Abstract
In species in which males care for young, testosterone (T) is often high during mating periods but then declines to allow for caregiving of resulting offspring. This model may apply to human males, but past human studies of T and fatherhood have been cross-sectional, making it unclear whether fatherhood suppresses T or if men with lower T are more likely to become fathers. Here, we use a large representative study in the Philippines (n = 624) to show that among single nonfathers at baseline (2005) (21.5 ± 0.3 y), men with high waking T were more likely to become partnered fathers by the time of follow-up 4.5 y later (P < 0.05). Men who became partnered fathers then experienced large declines in waking (median: −26%) and evening (median: −34%) T, which were significantly greater than declines in single nonfathers (P < 0.001). Consistent with the hypothesis that child interaction suppresses T, fathers reporting 3 h or more of daily childcare had lower T at follow-up compared with fathers not involved in care (P < 0.05). Using longitudinal data, these findings show that T and reproductive strategy have bidirectional relationships in human males, with high T predicting subsequent mating success but then declining rapidly after men become fathers. Our findings suggest that T mediates tradeoffs between mating and parenting in humans, as seen in other species in which fathers care for young. They also highlight one likely explanation for previously observed health disparities between partnered fathers and single men.
Keywords: challenge hypothesis, human evolution, hormones and behavior, paternal care, reproductive ecology
In male mammals, testosterone (T) stimulates the development and maintenance of traits and behaviors that contribute to male mating effort, including musculature, libido, conspecific aggressivity, and courtship (1–4). Although these T-driven traits factor into mating success, male reproductive fitness in some avian and mammalian species also depends on contributions to offspring care (5, 6). Because time and energy are finite (7), males in these species often face tradeoffs between conflicting behaviors related to mating and parenting. Adjustment of T production has been proposed as a physiological mechanism underlying this tradeoff, with males who focus on mating effort predicted to maintain elevated T, whereas males who cooperate with a female partner and invest in parental care should reduce T production (6, 8). This model is well supported by data from a variety of avian species (6, 8), but evidence for its applicability to mammalian species in which males provide direct care is mixed (9). It is presently unclear whether T mediates the tradeoff between mating and parenting effort in human males, who often express paternal care facultatively.
Humans have been described as serial monogamists who frequently engage in one or more long-lasting partnerships with females during reproductive life spans that last several decades (10–12). Humans are one of the few mammalian species in which paternal care is relatively common, with fathers often helping to raise multiple overlapping offspring who are dependent well into their second decade of life (5, 13–15). If T contributes to human male reproductive strategy, high initial T should enhance a man's mating success, but men who have succeeded in securing a mate and/or fathering a child should then down-regulate T, particularly if they frequently care for their children (6, 8, 13).
Past human work provides indirect support for these expectations largely using cross-sectional data. Multiple studies have shown that partnered men have lower T compared with single men (16, 17), and a large 10-y study of US servicemen found that T decreased in men who married during the study period (18). In comparisons of men varying in both relationship and parenting status, partnered fathers have been shown to have the lowest T overall, differing significantly from single nonfathers in some populations (19–21), including the present study population (22, 23). There is also increasing evidence that caregiving predicts which fathers have lowest T (20, 22, 24). Although these cross-sectional correlations are generally consistent with the presumed suppressive effect of partnering and fatherhood on T production (a “state” effect), such findings could alternatively result if men with low T are more likely to become partnered or fathers (a “trait” effect) (25). However, to date, no human study has monitored hormonal changes longitudinally as single nonfathers transition into stable partnerships and become fathers.
To clarify the role of T in human male reproductive strategy, we draw on data and biological samples collected in a large sample of men participating in the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a representative 1-y birth cohort study begun in the Philippines in 1983. In addition to longitudinal questionnaire data, we collected saliva samples for T measurement at waking (AM) and before bed (PM) in all participants (n = 624) when they were 21.5 (±0.3) y of age (baseline) and again when they were 26.0 (±0.3) y of age (follow-up). All participants live in or around Cebu City, the Philippines, where it is common for fathers to be involved in day-to-day care of their children (22). Focusing on the subsample of men who were single nonfathers at baseline (n = 465), we tested the hypotheses that (i) men with higher baseline T would have greater mating success as indicated by being in a stable partnership (married/cohabitating) and/or becoming a father by the time of follow-up and (ii) these newly partnered new fathers would subsequently show a greater decrease in T than men who remained single nonfathers. We also tested the hypothesis that (iii) fathers who reported spending more time in childcare would have lower T at follow-up than fathers who reported spending less time in childcare, as would be consistent with a direct suppressive role of caregiving on T among fathers.
In observational research, correlated nonmeasured factors can influence both predictor and outcome variables, and thus lead to confounded associations. Here, we use an econometric change model that minimizes the likelihood of such confounding, because any permanent or stable factors that differ among men but that have not changed in the follow-up period are included within the error term of both regression models, and thus are eliminated as potential influences on any change in T experienced during the period of follow-up (26).
Results
Table 1 summarizes sociodemographic and biological characteristics for the full sample and also stratified on a median split of AM T measured at follow-up (2009). When the full sample was considered, all men showed a modest but significant decrease in both AM and PM T between baseline and follow-up, consistent with age-related declines documented previously (27). Men with high T tended to have completed more years of education, whereas men with low and high T did not differ in anthropometric measures. Consistent with the results presented below, a greater proportion of partnered men and fathers had T below the median.
Table 1.
Sample characteristics stratified on low and high follow-up (2009) AM T (n = 624)
| All (n = 624) | Low AM T* (n = 316) | High AM T* (n = 308) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | P value | |
| Demographic characteristics† | ||||
| Age, y | 26.0 ± 0.3 | 26.0 ± 0.3 | 26.0 ± 0.3 | 0.57 |
| Highest grade completed | 11.5 ± 4.8 | 11.2 ± 4.8 | 11.9 ± 4.8 | 0.07 |
| T values | ||||
| AM T 2005, pg/mL | 192.8 ± 74.1 | 175.5 ± 65.7 | 210.5 ± 77.9 | 0.0001 |
| AM T 2009, pg/mL‡ | 162.0 ± 61.8 | 115.9 ± 30.8 | 209.2 ± 48.4 | 0.0001 |
| PM T 2005, pg/mL | 117.7 ± 51.9 | 112.3 ± 50.0 | 123.3 ± 53.2 | 0.0001 |
| PM T 2009, pg/mL‡ | 92.6 ± 39.2 | 79.3 ± 32.4 | 106.2 ± 40.9 | 0.0001 |
| Anthropometric measures† | ||||
| Body fat percentage | 20.1 ± 5.2 | 19.9 ± 5.3 | 20.2 ± 5.1 | 0.40 |
| Body mass index, kg/m2 | 22.7 ± 3.6 | 22.5 ± 3.8 | 22.9 ± 3.4 | 0.23 |
| Relationship characteristics§ | ||||
| Partnered 2005 | 19.7% | 20.6% | 18.8% | 0.59 |
| Partnered 2009 | 54.0% | 59.8% | 48.1% | 0.003 |
| Duration of relationship¶, y | 3.7 ± 2.4 | 3.6 ± 2.4 | 3.8 ± 2.4 | 0.45 |
| Fatherhood characteristics | ||||
| Father 2005 | 16.0% | 15.8% | 16.2% | 0.89 |
| Father 2009 | 50.0% | 56.0% | 43.8% | 0.002 |
| No. children∥ | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.7 ± 0.8 | 0.18 |
| Age of oldest child∥, y | 3.4 ± 2.2 | 3.2 ± 2.2 | 3.6 ± 2.2 | 0.16 |
*Test for significant differences by median split of 2009 AM T (low AM T < 155.6 pg/mL < high AM T); unpaired, two-tailed t test or χ2 test.
†2009.
‡Paired t tests comparing 2005 and 2009 AM and PM T (both P < 0.0001).
§Married/cohabitating.
¶Restricted to partnered men in 2009 (n = 337).
∥Restricted to fathers in 2009 (n = 312).
We first used logistic regression to test whether T at baseline predicted reproductive status at follow-up (n = 465; Table S1). Consistent with our hypothesis, single nonfathers with greater AM T at baseline were more likely to become newly partnered [odds ratio (OR) = 1.20, P = 0.044] and newly partnered new fathers (OR = 1.21, P = 0.048) by the time of follow-up in 2009.
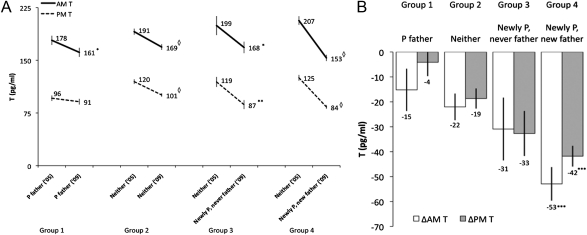
We next tested whether men who became newly partnered new fathers during the period of follow-up experienced a greater decline in T relative to men who remained single nonfathers between baseline and follow-up. Consistent with this hypothesis, men who were both newly partnered and new fathers showed the largest declines in AM and PM T between 2005 and 2009 (Fig. 1 A and B), and their declines in both AM (median: −26%) and PM (median: −34%) T were significantly greater than the modest age-related declines in AM (median: −12%) and PM (median: −14%) T observed among single nonfathers (P < 0.001, n = 465; Fig. 1B and Tables S2 and S3). Newly partnered men who remained nonfathers at follow-up showed declines in AM (median: −10%) and PM (median: −32%) T (Fig. 1A) that were not significantly different from those of single nonfathers [absolute change in AM T (ΔAM T), P = 0.499; ΔPM T, P = 0.167; Fig. 1B and Tables S2 and S3]. Effects of change in partnering and fatherhood status on ΔAM and ΔPM T were not substantially affected after adjusting for self-reported psychosocial stress and sleep quality, neither of which significantly predicted ΔT (all P > 0.3; Tables S2 and S3). In addition, men who were partnered fathers in 2005 and who already had low T at baseline showed only slight within-group declines in both ΔAM T (median: −7%, P = 0.048; n = 83) and ΔPM T (median: −3%, P = 0.374) by follow-up in 2009 (Fig. 1A and Table S4).
Fig. 1.
(A) Within-group changes in AM and PM T values between 2005 and 2009. Mean values of T, adjusted for time of saliva collection and usual wake time (AM), were compared using paired t tests. Group 1 (n = 83) comprised men who were partnered and fathers in 2005 and 2009. Group 2 (n = 257) comprised men who were not partnered in 2005 and 2009 and were never fathers. Group 3 (n = 46) comprised men who became partnered between 2005 and 2009 and were never fathers. Group 4 (n = 162) comprised men who became partnered and were first-time fathers between 2005 and 2009. *P < 0.05; **P < 0.01, ◇P < 0.0001. Error bars indicate SEM. P, partnered. (B) Between-group changes in AM and PM T values between 2005 and 2009 based on partnering and parenting status. Group descriptions are as in A. Values were adjusted for time of saliva collection and usual wake time (AM) and are derived from regressing the change in T on changes in partnering and parenting status, with group 2 as the comparison group, controlling for sleep quality and psychosocial stress (Tables S2 and S3). Partnered fathers are included for visual comparison but were not part of the regression analyses. ***P < 0.001. Error bars indicate SEM.
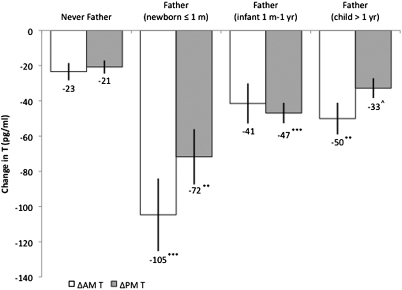
We next tested whether the large decrease in T among new fathers was contingent on the age of a man's youngest child. Although all new fathers, regardless of their youngest child's age, experienced a significant reduction in AM and/or PM T compared with nonfathers (Fig. 2 and Tables S5 and S6), fathers with newborns (1 mo old or less) at the time of follow-up hormone assessment showed significantly greater declines in AM (P = 0.023) and PM (P = 0.003) T compared with fathers whose youngest child was older than 1 y of age, which was not accounted for by reports of psychosocial stress, sleep quality, or involvement in caregiving (Tables S7 and S8). Men with newborns also differed significantly for ΔAM T compared with men with infants between 1 mo and 1 y of age (P = 0.007).
Fig. 2.
Between-group changes in AM and PM T values between 2005 and 2009 with fathers stratified by child age. Values are adjusted for time of saliva collection and usual wake time (AM) and are derived from regressing the change in T on fatherhood, stratified by child age, with men who were not fathers in 2005 and 2009 as the comparison group, and controlling for sleep quality and psychosocial stress (Tables S5 and S6). Fathers of newborns were men whose youngest child was in the perinatal period [1 mo (m) old or less]. Fathers of infants were men whose youngest child was older than 1 mo (m) but 1 y (yr) old or less. ^P < 0.10; **P < 0.01; ***P < 0.001. Error bars indicate SEM.
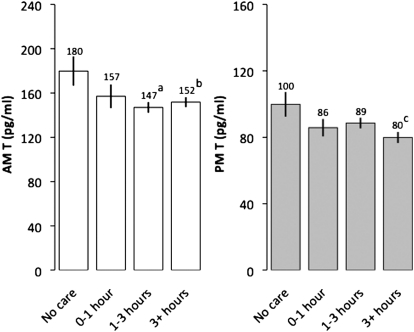
Finally, we evaluated whether men who were fathers at follow-up (n = 312) varied in T based on their self-reported involvement in childcare in 2009, controlling for sleep quality, psychosocial stress, and number of children. Men reporting 1–3 h of daily childcare had significantly lower AM T compared with fathers reporting not being involved with care (Fig. 3), whereas fathers reporting the highest involvement in childcare (3 h or more per day) showed significantly lower values of both AM and PM T compared with men reporting no care (Fig. 3). Consistent with the hypothesis that childcare suppresses T, among men who began as nonpartnered nonfathers at baseline (n = 162), time spent in childcare as fathers (at follow-up) was not predicted by either AM T [ordered logistic OR = 1.12, 95% confidence interval (CI): 0.84–1.48; P = 0.441] or PM T (OR = 0.89, 95% CI: 0.67–1.18; P = 0.417) measured at baseline.
Fig. 3.
2009 AM and PM T values among fathers varying in daily physical childcare. Values were derived from regressing T on daily paternal caregiving, controlling for time of saliva collection, usual wake time (AM), sleep quality, psychosocial stress, and number of children, with fathers who reported no involvement in childcare as the comparison group. No care (n = 34), 0–1 h (n = 37), 1–3 h (n = 139), 3+ h (n = 102). Regression models were calculated with robust SEs. aP = 0.020; bP = 0.044; cP = 0.015. AM model: R2 = 0.047; PM model: R2 = 0.046. Error bars indicate SEM.
Discussion
Among the men in our sample who were single nonfathers as young adults, those with higher waking T were more likely to have become a partnered father by the time of follow-up. Once these men entered stable partnerships and became new fathers, they subsequently experienced a large decline in T, which was greater than the comparably modest declines seen in single nonfathers during the same period. Finally, fathers who were most involved in childcare had lower T compared with fathers who did not participate in care. Using longitudinal data, these results demonstrate that high T not only predicts mating success (i.e., partnering with a female and fathering a child) in human males but that T is then greatly reduced after men enter stable relationships and become fathers. The finding that high involvement in childcare was associated with low T measured at follow-up but was not related to baseline T supports the hypothesis that direct care of dependent offspring suppressed T among the fathers in our sample (20, 22). Our findings suggest that human males have an evolved neuroendocrine architecture that is responsive to committed parenting, supporting a role of men as direct caregivers during hominin evolution (13, 14, 21).
Our results provide longitudinal evidence that high T predicts subsequent mating success in human males. Although we did not measure the behavioral or physical pathways linking T with mating success in this analysis, T has previously been shown to bolster traits related to mating effort and attractiveness, such as musculature (1, 28, 29), motivation to win during competition (30), and pursuit of social dominance (2, 31). Men with higher T have also been shown to have physical attributes deemed attractive by females and to have more recent and lifetime sexual partners (32–34). Although families traditionally played a primary role in arranging courtship and marriage in the Philippines, courting in recent decades has gradually moved toward males and females meeting independent of familial control (35). Men's romantic prospects may thus be increasingly contingent on male-male competition, particularly in social and economic domains (36). This trend has likely increased the potential for high T to factor into male mating success.
Although helpful in securing mates, many T-stimulated behaviors may conflict with partnership stability and parenting (4, 33). Indeed, men with higher T have been shown to be more likely to have marital problems and to be divorced (4, 18), whereas men with lower T have been found to spend more time with their wives (21). In an experimental setting, men with greater T also reported feeling less sympathy or need to respond to infant cries compared with men with lower T (37). Although prior cross-sectional studies have led to speculation that fatherhood decreases T in human males (19, 22, 24), our longitudinal results demonstrate that fatherhood causes T to decline and remain low. These findings were not substantively changed when covariates (psychosocial stress and sleep quality) that might be expected to mediate the relationship between fatherhood/marriage and T were included in models and are consistent with a previous longitudinal report that men who were married experienced decreased T (18).
We also found that T at follow-up was lowest among fathers reporting more hours spent in childcare. Although this finding could result if men with low T at baseline were more likely to get involved in childcare, instead, we found that childcare involvement was unrelated to T at baseline. Familial composition was not a confounding influence on the relationships that we documented, which is consistent with previous research from Cebu reporting that fathers did not alter their childcare participation based on their number of children (38). Together, these findings provide longitudinal support for the hypothesis that interacting with a dependent child suppresses T (20, 22, 24). In prior research conducted in two neighboring cultural groups in Tanzania, fathers in the population in which paternal care is the cultural norm had lower T, whereas this was not found among fathers in the group in which paternal care is absent (20). In a study of a polygynous Senegalese society, it was found that fathers who were highly invested in their children, as reported by the children's mothers, had lower T compared with fathers who were less invested (24). Lower T has also been associated with nurturing behaviors among fathers (37, 39).
Although all new fathers had lower T than men remaining single nonfathers, our results also suggest that fathers of newborns (1 mo old or less) experienced a large transient decline in T that was significantly greater than that of fathers whose youngest child was older than 1 mo of age. This finding is consistent with a previous cross-sectional study in which fathers of newborns were found to have lower T compared with a group of expectant fathers, whose T had been measured during their partners’ pregnancies (39). In another study in which expectant fathers were sampled for T multiple times during their partners’ pregnancies and after the women gave birth, those men with high T during the pregnancy showed a significant decline in the first week after birth (40). Viewed alongside these past findings, the steep transient T decrease that we document among fathers with newborns could indicate an anticipatory psychological component to men's T decline around the time of birth of their children. Alternatively, our control variables may not fully capture the scope of sleep disruption and psychosocial adjustments that accompany a family's accommodation of a newborn baby, which could contribute to this large short-term T decline in the postpartum period. Taken together, our findings suggest that anticipatory or other effects unique to the immediate period of parturition are likely additive to the more sustained effects of caregiving in suppressing paternal T.
Our results are consistent with findings from many bird species, among which fathers often show declines in T during periods in which they help raise young (6, 41). Relevant findings from other mammals are less consistent, with fathers having lower T in some (42–45) but not all (46–48) mammalian species in which fathers assist with offspring care. Analogies to bird and other mammalian species are somewhat constrained because humans are not seasonal breeders and have significantly longer interbirth intervals (3–4 y) and slowly developing dependent offspring (49, 50). Moreover, human males’ predisposition toward paternal care is likely a derived trait that emerged during hominin evolution (13–15, 51, 52). Thus, compared with other species with paternal care, men's T might decrease with fatherhood and then remain low over a longer and more sustained time period corresponding to the slow life history of humans and the prolonged dependency of offspring (50, 53, 54).
There is considerable interest in the health differentials between fathers and single men (55), and it is often reported that married men and fathers have lower risk for certain diseases and mortality (56–58). Our findings suggest that fathers are likely exposed to lower levels of T throughout much of their prime reproductive years, which could contribute to some of these health differentials. For instance, high T may increase risk for prostate cancer and adverse cholesterol profiles, and high T has also been linked to risk-taking behaviors that can affect men's health, such as drug and alcohol use and promiscuity (33, 59, 60). Our finding that men who end up as fathers tend to have higher T to begin with also suggests that some of the benefits of low T among fathers could be offset by higher T exposure among these men before becoming fathers, which could hinder efforts to identify the health impacts of being a partnered father. Thus, our findings point to likely health effects of fatherhood and also underscore some of the complexities of this exposure. The large reductions in circulating T among the new fathers in our sample provide a strong rationale to investigate linkages between fatherhood status and risk for diseases related to T exposure.
In sum, our results provide longitudinal confirmation that T exhibits a bidirectional relationship with reproductive strategy in human males. Single nonfathers with higher T at baseline were more likely to be partnered fathers 4.5 y later. After becoming partnered fathers, these men experienced dramatic reductions in both waking and evening T, which were substantially greater than the age-related declines observed in single nonfathers. Our finding that caregiving fathers had lower T than fathers who did not invest in care supports the hypothesis that father-child interaction likely contributes to suppressed paternal T among fathers. These results point to an important role of the hypothalamic-pituitary-gonadal axis as a mediator of the tradeoff between investments in parenting and mating in human males, similar to what is seen in other species in which paternal care is common. They also add to evidence that human males have an evolved neuroendocrine architecture shaped to facilitate their role as fathers and caregivers as a key component of reproductive success.
Methods
Study Population.
Data were collected in 2005 and 2009 as part of the CLHNS, a representative population-based birth cohort study of mothers and their infants born in 1983–1984 (61). Men (n = 624) were an average of 26.0 ± 0.3 (SD) y old at the time of data and sample collection in 2009. Socioeconomic, demographic, and behavioral data were collected during in-home interviews administered by Cebuano-speaking interviewers (61). Men were classified as “partnered” if they identified themselves as married or in a cohabitating relationship (22). Fathers were defined as men who reported having one or more biological children. Fathers of newborns were defined as men whose youngest child was in the perinatal period (1 mo old or less). Fathers of infants were defined as men whose youngest child was older than 1 mo of age but less than 1 y old. Paternal caregiving was assessed via the question, “How much time do you usually spend providing physical care to your children on a daily basis?” with men grouped by no contact/0 min, less than 1 h, 1–3 h, and 3+ h.
Weight (kg), height (cm), and triceps skinfold thickness (mm) were measured using standard anthropometric techniques (62). Body fat percentage was calculated from triceps skinfold thickness using body density estimates and a body composition predictive equation (63). The body mass index was calculated as the ratio of weight (kg)/height (m2). Self-reported psychosocial stress in the month preceding sampling was quantified via a modified version of the 10-item Perceived Stress Scale (PSS) (64). Sleep quality was assessed via self-reports of how many days per week subjects woke up feeling rested. This research was conducted under conditions of informed consent with human subject clearance from the Institutional Review Boards of the University of North Carolina at Chapel Hill and Northwestern University.
Salivary T Collection and Measurement.
The same saliva collection procedures were used in 2005 and 2009. Each participant was provided with instructions and two polypropylene tubes for saliva collection. The first sample was collected immediately before bed (PM) at mean sampling times of 10:14 PM ± 1:38 (SD) in 2005 and 10:04 PM ± 1:33 (SD) in 2009. The participants were instructed to collect the second sample immediately on waking the following morning (AM) and to report the time of saliva collection. Mean AM sampling times were 6:30 AM ± 1:13 (SD) in 2005 and 6:48 AM ± 1:28 (SD) in 2009. Saliva tubes were collected on the second day by an interviewer and stored at −35 °C until shipment on dry ice to Northwestern University, where they were stored at −80 °C.
Salivary T Assessment.
T concentrations were determined at the Laboratory for Human Biology Research at Northwestern University using an enzyme immunoassay protocol developed for use with saliva samples (kit no. 1-2402; Salimetrics). Interassay coefficients of variation were 13.7% and 11.5% for high and low control samples, respectively, in 2005 samples and 7.8% and 17.9% for high and low control samples, respectively, in 2009 samples.
Sample Selection.
During a 1-y period in 1983–1984, the CLHNS surveyed ∼28,000 households in randomly selected neighborhoods in metro Cebu City, inviting all pregnant women to participate (acceptance rate of 96%, n = 3,080 singleton liveborns). Thus, the original sample was representative of births during that year. Subsequent attrition has largely been attributable to out-migration, and the refusal rate for the subjects in adult surveys has typically been ∼5% (61). During the 2005 survey, 1,008 (62%) of the original cohort of 1,633 liveborn males were located and were willing to be interviewed, and 908 (56% of original cohort) men were located and enrolled in 2009. Subjects lost to attrition have generally been from higher socioeconomic status households (61), which is also true of the present sample (see below). Participants were compensated 100 pesos (∼$2 US) for their time. A final sample of 624 individuals had all required data and met all criteria for inclusion. Seventy-three men were excluded from this analysis because they were nightshift workers or had sleep patterns consistent with nightshift work, which is associated with disrupted circadian rhythms for T (65, 66). Four subjects were excluded as outliers because their T values were very high (all were 6+ SD above the sample mean), suggesting contamination of the saliva sample by blood, and one subject was excluded because of a T value below the assay detection limit. Because this sample is drawn from a cultural setting in which it is rare for men to become new fathers outside of stable romantic partnerships or to file for divorce, there were few single new fathers (n = 12) or divorced men (n = 9), who therefore were excluded from longitudinal analyses. We assessed whether subjects in the analysis differed from excluded men. Excluded individuals were born to mothers (P < 0.10) and fathers (P < 0.01) who were more educated. However, there were no significant differences between the subsample used here and the original baseline cohort in birth weight, birth length, birth order, household income, parental height, or mother or father's age at baseline (all P > 0.10).
Statistical Analysis.
All analyses were conducted using version 10 of Stata (Stata Corporation). AM T (pg/mL), PM T (pg/mL), sleep quality, and PSS were all analyzed as continuous variables. AM and PM T were each adjusted for time of sampling (AM and PM) and usual wake time (AM) before calculating absolute change in T (ΔT) between baseline (2005) and follow-up (2009). All other models were also adjusted for time of sampling (AM and PM) and usual wake time (AM). Average self-reported stress and sleep quality were calculated as the mean of 2005 and 2009 values (26).
Paired t tests were used to compare adjusted values of baseline T and follow-up T. Multiple logistic regression was used to predict 2009 partnership and fatherhood status from baseline T (z-scored) among men who were single or were not fathers in 2005. Multiple linear regression was used to predict ΔT based on partnership and fatherhood status changes between 2005 and 2009, controlling for sleep quality and self-reported stress, among men who were single nonfathers at baseline. Multiple linear regression was used to predict ΔT among men who were single nonfathers at baseline, based on the age of fathers’ youngest child at follow-up, with child age stratified according to whether the youngest child was a perinatal infant (1 mo old or less), nonperinatal infant (older than 1 mo but less than 1 y), or noninfant (older than 1 y). Multiple linear regression was also used to assess differences in fathers’ T values (2009) based on self-reported hours spent in direct physical childcare. Ordered logistic regression was used to predict men's self-reported hours spent in direct physical childcare (2009) from baseline T (z-scored) among men who were single nonfathers in 2005, with all models meeting the parallel regression assumption based on the Brant test. Statistical significance was evaluated at P < 0.05, with relationships with 0.05 < P < 0.10 interpreted as a borderline statistical trend. All regression models were tested for heteroscedasticity using the Breusch–Pagan/Cook–Weisberg test and were calculated with robust SEs where appropriate.
Supplementary Material
Acknowledgments
Linda Adair played a central role in designing and implementing the CLHNS survey from which these data and samples were obtained. Greg Duncan provided statistical advice. Jeffrey Huang helped with laboratory work. We thank the Office of Population Studies, University of San Carlos, Cebu, Philippines, for its role in study design and data collection and the Filipino participants who provided their time for this study. This work was supported by the Wenner Gren Foundation (Grants 7356 and 8186), National Science Foundation (Grants BCS-0542182 and BCS-0962212), Interdisciplinary Obesity Center (Grant RR20649), and Center for Environmental Health and Susceptibility (Grant ES10126; Project 7-2004-E). L.T.G. was supported by a National Science Foundation Graduate Research Fellowship during write-up.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 16141.
This article is a PNAS Direct Submission. A.E.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105403108/-/DCSupplemental.
References
- 1.Bribiescas RG. Reproductive ecology and life history of the human male. Am J Phys Anthropol. 2001;44(Suppl 33):148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- 2.Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Hart BL. Gonadal androgen and sociosexual behavior of male mammals: A comparative analysis. Psychol Bull. 1974;81:383–400. doi: 10.1037/h0036568. [DOI] [PubMed] [Google Scholar]
- 4.Booth A, Dabbs JM. Testosterone and men's marriages. Soc Forces. 1993;72:463–477. [Google Scholar]
- 5.Kleiman DG, Malcolm JR. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. New York: Plenum; 1981. pp. 347–387. [Google Scholar]
- 6.Wingfield JC, Hegner RE, Ball GF, Duffy AM. The ‘challenge hypothesis’: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- 7.Stearns S. Trade-offs in life-history evolution. Funct Ecol. 1989;3:259–268. [Google Scholar]
- 8.Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: Meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- 9.Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Quinlan RJ. Human pair-bonds: Evolutionary functions, ecological variation, and adaptive development. Evol Anthropol. 2008;17:227–238. [Google Scholar]
- 11.Fisher HE. Evolution of human serial pairbonding. Am J Phys Anthropol. 1989;78:331–354. doi: 10.1002/ajpa.1330780303. [DOI] [PubMed] [Google Scholar]
- 12.Winking J, Kaplan H, Gurven M, Rucas S. Why do men marry and why do they stray? Proc Biol Sci. 2007;274:1643–1649. doi: 10.1098/rspb.2006.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray PB, Anderson KG. Fatherhood: Evolution and Human Paternal Behavior. Cambridge, MA: Harvard Univ Press; 2010. [Google Scholar]
- 14.Gettler LT. Direct male care and hominin evolution: Why male-child interaction is more than a nice social idea. Am Anthropol. 2010;112:7–21. [Google Scholar]
- 15.Lancaster JB, Lancaster CS. Parental investment: The hominid adaptation. In: Ortner DJ, editor. How Humans Adapt: A Biocultural Odyssey. Washington, DC: Smithsonian Institution Press; 1983. pp. 33–66. [Google Scholar]
- 16.van Anders SM, Watson NV. Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data. Psychoneuroendocrinology. 2006;31:715–723. doi: 10.1016/j.psyneuen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre M, et al. Romantic involvement often reduces men's testosterone levels—But not always: The moderating role of extrapair sexual interest. J Pers Soc Psychol. 2006;91:642–651. doi: 10.1037/0022-3514.91.4.642. [DOI] [PubMed] [Google Scholar]
- 18.Mazur A, Michalek J. Marriage, divorce, and male testosterone. Soc Forces. 1998;77:315–330. [Google Scholar]
- 19.Gray PB, Yang C-FJ, Pope HG., Jr Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc Biol Sci. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proc Biol Sci. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evol Hum Behav. 2002;23:193–201. [Google Scholar]
- 22.Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding and testosterone in the Philippines. Horm Behav. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: Evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011;23(5):609–620. doi: 10.1002/ajhb.21187. [DOI] [PubMed] [Google Scholar]
- 24.Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: Insight from a polygynous human population. Horm Behav. 2009;56:491–497. doi: 10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 25.van Anders SM, Watson NV. Social neuroendocrinology—Effects of social contexts and behaviors on sex steroids in humans. Hum Nat. 2006;17:212–237. doi: 10.1007/s12110-006-1018-7. [DOI] [PubMed] [Google Scholar]
- 26.Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? Am Sociol Rev. 1998;63:406–423. [Google Scholar]
- 27.Mazur A. The age-testosterone relationship in black, white, and Mexican-American men, and reasons for ethnic differences. Aging Male. 2009;12:66–76. doi: 10.1080/13685530903071802. [DOI] [PubMed] [Google Scholar]
- 28.Gettler LT, Agustin SS, Kuzawa CW. Testosterone, physical activity, and somatic outcomes among Filipino males. Am J Phys Anthropol. 2010;142:590–599. doi: 10.1002/ajpa.21282. [DOI] [PubMed] [Google Scholar]
- 29.Ellison PT. On Fertile Ground: A Natural History of Human Reproduction. Cambridge, MA: Harvard Univ Press; 2001. [Google Scholar]
- 30.Salvador A, Suay F, González-Bono E, Serrano MA. Anticipatory cortisol, testosterone and psychological responses to judo competition in young men. Psychoneuroendocrinology. 2003;28:364–375. doi: 10.1016/s0306-4530(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 31.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21:353–363. discussion 363–397. [PubMed] [Google Scholar]
- 32.Pollet TV, van der Meij L, Cobey KD, Buunk AP. Testosterone levels and their associations with lifetime number of opposite sex partners and remarriage in a large sample of American elderly men and women. Horm Behav. 2011;60:72–77. doi: 10.1016/j.yhbeh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Dabbs JM, Morris R. Testosterone, social class, and antisocial behavior in a sample of 4,462 men. Psychol Sci. 1990;1:209–211. [Google Scholar]
- 34.Roney JR, Hanson KN, Durante KM, Maestripieri D. Reading men's faces: Women's mate attractiveness judgments track men's testosterone and interest in infants. Proc Biol Sci. 2006;273:2169–2175. doi: 10.1098/rspb.2006.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina B. The Filipino Family. Quezon City, Philippines: Univ of the Philippines Press; 2001. [Google Scholar]
- 36.Williams L, Kabamalan M, Ogena N. Cohabitation in the Philippines: Attitudes and behaviors among young women and men. J Marriage Fam. 2007;69:1244–1256. [Google Scholar]
- 37.Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- 38.Tiefenthaler J. Fertility and family time allocation in the Philippines. Popul Dev Rev. 1997;23:377–397. [Google Scholar]
- 39.Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 40.Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler TE. Hormones associated with non-maternal infant care: A review of mammalian and avian studies. Folia Primatol (Basel) 2000;71:6–21. doi: 10.1159/000021726. [DOI] [PubMed] [Google Scholar]
- 42.Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- 43.Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner FH. Prolactin's mediative role in male parenting in parentally experienced marmosets (Callithrix jacchus) Horm Behav. 2009;56:436–443. doi: 10.1016/j.yhbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- 46.Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler TE, Wegner FH, Carlson AA, Lazaro-Perea C, Snowdon CT. Prolactin levels during the periparturitional period in the biparental cotton-top tamarin (Saguinus oedipus): Interactions with gender, androgen levels, and parenting. Horm Behav. 2000;38:111–122. doi: 10.1006/hbeh.2000.1606. [DOI] [PubMed] [Google Scholar]
- 48.Luis J, et al. Paternal behavior and testosterone plasma levels in the Volcano Mouse Neotomodon alstoni (Rodentia: Muridae) Rev Biol Trop. 2009;57:433–439. doi: 10.15517/rbt.v57i1-2.11360. [DOI] [PubMed] [Google Scholar]
- 49.Aiello LC, Key C. Energetic consequences of being a Homo erectus female. Am J Hum Biol. 2002;14:551–565. doi: 10.1002/ajhb.10069. [DOI] [PubMed] [Google Scholar]
- 50.Robson SL, Van Schaik CP, Hawkes K. In: The Derived Features of Human Life History. The Evolution of Human Life History, School of American Research Advanced Seminar Series. 1st Ed. Hawkes K, Paine RR, editors. Santa Fe, NM: School of American Research Press; 2006. pp. 17–45. [Google Scholar]
- 51.DeSilva JM. A shift toward birthing relatively large infants early in human evolution. Proc Natl Acad Sci USA. 2011;108:1022–1027. doi: 10.1073/pnas.1003865108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geary DC. Evolution and proximate expression of human paternal investment. Psychol Bull. 2000;126:55–77. doi: 10.1037/0033-2909.126.1.55. [DOI] [PubMed] [Google Scholar]
- 53.Bogin B. Patterns of Human Growth. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 54.Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proc Biol Sci. 2006;273:835–841. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garfield CF, Clark-Kauffman E, Davis MM. Fatherhood as a component of men's health. JAMA. 2006;296:2365–2368. doi: 10.1001/jama.296.19.2365. [DOI] [PubMed] [Google Scholar]
- 56.Kobrin FE, Hendershot GE. Do family ties reduce mortality? Evidence from the United States, 1966-1968. J Marriage Fam. 1977;39:737–745. [Google Scholar]
- 57.Ringbäck Weitoft G, Burström B, Rosén M. Premature mortality among lone fathers and childless men. Soc Sci Med. 2004;59:1449–1459. doi: 10.1016/j.socscimed.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 58.Smith KR, Zick CD. Linked lives, dependent demise? Survival analysis of husbands and wives. Demography. 1994;31:81–93. [PubMed] [Google Scholar]
- 59.Parsons JK, et al. Serum testosterone and the risk of prostate cancer: Potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–2260. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 60.Bhasin S. Effects of testosterone administration on fat distribution, insulin sensitivity, and atherosclerosis progression. Clin Infect Dis. 2003;37(Suppl 2):S142–S149. doi: 10.1086/375878. [DOI] [PubMed] [Google Scholar]
- 61.Adair LS, et al. Cohort profile: The Cebu longitudinal health and nutrition durvey. Int J Epidemiol. 2010;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 63.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 64.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 65.Touitou Y, et al. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur J Appl Physiol Occup Physiol. 1990;60:288–292. doi: 10.1007/BF00379398. [DOI] [PubMed] [Google Scholar]
- 66.Axelsson J, Ingre M, Akerstedt T, Holmbäck U. Effects of acutely displaced sleep on testosterone. J Clin Endocrinol Metab. 2005;90:4530–4535. doi: 10.1210/jc.2005-0520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.