell morphology was analyzed microscopically (using the 10× objective) after 2 h. (D) Intoxication of non- and FLAG-LSR–transduced H1-HeLa cells [H1-HeLa (+LSR)] was performed with increasing concentrations of CDT as indicated, and cell detachment was analyzed by Crystal violet staining of nondetached cells after 24 h of intoxication.
LSR Mediates Binding of CDT to the Surface of HeLa Cells.
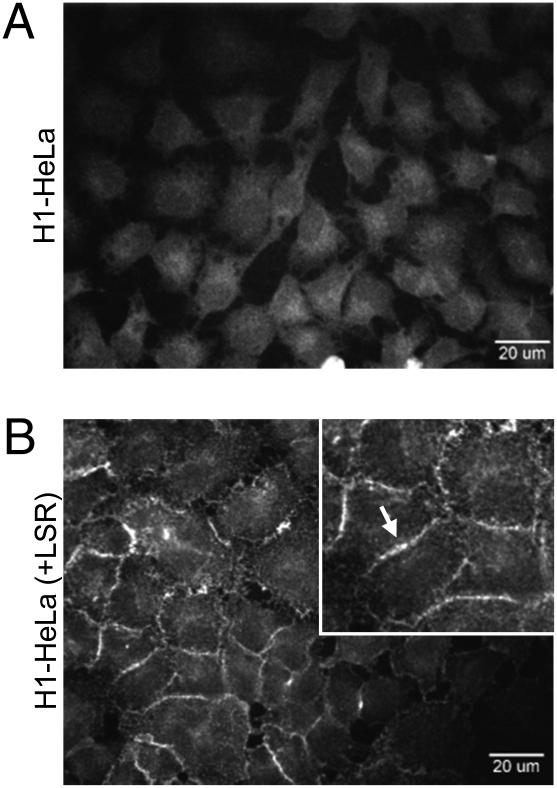
To prove that LSR is involved in binding of CDT to the cell membrane of target cells, the Alexa-coupled binding component of CDT (CDTb) was added to FLAG-LSR–transduced and nontransduced HeLa cells at 4 °C to prevent endocytosis, and surface-bound CDTb was visualized by confocal fluorescence microscopy. As expected, fluorescence staining at the cell periphery was observed exclusively with FLAG-LSR–transduced HeLa cells and not with nontransduced cells. These findings indicated that LSR is required for binding of CDT to the cell surface (Fig. 4).
Fig. 4.
Ectopic expression of LSR promotes binding of CDTb to the cell surface of H1-HeLa cells. Ten micrograms (100 nM) Alexa-coupled CDTb was incubated with (A) non- and (B) FLAG-LSR–transduced H1-HeLa cells [H1-HeLa (+LSR)] at 4 °C for 90 min. After washing, CDTb binding to the cell surface was visualized by confocal fluorescence microscopy.
Binding Component of CDT Interacts Directly with the LSR.
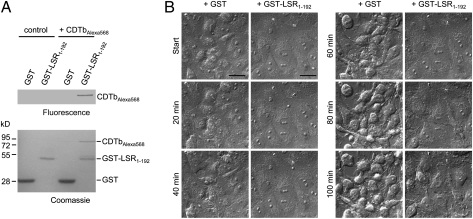
To answer the question whether the binding component of CDT interacts directly with the LSR, pull-down assays with a GST-tagged extracellular domain of LSR (GST-LSR1–192) were performed. GST-LSR1–192 coprecipitated with Alexa-coupled CDTb, indicating that the LSR is directly involved in binding of CDT (Fig. 5A). Moreover, the requirement of this interaction was further demonstrated by showing that preincubation of CDTb with GST-LSR1–192, but not with GST alone, inhibited the intoxication process when added together with CDTa to cultured Vero cells (Fig. 5B). Thus, soluble GST-LSR1–192 was able to compete with the endogenous membrane-bound LSR, thereby delaying intoxication.
Fig. 5.
The binding component of CDT interacts directly with LSR. (A) Pull-down interaction analysis. Glutathione beads were preloaded with GST-LSR1–192 or GST alone and Alexa568-coupled CDTb was added for 1 h at 4 °C. Thereafter, beads were isolated by centrifugation, washed, and bound proteins were analyzed by SDS/PAGE followed by fluorescence imaging and Coomassie staining. (B) Competitive inhibition assay. CDTb (5 nM) was preincubated for 30 min with 1,000-fold molar excess of GST or GST-LSR1–192 protein at 4 °C and then applied to cultured Vero cells together with CDTa (5 nM). Cells were further incubated at room temperature and intoxication (cell rounding) was monitored by differential interference contrast time-lapse microscopy. Representative images at indicated time points are shown. (Scale bars, 10 μm.)
C. perfringens Iota Toxin Shares the LSR for Cell Entry.
The binding components of iota and C2 toxin are ∼80% and ∼40%, respectively, identical with the binding component of CDT. Therefore, we asked whether iota toxin and CDT share the same cell receptor for toxin uptake. To this end, FLAG- and FLAG-LSR–transduced LSRGT cells, as well as FLAG-LSR– and nontransduced HeLa cells, were incubated with iota toxin. As observed with CDT, exclusively LSRGT and HeLa cells that ectopically expressed LSR protein were sensitive toward iota toxin (Fig. 6). Thus, CDT and iota toxin share the LSR as membrane receptor for toxin uptake. In contrast, the more distantly related binary C. botulinum toxin C2 toxin, which was still capable of intoxicating LSR-deficient HAP1 cells, uses a different receptor (Fig. S4).
Fig. 6.
Iota toxin shares the same cellular receptor with CDT. (A) FLAG- and FLAG-LSR–transduced LSRGT cells, respectively, and (B) non- and FLAG-LSR–transduced H1-HeLa cells were incubated without (w/o toxin) or with 20 nM iota toxin (+iota toxin) for 24 h, before visualization of intoxication (cell rounding) by microscopy.
Discussion
The present study identifies the LSR as the cell membrane receptor for C. difficile transferase CDT and C. perfringens iota toxin, thereby opening previously unexplored perspectives for the understanding of the cellular uptake of CDT and of other clostridial actin-ADP ribosylating toxins. Several lines of evidence support our conclusion that the LSR is the main receptor for cell entry of CDT. First, a lack of LSR expression in LSRGT and HeLa cells renders the cells resistant toward CDT and, second, ectopic expression of LSR in these cells restores sensitivity toward the toxin. Third, the soluble, extracellular part of the LSR binds directly to the binding component of CDT in GST pull-down assays and competes with binding of CDT to endogenous, membrane-bound LSR, thereby causing a delay in the intoxication of Vero cells by CDT. In full agreement with our findings, earlier studies on the CDT-related C. perfringens iota toxin suggested the presence of a proteinous receptor that mediates binding of the toxin to the cell surface of Vero cells (25). Moreover, we observed that C. botulinum C2 toxin, which is related to CDT and iota toxin, does not share LSR for cell entry. These findings are in line with a previous report indicating that C2 toxin but not iota toxin is taken up via asparagine-linked complex and hybrid carbohydrate structures (26). This difference is explained by the low sequence similarity of 12% of the C-terminal receptor binding domain of CDT with the same region of C2II.
LSR is a type I single-pass transmembrane protein of the cell membrane and is mainly expressed in the liver, but also in the intestine and various other tissues (27, 28). A role in the cellular uptake of triglyceride-rich and low-density lipoproteins for clearance of chylomicron remnants from circulation has been attributed to this protein (29, 30). Disruption of the LSR gene causes embryonic lethality in mice (28), revealing an important role during development, which has to be clarified in the future. Heterozygous mice have increased levels of plasma triacylglyceride and cholesterol after food intake (31).
It is remarkable that LSR is not only involved in triacylglyceride-rich lipoprotein uptake (31), but may also play an essential role in organization of three-cellular tight junctions that are involved in epithelial barrier function (32). Tight-junction proteins are well-known receptors of toxins and viruses; for example, claudin is the membrane receptor for C. perfringens enterotoxin (33) and, together with the tight-junction protein occludin, it functions as membrane receptor for hepatitis-C virus (34). It remains to be studied whether the alteration of the actin cytoskeleton by the actin-ADP ribosylating toxins CDT and iota toxin or binding of the toxins to LSR influences also the function of LSR as a tight-junction protein and whether this is important for CDI.
An Ig-like V-type domain is annotated in the N-terminal, extracellular portion of LSR. Notably, paired Ig-like type 2 receptor-α, a coreceptor for glycoprotein B of Herpes simplex virus-1, or T-cell Ig and mucin domain 1, which serves as a receptor for Hepatitis A, Ebola, and Marburg viruses, each contain Ig-like V-type domains in their extracellular portions (35–37). We are currently investigating whether the Ig-like domain of LSR is directly implicated in CDT binding.
A recent study associated the endocytic uptake of the C. perfringens iota toxin with a Rho-GDI–regulated, clathrin-independent pathway, which is also followed by the IL2 receptor (38). Our findings will now facilitate further studies that aim to clarify whether binding of the toxins to the LSR triggers the IL2-like endocytic route or whether this uptake mechanism represents the intrinsic recycling process of the receptor even in the absence of CDT or iota toxin.
Cell-surface receptors have been described previously also for other binary bacterial toxins, such as the anthrax toxin. Here, two homologous membrane proteins, namely tumor-endothelium marker-8 and capillary morphogenesis protein 2 (CMG2), have been identified that bind to the cell-binding component of the anthrax toxin, called protective antigen (PA) (39, 40). Interestingly, the reported structure of the PA/CMG2 complex suggests that CMG2 acts as pH-dependent brace, allowing proper and timely insertion of PA into acidified endosomal membranes (41). It remains to be studied, although the LSR shares no obvious homology to CMG2, whether pore formation of CDT (and iota toxin) in endosomal membranes is regulated by its cellular receptor.
To our knowledge, LSR is unique in being a cell-membrane receptor that has been identified for C. difficile toxins and for members of the family of clostridial actin-ADP ribosylating toxins. Moreover, the identification of the host cell-membrane receptor of CDT allows the development of new therapeutic strategies against CDT and related toxins.
Materials and Methods
Haploid Genetic Screen.
The derivation of the adherent haploid HAP1 cell line through reprogramming and the generation of a mutagenized knockout library in HAP1 cells have been described previously (24). In short, gene-trap virus was produced by transfection of 293T cells with a retroviral gene-trap vector (23) and packaging plasmids. Concentrated gene-trap virus was used to infect ∼100 million HAP1 cells. After a brief period of expansion, 100 million mutagenized cells were used for the screen by addition of 1 nM CDTa and 1 nM CDTb in the medium and continued incubation at 37 °C. After 4 d, dead cells were removed by medium exchange and fresh medium supplemented with toxin was added to the surviving cell clones that remained attached in the flasks. After 1 wk, visible colonies of toxin-resistant cell clones were collected by trypsinization, unified in new flasks, and grown at 37 °C to increase total cell number.
Sequence Analysis of Gene-Trap Insertion Sites.
Insertion sites were identified en masse by sequencing the genomic DNA flanking gene-trap proviral DNA as described previously (42, 24). In short, insertions in the mutagenized HAP1 cells after selection with CDT were identified using an inverse PCR protocol followed by sequencing using the Genome Analyzer platform (Illumina). Sequences were aligned to the human genome to determine insertions sites. To identify genomic regions with a high density of insertions, we defined the proximity index for a given insertion as the inverse value of the average distances with its neighboring insertion sites. This method provides a graphic illustration of insertion site clustering. We also analyzed the experimental dataset using an alternative method where we compare the number of insertions per gene in the experimental dataset of cells resistant to CDT to a previously described control dataset (24) of unselected control cells. Enrichment of a gene in the screen was calculated by comparing how often that gene was mutated in the screen compared with how often the gene carries an insertion in the control dataset. For each gene a P value (corrected for false-discovery rate) was calculated using the one-sided Fisher exact test.
Isolation of a Clonally Derived LSR-Deficient HAP1 Mutant Cell Line.
A mixture of CDT-resistant HAP1GT cells was subcloned sequentially in 96-well plates where wells contained initially 100 cells per well, in the second round 30 to 50 cells per well, in the third round 5 to 15 cells per well, and finally 1 cell per well. Subpopulations were increased in cell number and analyzed by PCR for the presence of a gene-trapped LSR clone. For this purpose, genomic DNA was isolated from 5 million cells using the QiaAmp DNA mini kit (Qiagen) and a nested PCR was performed with forward primers that hybridize to the DNA sequence of the gene-trap insertion (5′-tctccaaatctcggtggaac-3′ and 5′-ctcggtggaacctccaaat-3′) and reverse primers that bind to sequences in the first intron of the LSR (5′-gggaaagacctcatcaccac-3′ and 5′-ccaaatatcccatccaggtg-3′). Only subpopulations yielding a PCR product (indicating the presence of a gene-trapped LSR clone) were processed to the next subcloning round. Finally, a subregion of the LSR gene (bp 349–662) was amplified by nested PCR (forward primers: 5′-ggaactgtagagggggatgg-3′ and 5′-gtctcagaggctgggacctt-3′; reverse primers: 5′-aggcctaggcattgttcctt-3′ and 5′-ctctaggaagcgtctgatcca-3′) and by using genomic DNA from a clonal LSR clone (LSRGT) as template, followed by DNA sequencing of the PCR product to verify the presence of the gene-trap insertion.
Retrovirus Production and Transduction of Cultured Cells.
Phoenix Ampho cells (retroviral packaging cell line) were transfected with pMXs-IRES-Blasticidin/FLAG-LSR or pMXs-IRES-Blasticidin/FLAG using Lipofectamine 2000 reagent (Invitrogen) and by following the manufacturer's recommendations. Transfected cells were incubated in DMEM (+10% FCS) at 32 °C for 48 h and retrovirus-containing supernatants were collected for transduction of H1-HeLa and LSRGT cells. Briefly, cells were grown to near confluency and retrovirus-containing supernatant was added directly to the growth medium supplemented with 4 μg/mL polybrene. Following an initial incubation for 3 h at 32 °C, cells were incubated at 37 °C for 48 h, before selection of transduced cells by exchange of medium supplemented with 20 μg/mL blasticidin.
Plasmids, Proteins, and Toxins Used in This Study.
An LSR cDNA clone from a human pancreas adenocarcinoma cDNA library (IMAGE:3641235, GenBank protein accession number: AAH04381.2) was used as template for cloning of an N-terminally FLAG-tagged version of full-length LSR into a pMXs-IRES-Blasticidin retroviral vector (Cell Biolabs).
GST-LSR1–192 was expressed in Escherichia coli (strain C43) by using the vector backbone pGEX-4T3 (GE Healthcare) and pMXs-IRES-Blasticidin/FLAG-LSR as template for cloning of the extracellular part of LSR (amino acids 1–192). Briefly, bacteria were grown in LB (Luria-Bertani) medium at 37 °C to an OD600 of 0.8 to 1.2 and protein expression was induced by addition of 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and overnight incubation at 16 °C. Bacteria were harvested by centrifugation and resuspended in ice-cold PBS, supplemented with 10 mM 2-mercaptoethanol, 0.1% vol/vol Triton X-100 and Complete protease inhibitor mixture (Roche) for lysis by using a microfluidizer (Microfluidics) at 15,000 psi and 4 °C. Cell debris was removed by centrifugation (164,000 × g, 1 h, 4 °C) and supernatant applied to a pre-equilibrated glutathione Sepharose 4B column (GE Healthcare). Bound proteins were eluted with buffer, containing 50 mM Tris-HCl/pH 8.0 and 10 mM reduced glutathione. Protein was further purified by size-exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare), equilibrated with Tris-buffered saline (TBS).
CDTa and CDTb (C. difficile strain 196) were produced recombinantly as C-terminally His-tagged proteins in the expression host Bacillus megaterium with protocols as described for clostridial glucosylating toxins by others previously (43, 44).
The components of C. botulinum C2 toxin (C2I and C2II) and C. perfringens iota toxin (Ia and Ib) were purified as described elsewhere (45, 46). All binding components (CDTb, C2II, Ib) were activated by protease treatment (45, 46).
Additional methods were applied in this study and are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Otilia Wunderlich, Sven Hornei, and Lars Ellenrieder for excellent technical assistance, and Hidde Ploegh, Thomas Jank, Selda Genisyuerek, and Alexander Lang for valuable discussions. This work was supported by the Deutsche Forschungsgemeinschaft AK6/20-1 and AK6/16-3 (to K.A. and P.P.) and National Institutes of Health Grant R21-HG004938-01 (to T.R.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109772108/-/DCSupplemental.
References
- 1.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12:409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: An overview of the disease and its pathogenesis, epidemiology and interventions. Gut Microbes. 2010;1:234–242. doi: 10.4161/gmic.1.4.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald LC, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP, LaMont JT. Clostridium difficile—More difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 6.Bakker D, et al. Relatedness of human and animal Clostridium difficile PCR ribotype 078 isolates determined on the basis of multilocus variable-number tandem-repeat analysis and tetracycline resistance. J Clin Microbiol. 2010;48:3744–3749. doi: 10.1128/JCM.01171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyne L. Clostridium difficile—Beyond antibiotics. N Engl J Med. 2010;362:264–265. doi: 10.1056/NEJMe0910055. [DOI] [PubMed] [Google Scholar]
- 8.Burns K, et al. Infection due to C. difficile ribotype 078: First report of cases in the Republic of Ireland. J Hosp Infect. 2010;75:287–291. doi: 10.1016/j.jhin.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–1924. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 10.Perelle S, Gibert M, Bourlioux P, Corthier G, Popoff MR. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect Immun. 1997;65:1402–1407. doi: 10.1128/iai.65.4.1402-1407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson LL, Stiles BG, Zepeda HH, Wilkins TD. Molecular basis for the pathological actions of Clostridium perfringens iota toxin. Infect Immun. 1987;55:118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson LL, Stiles BG, Zepeda H, Wilkins TD. Production by Clostridium spiroforme of an iotalike toxin that possesses mono(ADP-ribosyl)transferase activity: Identification of a novel class of ADP-ribosyltransferases. Infect Immun. 1989;57:255–261. doi: 10.1128/iai.57.1.255-261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohishi I, DasGupta BR. In: Avian Botulism. Eklund MW, Dowell VR, editors. Springfield: Charles C. Thomas; 1987. pp. 223–247. [Google Scholar]
- 14.Han S, Craig JA, Putnam CD, Carozzi NB, Tainer JA. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat Struct Biol. 1999;6:932–936. doi: 10.1038/13300. [DOI] [PubMed] [Google Scholar]
- 15.Barth H, Aktories K, Popoff MR, Stiles BG. Binary bacterial toxins: Biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol Mol Biol Rev. 2004;68:373–402. doi: 10.1128/MMBR.68.3.373-402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aktories K, Barth H. Clostridium botulinum C2 toxin—New insights into the cellular up-take of the actin-ADP-ribosylating toxin. Int J Med Microbiol. 2004;293:557–564. doi: 10.1078/1438-4221-00305. [DOI] [PubMed] [Google Scholar]
- 17.Gülke I, et al. Characterization of the enzymatic component of the ADP-ribosyltransferase toxin CDTa from Clostridium difficile. Infect Immun. 2001;69:6004–6011. doi: 10.1128/IAI.69.10.6004-6011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aktories K, et al. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 19.Vandekerckhove J, Schering B, Bärmann M, Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987;225:48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- 20.Wiegers W, et al. Alteration of the cytoskeleton of mammalian cells cultured in vitro by Clostridium botulinum C2 toxin and C3 ADP-ribosyltransferase. Eur J Cell Biol. 1991;54:237–245. [PubMed] [Google Scholar]
- 21.Hilger H, et al. The long-lived nature of clostridium perfringens iota toxin in mammalian cells induces delayed apoptosis. Infect Immun. 2009;77:5593–5601. doi: 10.1128/IAI.00710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwan C, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carette JE, et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science. 2009;326:1231–1235. doi: 10.1126/science.1178955. [DOI] [PubMed] [Google Scholar]
- 24.Carette JE, et al. Ebola virus entry requires the cholesterol transporter Niemann Pick-C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiles BG, Hale ML, Marvaud JC, Popoff MR. Clostridium perfringens iota toxin: Binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect Immun. 2000;68:3475–3484. doi: 10.1128/iai.68.6.3475-3484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckhardt M, Barth H, Blöcker D, Aktories K. Binding of Clostridium botulinum C2 toxin to asparagine-linked complex and hybrid carbohydrates. J Biol Chem. 2000;275:2328–2334. doi: 10.1074/jbc.275.4.2328. [DOI] [PubMed] [Google Scholar]
- 27.Yen FT, et al. Molecular cloning of a lipolysis-stimulated remnant receptor expressed in the liver. J Biol Chem. 1999;274:13390–13398. doi: 10.1074/jbc.274.19.13390. [DOI] [PubMed] [Google Scholar]
- 28.Mesli S, et al. Distribution of the lipolysis stimulated receptor in adult and embryonic murine tissues and lethality of LSR−/− embryos at 12.5 to 14.5 days of gestation. Eur J Biochem. 2004;271:3103–3114. doi: 10.1111/j.1432-1033.2004.04223.x. [DOI] [PubMed] [Google Scholar]
- 29.Yen FT, et al. Identification of a lipolysis-stimulated receptor that is distinct from the LDL receptor and the LDL receptor-related protein. Biochemistry. 1994;33:1172–1180. doi: 10.1021/bi00171a017. [DOI] [PubMed] [Google Scholar]
- 30.Bihain BE, Yen FT. The lipolysis stimulated receptor: A gene at last. Curr Opin Lipidol. 1998;9:221–224. doi: 10.1097/00041433-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Yen FT, et al. Lipolysis stimulated lipoprotein receptor: A novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J Biol Chem. 2008;283:25650–25659. doi: 10.1074/jbc.M801027200. [DOI] [PubMed] [Google Scholar]
- 32.Masuda S, et al. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–555. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 33.Kimura J, et al. Clostridium perfringens enterotoxin interacts with claudins via electrostatic attraction. J Biol Chem. 2010;285:401–408. doi: 10.1074/jbc.M109.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisel MB, Fofana I, Fafi-Kremer S, Baumert TF. Hepatitis C virus entry into hepatocytes: Molecular mechanisms and targets for antiviral therapies. J Hepatol. 2011;54:566–576. doi: 10.1016/j.jhep.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Satoh T, et al. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondratowicz AS, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci USA. 2011;108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibert M, et al. Endocytosis and toxicity of clostridial binary toxins depend on a clathrin-independent pathway regulated by Rho-GDI. Cell Microbiol. 2011;13:154–170. doi: 10.1111/j.1462-5822.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- 39.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santelli E, Bankston LA, Leppla SH, Liddington RC. Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430:905–908. doi: 10.1038/nature02763. [DOI] [PubMed] [Google Scholar]
- 42.Carette JE, et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat Biotechnol. 2011;29:542–546. doi: 10.1038/nbt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang G, et al. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 2008;8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papatheodorou P, Zamboglou C, Genisyuerek S, Guttenberg G, Aktories K. Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PLoS ONE. 2010;5:e10673. doi: 10.1371/journal.pone.0010673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barth H, et al. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J Biol Chem. 2000;275:18704–18711. doi: 10.1074/jbc.M000596200. [DOI] [PubMed] [Google Scholar]
- 46.Blöcker D, Behlke J, Aktories K, Barth H. Cellular uptake of the Clostridium perfringens binary iota-toxin. Infect Immun. 2001;69:2980–2987. doi: 10.1128/IAI.69.5.2980-2987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.