Abstract
Forkhead box O1 (FoxO1), a member of the Forkhead box-containing O family of transcription factors, is a key regulator of numerous genes that govern a wide array of cellular functions, including differentiation, homeostasis, and survival. However, the role of FoxO1 in development remains elusive. Here, we describe an essential and previously undefined role for FoxO1 in placental development. We demonstrate that FoxO1-null embryos up to embryonic day 9.0 (E9.0) are indistinguishable, including their morphology, cardiovascular structure, and vascular gene expression, from wild-type (WT) littermates. However, FoxO1-nulls manifested a profoundly swollen/hydropic allantois, which failed to fuse with the chorion, a phenotype that leads to subsequent cardiovascular malformation, progressive apoptotic cell death, and embryonic lethality at E10.5. Quantitative RT-PCR analysis of genes involved in placental development revealed significant attenuation of VCAM1 expression in FoxO1-null embryos. Using immunohistochemical, transcriptional, and chromatin immunoprecipitation assays, we further discovered that FoxO1 is an essential upstream regulator of the VCAM1 gene. Collectively, our findings provide critical molecular insight into a unique FoxO1–VCAM1 axis that governs placental morphogenesis, a process that is essential for subsequent normal cardiovascular development and fetal life.
FoxO factors are a subclass of the large family of Forkhead transcriptional regulators characterized by a conserved 110-amino-acid DNA-binding motif called the “forkhead box” or “winged helix” domain (1–3). On the basis of homologies within the forkhead box domain, the 39 distinct members of the human Forkhead family are divided into 19 subclasses (FoxA–FoxS) (3, 4). Four members of the FoxO subgroup (FoxO1, FoxO3, FoxO4, and FoxO6) recognize a consensus DNA-binding element, 5′-RYAAAYA-3′ (where R = A/G, Y = C/T), in the promoter/enhancer region of numerous genes to regulate a wide array of cellular functions, including cell differentiation, proliferation, survival, metabolism, and homeostasis of stem/progenitor cells (5–8); their role in development is just emerging. For example, mice lacking FoxO3 are viable but manifest age-dependent female infertility (9, 10), whereas targeted disruption of FoxO1, but not FoxO3 or FoxO4, leads to midgestational lethality (6, 9, 11). Although embryonic lethality of FoxO1-deficient mice has been attributed to abnormal vascular/cardiovascular morphogenesis (9, 11), mechanisms underlying these defects are currently unknown. Expression of FoxO1 in developing umbilical cord (9) suggests that, apart from its putative role in the cardiovascular system, FoxO1 may play a role in other contexts, such as development of the placenta.
The placenta is the first organ to form during mammalian embryogenesis, establishing the maternal–fetal circulatory system for the exchange of gases, nutrients, waste products, and growth factors (12, 13). Therefore, subtle perturbations in its morphogenesis and function due to either genetic or environmental insult underlie many aspects of organ malformation, pregnancy complications, and early pregnancy loss (12, 13). Although the human and rodent placentas differ in specific aspects of their architecture, the overall molecular mechanisms and distinct stages of placental development are similar (12). Among these stages, attachment of the allantois, a mesodermal extraembryonic sac that arises at the posterior end of the embryo, with its counterpart, the chorion, at E8.5 is believed to be one of the most critical aspects of functional placental morphogenesis (12, 13). Therefore, defects in chorioallantoic attachment are one of the most common causes of midgestation embryonic lethality (12, 13).
Precise mechanisms governing chorioallantoic attachment are unknown, but the general consensus is that interaction between the vascular cell adhesion molecule-1 (Vcam-1) of the allantois with its counterpart, α4 integrin, in the chorion, is critically involved (14–16). Given the midgestational lethality of FoxO1-null embryos (6, 9, 11) and FoxO1's expression in developing umbilical cord (9), we set out to investigate whether FoxO1 participates in placental development.
In the present study, we have defined the molecular mechanism underlying midgestation lethality of FoxO1-null embryos. We demonstrate that early developing FoxO1-null embryos are morphologically and anatomically indistinguishable from their wild-type (WT) littermates, and cardiovascular structures are intact. However, mutant embryos at this stage manifest a profoundly swollen/hydropic allantois, which does not fuse with the chorion. Using immunohistochemical, histological, and gene expression analyses, we demonstrate that this lack of chorioallantoic attachment in FoxO1-null embryos was not due to cardiovascular malformations, but rather arises due to dysregulation of VCAM1 gene expression. Indeed, using transcriptional, chromatin immunoprecipitation (ChIP), and TUNEL assays, we report that FoxO1 is an essential upstream regulator of the VCAM1 gene, and that a FoxO1–VCAM1 axis is critical for cell survival and subsequent growth and development of the placenta and the cardiovascular system. Collectively, our findings provide unique mechanistic and molecular insight into a previously unrecognized FoxO1–VCAM1 axis that governs placental morphogenesis, which is essential for subsequent cardiovascular development and fetal life.
Results and Discussion
FoxO1 Is Essential for Allantois and Cardiovascular Morphogenesis.
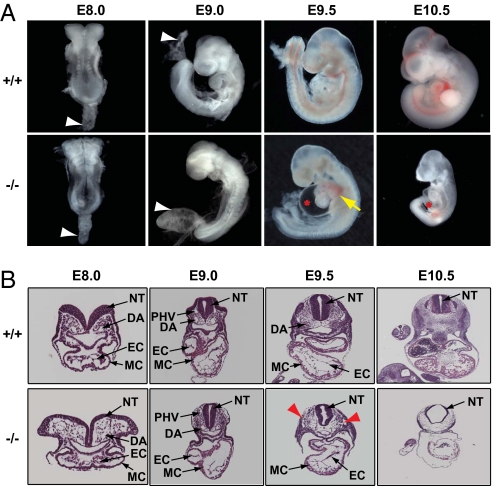
To gain insight into the role of FoxO1 in development, we mated FoxO1 heterozygous mice (6) and isolated FoxO1-WT, -heterozygous, and -null embryos from timed pregnant females at distinct developmental stages (17). Consistent with previously reported studies (6, 9, 11), FoxO1-null embryos were dead at E10.5, and multiple cardiovascular malformations, including looping defects, pericardial edema, and hemorrhage, were apparent at E9.5 (Fig. 1A). By contrast, FoxO1-heterozygous embryos were viable and morphologically indistinguishable from their WT littermates at all developmental stages (Fig. S1A), suggesting that at least one allele of the FoxO1 gene is sufficient for normal growth and survival of developing and adult mice. Histological analyses of transverse sections of WT and null embryos at E9.5 revealed abnormal morphogenesis of endocardium and major vessels, including dorsal aorta, in FoxO1-null embryos (Fig. 1B and Fig. S2A).
Fig. 1.
FoxO1 is essential for allantois and cardiovascular morphogenesis. (A) Morphological appearances of FoxO1-WT (+/+) and -null (−/−) embryos isolated at the indicated developmental stages. Note that null embryos died at E10.5 and manifested pericardial edema (red asterisk) and hemorrhage (yellow arrow) at E9.5 and E10.5. White arrowheads denote the allantois of E8.0 and E9.0 embryos. Note the swollen/hydropic allantois in FoxO1-null embryos at E9.0. (B) Hematoxylin and eosin (H&E) staining of transverse sections of E8.0–E10.5 embryos revealed normal cardiovascular development in FoxO1-null embryos isolated between E8.0 and E9.0 of development, whereas FoxO1-null embryos at E9.5 manifested cardiovascular malformations, including a looping defect, aberrant attachment of endocardial endothelium, and ruptured vessels (red arrowheads). DA, dorsal aorta; PHV, primary head vein; EC, endocardium; MC, myocardium; NT, neural tube.
Next, we isolated embryos at slightly earlier stages of gestation, E8.0–E9.0, and noted that FoxO1-WT and -null littermates were morphologically and anatomically indistinguishable (Fig. 1). However, compared with the normal, funnel-shaped configuration of the allantois in FoxO1-WT and -heterozygous embryos at E9.0, FoxO1-null littermates manifested a profoundly swollen/hydropic allantois (Figs. 1A and 2 A and D and Fig. S1A). Further, the configuration of the allantois was different in FoxO1-WT and -null littermates at E8.0 as well (Fig. 1A and Fig. S1B). RT-PCR analysis of RNA isolated from embryonic and extraembryonic tissues established FoxO1 expression in both WT allantois and total embryo at these early stages (Fig. S1C). Collectively, these data suggest strongly that FoxO1 is essential for normal growth and morphogenesis of both the allantois and the cardiovascular system.
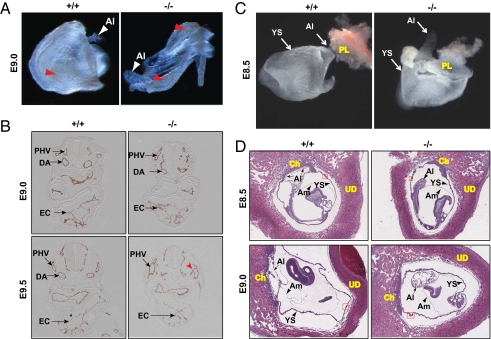
Fig. 2.
Lack of chorioallantoic attachment in FoxO1-null embryos was not due to cardiovascular malformations. (A) Normal vascular development in FoxO1-WT (+/+) and -null (−/−) YS at E9.0. Note a blood-containing honeycomb-like vascular plexus (red arrowheads) in both WT and null YS. (B) Immunohistochemical analyses for α-endomucin in WT (+/+) and FoxO1-null (−/−) embryos at the indicated developmental stages revealed normal cardiovascular development at E9.0. Note abnormal vascular (such as DA) and endocardial morphogenesis in null embryo compared with WT littermates at E9.5. Red arrowhead denotes a disrupted vessel in null mice. DA, PHV, and EC are as in Fig. 1. (C) FoxO1-WT (+/+) and -null (−/−) embryos were isolated at E8.5 and photographed without detaching from placenta (PL) and yolk sac (YS). Note that the allantois (Al) of WT, but not the null littermates is attached to the chorion (arrow). (D) H&E staining of E8.5 and E9.0-WT and -null littermates within uteri revealed lack of chorioallantoic attachment in FoxO1-null embryos. Small arrow(s) indicates chorioallantoic attachment in FoxO1-WT embryos. Al, YS, chorion (Ch), amnion (Am), uterine decidua (UD), and blood island (red box, magnified in Fig. S3) are indicated.
Failure of Chorioallantoic Attachment in FoxO1-Deficient Embryos Is Not Due to Cardiovascular Malformation.
Having detected the abnormal allantois and dysregulated cardiovascular morphogenesis in FoxO1-null mice (Fig. 1), we first sought to determine whether the misshapen allantois in early developing null embryos was associated with vascular and/or cardiovascular malformation. To compare embryonic and extraembryonic vascular development in FoxO1-WT and -null embryos, we examined embryos without detaching them from the yolk sac (YS). We observed a honeycomb-like vascular plexus containing blood in the YS at E8.5 (Fig. 2C) and E9.0 (Fig. 2A) of both FoxO1-WT and -null embryos. The presence of erythrocytes within the dorsal aorta of FoxO1-WT and -null embryos at E9.0 (Fig. 1B and Fig. S2A) further supported the notion that both embryonic and extraembryonic vascular structures were normal in early (between E8.5 and E9.0) developing WT and FoxO1-null littermates. This conclusion is consistent with a previous study (11) and was further corroborated by immunohistochemical analyses for the endothelial/endocardial marker α-endomucin (Fig. 2B) (17) and measurements of transcript levels of two endothelial-specific genes, Cd31/Pcam1 and Tie2 (Fig. S2B) (18), each of which was essentially similar in both genotypes. In contrast with these early observations, a well-developed YS and head vasculature containing blood, readily seen in FoxO1-WT embryos, was absent in null littermates at E9.5 (Fig. 1A and Fig. S2C). Although endothelial/endocardial cells were detected in E9.5 embryos (Fig. 2B), aberrant association of endocardial endothelium and ruptured vessels were each seen exclusively in FoxO1-null embryos (Figs. 1B, 2B, and 3B); this was further evident by the presence of hemorrhage (Fig. 1A) and absence of erythrocytes within the dorsal aorta of FoxO1-null, but not -WT embryos (Fig. S2A). Collectively, these data strongly suggest that the misshapen allantois observed in E8.0 and E9.0 null embryos (Figs. 1A and 2) was not associated with vascular/cardiovascular malformation. Indeed, early developing embryos deficient of the ER71 gene, a master regulator of the genesis of the cardiovascular system, do not manifest a misshapen allantois (17). On the basis of these data, we hypothesized that FoxO1 plays an essential role in placental development.
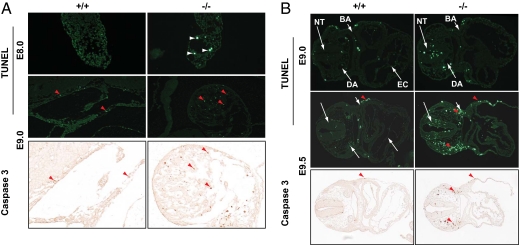
Fig. 3.
FoxO1-deficient embryos manifest progressive apoptotic cell death. The DeadEnd fluorometric TUNEL system and immunohistochemical analyses for activated caspase-3 were used to compare programmed cell death in FoxO1-WT (+/+) and -null (−/−) allantois (A) and embryo proper (B). Note an increase of apoptotic cell death in allantois (white arrowheads) and embryos of FoxO1-null mice. Red arrowheads indicate TUNEL- and corresponding caspase-3–positive cells. All images were captured using a 10× objective, except that the allantois with caspase-3 staining was at 20× magnification. BA, branchial arch; DA, PHV, EC, and NT are as in Fig. 1 and indicated with white arrows.
To test this hypothesis, we next sought to determine whether the misshapen allantois in FoxO1-null embryos was associated with failure of chorioallantoic attachment. In FoxO1-WT embryos, which were either isolated (Fig. 2C) or identified within uteri (Fig. 2D) at E8.5, the allantois had fused normally with the chorion. Similarly, the allantois of FoxO1-WT embryos was fused with the chorion at E9.0 (Fig. 2D). By contrast, we noted a striking absence of chorioallantoic attachment in FoxO1-null littermates at each of these developmental stages (Fig. 2 C and D), whereas formation of the amnion (Fig. 2D) and blood islands (Fig. S3) were each normal in FoxO1-null and -WT littermates. These data further suggest that the lack of chorioallantoic attachment in FoxO1-null embryos was not associated with a developmental delay or abnormal cardiovascular morphogenesis. Collectively, these findings support our hypothesis that FoxO1 is essential for chorioallantoic attachment/placental development, a process that is critical for subsequent normal vascular/cardiovascular development, fetal growth, and survival.
FoxO1-Deficient Embryos Manifest Progressive Apoptotic Cell Death.
Having established that FoxO1 is essential for chorioallantoic attachment (Fig. 2), and that this chorioallantoic attachment is critical in establishing placental–fetal circulatory systems for the exchange of nutrients, cell–cell communication, and homeostasis (12, 13), we set out to determine whether disruption of placental–fetal circulatory interactions in FoxO1-null embryos contributes to their embryonic lethality. Immunohistochemical analyses for Ki67 demonstrated normal, active cellular proliferation in FoxO1-null and -WT littermates (Fig. S4), suggesting that lethality was not due to a lack of cell proliferation. Using TUNEL assays, we next probed for evidence of apoptotic cell death in FoxO1-WT and –null embryos. Compared with WT allantois, apoptotic cell death in FoxO1-null allantois was significantly increased at E8.0 and E9.0 (Fig. 3A). By contrast, we did not detect evidence of increased cell death in other regions of E8.0 FoxO1-null embryos (Fig. S5A). At later stages (E9.0 and E9.5), however, apoptotic cell death was progressively increased in FoxO1-null embryos compared with WT littermates (Fig. 3B and Fig. S5B). Apoptotic cell death in FoxO1-null allantois and embryos was further confirmed by immunohistochemical analyses for activated caspase-3 (Fig. 3). It is worth noting that the apoptotic cells were detected primarily in the allantois and the neural tube, dorsal aorta, and branchial arches of embryo proper (Fig. 3), regions which are the primary sites of FoxO1 gene expression early during development (9, 11). We conclude that FoxO1 is a critical upstream regulator of transcriptional networks governing cell–cell communication in chorioallantoic attachment, a process that is critical for fetal cell survival, homeostasis, and subsequent normal organ, including vascular/cardiovascular, development.
FoxO1 Is an Essential Upstream Regulator of the VCAM1 Gene.
Next, we sought to define mechanisms of FoxO1-dependent transcriptional regulation of placental morphogenesis. Numerous studies based on gene mutation strategies have identified a host of cellular factors, including, but not limited to, diverse transcription factors, cell signaling, and adhesion proteins as essential regulators for placental morphogenesis (12, 13). Interestingly, mutation of several genes that are expressed in developing heart but are essentially absent in allantois, such as Itga4, which encodes α4 integrin (16) and Mrj/Dnajb6, which encodes a cochaperone protein (19), are reported to manifest phenotypic consequences similar to those observed in FoxO1-deficient mice (Figs. 1 and 2). To define the transcriptional circuitry governed by FoxO1 during placental development, we used an unbiased strategy and examined expression of numerous genes that are essential at discrete stages of placental morphogenesis. Quantitative RT-PCR (qRT-PCR) analyses of RNA from hearts of FoxO1-WT and -null embryos revealed that expression of the Itga4 gene was not repressed in FoxO1-null heart (Fig. 4A), which was consistent with immunohistochemical analyses revealing similar α4 integrin protein levels in WT and FoxO1-null chorion (Fig. S6). Similarly, transcripts of the Mrj gene, which encodes a cochaperone protein (19), and Hand1, which encodes a basic helix-loop-helix transcription factor, each essential for early placental and late cardiovascular development (20–22), were not attenuated in FoxO1-null heart (Fig. 4A). These data strongly suggest that defects in placental morphogenesis in FoxO1-null mice were not associated with altered expression of these genes. By contrast, we noted significant attenuation of VCAM1 transcripts in FoxO1-null heart (Fig. 4A), which was further corroborated with immunohistochemical analyses revealing significantly diminished Vcam-1 protein levels in FoxO1-null heart (Fig. 4C). These data, then, suggest that FoxO1 regulates placental morphogenesis by modulating VCAM1 gene expression. Additionally, it has been reported that attenuated expression of BMP4, but not VCAM1, is associated with placental malformations in Foxf1-deficient mouse embryos (23), suggesting that mechanisms of placental morphogenesis regulated by FoxO1 are distinct from those of Foxf1.
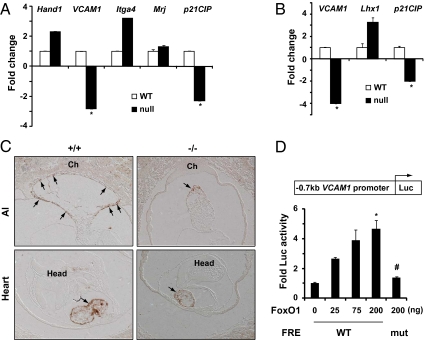
Fig. 4.
FoxO1 is an upstream regulator of VCAM1 gene expression. Quantitative RT-PCR analyses for fold change of the transcript of indicated genes using RNA isolated from FoxO1-WT and -null heart (A) and allantois (B) (n = 3–4). Gene expression in WT heart and allantois was normalized to 1. Note the significant dysregulation of VCAM1 gene expression in FoxO1-null heart and allantois (#P < 0.001 versus WT). (C) Immunohistochemical analyses for Vcam-1 in E8.5 FoxO1-WT (+/+) and -null (−/−) embryos revealed significant attenuation of Vcam-1 levels (arrows) in FoxO1-null heart and allantois (Al) compared with WT littermates. Head and chorion (Ch) are indicated. (D) Schematic of the VCAM1 upstream promoter region (−0.7 kb) fused to a luciferase (Luc) reporter. Coexpression of a constitutively active FoxO1 (caFoxO1) elicited dose-dependent activation of VCAM1 reporter activity harboring WT, but not mutated, FoxO-responsive elements (FREs) (*P < 0.001 versus control; #P < 0.01 versus WT FREs). Error bars represent mean ± SD, and P values were calculated by Student's t test.
Vcam-1 is a member of the transmembrane glycoprotein Ig superfamily expressed in a variety of vascular and nonvascular cells to mediate cell–cell interactions (24). Although Vcam-1 function was originally thought to be associated primarily with white cell–endothelial cell interaction in response to inflammatory cytokines (25), gene disruption analyses uncovered its pivotal role in placental and subsequent cardiovascular development (14, 15). Consistent with this, the VCAM1 gene is expressed predominantly in the allantois and heart of early developing embryos (15). Given this, we asked whether VCAM1 expression was also attenuated in FoxO1-null allantois. Indeed, qRT-PCR, and immunohistochemical analyses, revealed significant attenuation of VCAM1 transcripts (Fig. 4B) and protein levels (Fig. 4C) in FoxO1-null allantois compared with WT embryos. In contrast, expression of Lhx1, a Lim-domain transcription factor involved in chorioallantoic attachment (12, 13), was not attenuated in FoxO1-null allantois (Fig. 4B), suggesting that attenuation of VCAM1 gene expression was FoxO1 specific. This was further corroborated by immunohistochemical analyses revealing co-localization of FoxO1 and Vcam-1 in developing allantois (Fig. S7A). Also, coexpression of FoxO1 and VCAM1 genes was confirmed in endothelial progenitor cells using qRT-PCR analyses of RNA extracted from Tie2-GFP+ cells (Fig. S7B) (17). At the same time, significant dysregulation of p21CIP, a known downstream target gene of FoxO1 (26), in FoxO1-null heart (Fig. 4A) and allantois (Fig. 4B) further suggested that the regulation of VCAM1 abundance occurred at the level of gene transcription. On the basis of these data, we hypothesized that VCAM1 is a unique downstream target gene of FoxO1.
To complement these in vivo data, we undertook in vitro analyses to test further whether VCAM1 is a downstream target gene of FoxO1. Sequence analysis of the VCAM1 gene revealed two conserved FoxO-responsive elements (FREs) in its upstream promoter region (Fig. S8A). We next fused the VCAM1 promoter to a luciferase reporter and conducted transcriptional assays to define the specificity of FoxO1 as a transcriptional regulator of the VCAM1 gene. We observed a significant and dose-dependent induction of luciferase activity by FoxO1 from a VCAM1 promoter harboring WT-FREs (Fig. 4D), whereas mutation of the FREs (Fig. S8B) abrogated transcriptional activity (Fig. 4D), suggesting that FoxO1 binding to the FREs is essential for its transcriptional activity. Using ChIP assays (17), we confirmed the in vivo occupancy of FoxO1 at the VCAM1 promoter harboring FREs (Fig. S8C). Thus, given the striking similarities between the phenotypes of FoxO1- (Figs. 1 and 2) and VCAM1-null (14, 15) mice, and evidence of transcriptional regulation of VCAM1 gene expression by FoxO1, we concluded that FoxO1 is an essential upstream regulator of the VCAM1 gene and that this FoxO1–VCAM1 transcriptional axis is critical for normal allantois development and establishment of maternal–fetal circulatory interaction during embryogenesis. To our knowledge, this is a unique report that has established FoxO1 as an essential transcriptional regulator of the VCAM1 gene in vivo and unveiled its physiological significance in fetal growth and survival.
In this study, we report an essential and previously unrecognized FoxO1–VCAM1 network that is critical to early placental development and establishment of maternal–fetal circulatory interactions for subsequent normal cardiovascular morphogenesis. Further, our study provides insight into the mechanism of embryonic lethality of FoxO1-null embryos; inactivation of the FoxO1 gene did not affect early vascular and/or cardiovascular development (Figs. 1 and 2), but rather disrupted placentation (Fig. 2), triggered progressive progenitor cell death (Fig. 3), and vascular leakage leading to midgestational lethality due to hemorrhage and cardiovascular malformations (Fig. 1). In other words, these findings have not only unveiled a pivotal and previously unrecognized role for FoxO1 in development but they also provide key mechanistic insight into a FoxO1–VCAM1 axis, which is critical to normal cell–cell communication and cell viability within the developing embryo and placenta.
Conditional inactivation of FoxO1 in the developing cardiovascular system using Cre-mediated recombination strategies is not lethal (27). However, expression of FoxO1 in the developing cardiovascular system (28) suggests a putative role for FoxO1 and/or a FoxO1–VCAM1 axis in cardiovascular development. The fact that the cardiac phenotype in VCAM1-null (15) and other mutant mouse models precedes placental malformation (14–16, 19, 20), confounds analysis of their autonomous role in cardiovascular development. Similarly, the relative contributions to embryonic cardiovascular malformation (Fig. 1) afforded by placental malformation versus FoxO1 gene inactivation within the cardiovascular system itself is unknown. Future work involving conditional and combinatorial inactivation of FoxO1 in cardiac lineages will be needed to unveil a potentially cardiovascular-autonomous role of FoxO1. However, the fact that more than half a million pregnancy losses occur annually due to miscarriage in the United States alone highlights the significance of the pathway described here in normal placental development and fetal growth and survival. In addition, our findings have implications regarding potential danger associated with use of Vcam-1 and FoxO1 antagonists during pregnancy.
Materials and Methods
Plasmids.
A 0.7-kb upstream promoter fragment of the VCAM1 gene was PCR amplified from mouse genomic DNA and cloned into MluI and XhoI sites of the pGL3-luc reporter plasmid (Promega) and confirmed by sequencing. The conserved FREs in the VCAM1 promoter were mutated by serial PCR amplification of promoter fragments using mutant primer pairs as described (17) and sequence confirmed. A constitutively active FoxO1-expressing plasmid was generously provided by D. H. Castrillon (University of Texas Southwestern, Dallas, TX) (29).
Mouse and Embryo Isolations.
FoxO1 knockout mice were described previously (6). The staging and processing of embryos and genotyping using genomic DNA, extracted either from yolk sacs or embryo proper, were described previously (17). All mice were maintained in the animal facility at University of Texas Southwestern Medical Center according to the guidelines of the institutional animal care and use committee and the Animal Resource Center. A detailed procedure is provided in SI Materials and Methods.
Histological, Immunohistochemical, and TUNEL Analyses.
Histological and immunostaining analyses for α-endomucin and Ki67 were described previously (17). Immunostaining for activated caspase-3 (Cell Signaling; no. 9661S) and α4 integrin (Antibodies-Online; no. 03F01) was carried out as described for α-endomucin, except that 0.05% citraconic anhydride was used for antigen retrieval, and the primary antibody was used at 1:50 and 1:40 dilution, respectively. Immunostaining for Vcam-1 (Santa Cruz Biotechnology; sc-8304) was performed as described (17), except that the signal was amplified using the Tyramide Signal Amplification kit (Perkin-Elmer) according to the manufacturer's instructions and detected by streptavidin-peroxidase (Vector Laboratories) and diaminobenzidine (Dako-Cytomation). Apoptotic cell death in FoxO1-WT and -null embryos was analyzed according to previously published methods (17).
RT-PCR and Quantitative RT-PCR Analyses.
Total RNA from FoxO1-WT and -null embryos, hearts, and extraembryonic tissues (yolk sac and allantois) was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. Equal amounts of RNA was used to prepare cDNA and used for RT-PCR and qRT-PCR analyses as described (17). A detailed procedure is provided in SI Materials and Methods.
Reporter Gene Assays.
Transcriptional assays using VCAM1 reporter constructs, harboring WT or mutated FoxO-response elements, were performed as described (17, 30, 31), except that COS1 cells were used, and DNA (310 ng) was transfected using Fugene-HD (Roche) according to the manufacturer's instructions. Luciferase activity was measured using dual-luciferase kit (Promega), and luciferase activity of pTK-renilla (control) was used to normalize firefly luciferase activity. Each assay was performed in triplicate and repeated three times. Luciferase activity in the absence of FoxO1 was normalized to 1 to determine FoxO1-dependent fold activation of luciferase activity.
Supplementary Material
Acknowledgments
We thank Drs. Diego H. Castrillon for providing FoxO1 knockout mice and the constitutively active FoxO1 expression plasmid and Eric N. Olson for antibody. We acknowledge John Shelton for assistance with the TUNEL assays. We also thank Drs. Beverly Rothermel, Sergio Lavandero, and Thomas Gillette for critical reading of the manuscript. Funding for these studies was provided by the National Institutes of Health (HL-075173, HL-080144, HL-090842, U01-HL100401-01, and P30-HL101254-01), American Heart Association (0640084N), American Diabetes Association (7-08-MN-21-ADA), the AHA–Jon Holden DeHaan Foundation (0970518N), and the March of Dimes (5-FY09-21).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107341108/-/DCSupplemental.
References
- 1.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson P, Mahlapuu M. Forkhead transcription factors: Key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 3.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 4.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: Of mice, men and foxes. Biochem J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 6.Paik JH, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik JH, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferdous A, Battiprolu PK, Ni YG, Rothermel BA, Hill JA. FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res. 2010;3:355–364. doi: 10.1007/s12265-010-9200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosaka T, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 11.Furuyama T, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 12.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 13.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 14.Gurtner GC, et al. Targeted disruption of the murine VCAM1 gene: Essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Kwee L, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 16.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 17.Ferdous A, et al. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci USA. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 19.Hunter PJ, Swanson BJ, Haendel MA, Lyons GE, Cross JC. Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development. 1999;126:1247–1258. doi: 10.1242/dev.126.6.1247. [DOI] [PubMed] [Google Scholar]
- 20.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 21.Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- 22.Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 24.Osborn L, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993;143:1551–1559. [PMC free article] [PubMed] [Google Scholar]
- 26.Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Han Y, Ferdous A, Corey DR, Kodadek T. Transcription activation by a PNA-peptide chimera in a mammalian cell extract. Chem Biol. 2003;10:909–916. doi: 10.1016/j.chembiol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Martin CM, et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.