Abstract
Gram-positive bacteria of the genus Streptomyces are industrially important microorganisms, producing >70% of commercially important antibiotics. The production of these compounds is often regulated by low-molecular-weight bacterial hormones called autoregulators. Although 60% of Streptomyces strains may use γ-butyrolactone–type molecules as autoregulators and some use furan-type molecules, little is known about the signaling molecules used to regulate antibiotic production in many other members of this genus. Here, we purified a signaling molecule (avenolide) from Streptomyces avermitilis—the producer of the important anthelmintic agent avermectin with annual world sales of $850 million—and determined its structure, including stereochemistry, by spectroscopic analysis and chemical synthesis as (4S,10R)-10-hydroxy-10-methyl-9-oxo-dodec-2-en-1,4-olide, a class of Streptomyces autoregulator. Avenolide is essential for eliciting avermectin production and is effective at nanomolar concentrations with a minimum effective concentration of 4 nM. The aco gene of S. avermitilis, which encodes an acyl-CoA oxidase, is required for avenolide biosynthesis, and homologs are also present in Streptomyces fradiae, Streptomyces ghanaensis, and Streptomyces griseoauranticus, suggesting that butenolide-type autoregulators may represent a widespread and another class of Streptomyces autoregulator involved in regulating antibiotic production.
Keywords: structure elucidation, actinomycetes, intercellular communications, microbial hormones
Bacteria commonly use small extracellular signaling molecules to control complex physiological traits such as biofilm formation, pathogenicity, and antibiotic production (1, 2). Autoregulators are signaling molecules that are able to trigger antibiotic production in the genus Streptomyces, Gram-positive bacteria that have the ability to produce a plethora of antibiotics with important medical and agricultural applications that are encoded by biosynthetic gene clusters. One well-studied family of autoregulators consists of the γ-butyrolactones, which are active at nanomolar concentrations and elicit antibiotic production by modulating the DNA binding activity of cognate receptor proteins; hence, they have been referred to as bacterial hormones (3). The most characterized γ-butyrolactone autoregulator is A-factor (compound 2) (Fig. 1A), discovered as the first example in the early 1960s, which triggers both streptomycin production and sporulation in Streptomyces griseus (4, 5). Fourteen γ-butyrolactone autoregulators have since been identified in several Streptomyces species (3, 6). At least 60% of Streptomyces species appear to synthesize this type of signaling molecule (7), whereas signaling molecules produced by many other species still remain to be unraveled, largely because of extremely low concentrations in the culture medium. Recently, a furan-type autoregulator, methylenomycin furan (MMF), was shown to act as an additional class of Streptomyces autoregulator, although the minimum concentration required for inducing antibiotic production was not reported (8). Unlike γ-butyrolactone–type autoregulators, furan autoregulators are produced by enzymes encoded by plasmid-borne genes, and their distribution across the genus Streptomyces remains unclear.
Fig. 1.
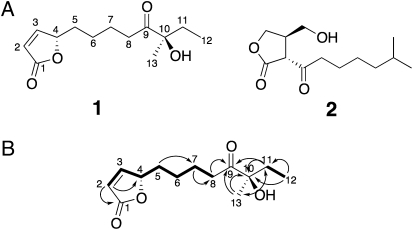
Chemical structures of natural avenolide (1) and A-factor (2) as a representative γ-butyrolactone autoregulator (A). COSY (bold lines) and HMBC (arrows) correlations of avenolide (1) (B). Double arrow indicates cross peak in HMBC of 1.
Streptomyces avermitilis is used for the industrial production of the important anthelmintic agent avermectin. A semisynthetic derivative of avermectin, ivermectin (22,23-dihydroavermectin B1), has been used as an agricultural pesticide and antiparastic agent since 1985. Ivermectin plays a major role in animal health and is also being used in humans to eradicate onchocerciasis, one of the neglected tropical diseases in Africa (9, 10), and strongyloidiasis in Asia (11). However, little was known about the extracellular signaling molecule that might regulate avermectin production. Here we report the isolation and structural elucidation of another type of signaling molecule termed “avenolide” for antibiotic production in the industrial microorganism S. avermitilis.
Results
Decreased Levels of Avermectin in aco Mutant Are Restored by Addition of Organic Solvent Extracts of Wild-Type Culture Broth.
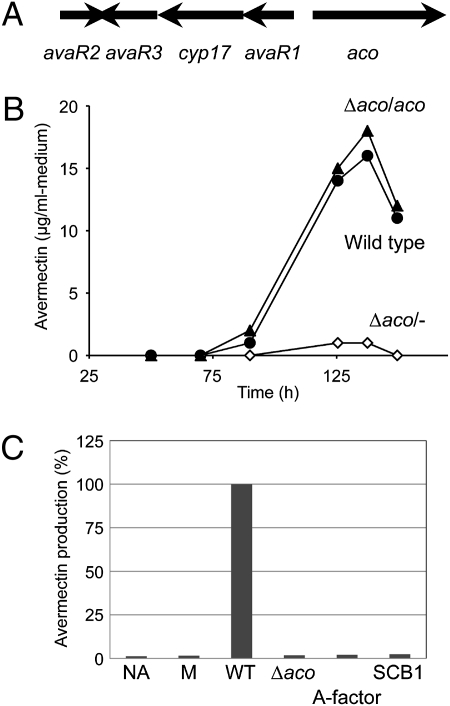
Genes involved in autoregulator biosynthesis and that encode the autoregulator receptor protein are frequently clustered at the same locus in various Streptomyces species (12). sav3702-3706 of S. avermitilis, which is located far away from the avermectin gene cluster (sav935-953), consists of three genes, avaR1 (sav3705), avaR2 (sav3702), and avaR3 (sav3703), that encode homologs of γ-butyrolactone–autoregulator receptor proteins and two genes, aco (sav3706) and cyp17 (sav3704), that encode an acyl-CoA oxidase and a cytochrome P450 hydroxylase (CYP154B2), respectively (Fig. 2A). Loss of the S. fradiae aco and cyp17 homologs abolished production of the antibiotic tylosin together with an unidentified compound that interacts with the autoregulator receptor (13). To determine whether S. avermitilis aco is important in avermectin production, a knockout mutant was constructed (SI Materials and Methods) and evaluated for avermectin production. The level of avermectin production in the aco mutant was markedly reduced to ∼6% of wild-type levels at 138 h of cultivation (Fig. 2B). The introduction of an intact copy of aco with its upstream region into the aco mutant restored avermectin production to the level of the wild-type strain (Fig. 2B). These results confirmed that aco is indeed involved in avermectin production, although aco has no assigned functions in the context of avermectin biosynthesis itself. Thus, impaired pathways of signal transduction for the avermectin production could explain why the remarkable reduction was observed in the aco mutant. To test this hypothesis, we added organic solvent extracts from the culture broth into the aco mutant (Fig. 2C). The defect in the avermectin production was restored completely by the addition of an ethyl acetate (AcOEt) extract of the wild-type culture broth. Interestingly, an AcOEt extract of the aco mutant was unable to induce avermectin production, which suggests that signaling molecule(s) eliciting avermectin production is present in the wild-type strain, but not in the aco mutant. Our subsequent hypothesis was that the signaling molecule might belong to the family of γ-butyrolactone autoregulators. To test this hypothesis, we added representative γ-butyrolactone autoregulators (A-factor, IM-2, virginiae butanolides, or SCB1) (3) into the culture of the aco mutant, resulting in no recovery of avermectin production. All of these results implied that a low-molecular-weight (low-MW) factor distinct from known γ-butyrolactone autoregulators was likely to be involved in avermectin biosynthesis.
Fig. 2.
Avermectin production in the aco mutant. (A) Organization of the avaR genes and the avenolide biosynthetic genes in S. avermitilis. (B) Production profiles of avermectin in the S. avermitilis wild-type strain and aco mutant. Filled circles, open diamonds, and filled triangles indicate time courses of avermectin production in the wild-type strain, the aco mutant (Δaco/−), and aco-complemented aco mutant (Δaco/aco), respectively. (C) Restoration of avermectin production in the aco mutant with the AcOEt extracts from S. avermitilis strains and γ-butyrolactone autoregulators. NA, no addition; M, AcOEt extract from production medium. The AcOEt extract or synthetic γ-butyrolactone autoregulator (A-factor and SCB-1, 246 nM) were added into the culture of the aco mutant at 48 h of cultivation, and the amounts of avermectin were measured in the culture broth after 144 h of cultivation.
Influence of Organic Solvent Extracts on DNA Binding Activity of AvaR1.
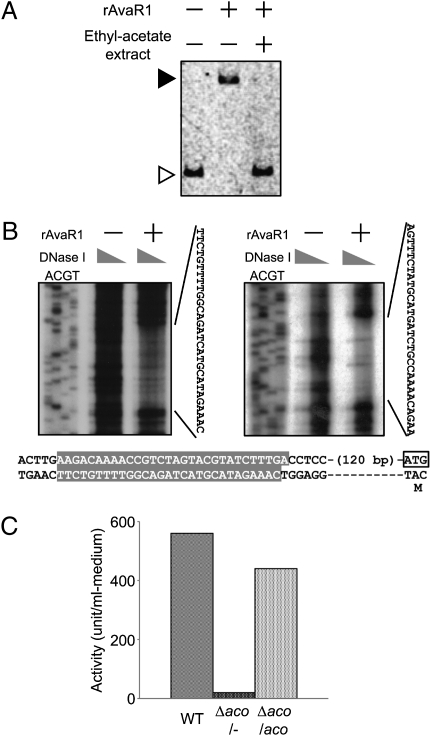
The deduced gene product of avaR1, divergently situated from aco, showed the highest sequence similarity to other Streptomyces autoregulator receptors such as ArpA, BarA, and ScbR (14). These autoregulator receptors typically bind to autoregulator responsive element (ARE) sequences that are frequently found in the promoter region of the direct target genes (15). We found a 26-bp ARE-like sequence in the 128 bp upstream of the aco gene, suggesting that AvaR1 may bind directly to the aco upstream region. To confirm whether AvaR1 has DNA binding activity toward the aco upstream region, we performed a gel-shift assay as shown in Fig. 3A. The recombinant AvaR1 (rAvaR1) protein showed clear binding to the upstream region of aco, which suggests that AvaR1 recognizes the ARE-like sequence upstream of aco. To precisely identify the location and sequence of the AvaR1 binding site, DNase I footprinting analysis was carried out, which revealed that AvaR1 protected the region of 30-bp sequences, situated 126 bp upstream from the translational start codon of aco (Fig. 3B) and designated as aco-ARE. These results demonstrated that DNA binding activity of AvaR1 is sequence-specific.
Fig. 3.
Influence of AcOEt extract on DNA binding activity of rAvaR1 and avermectin production. (A) Effect of S. avermitilis extract on DNA binding activity of rAvaR1. The AcOEt extract prepared from the wild-type culture broth after 70 h of cultivation was used in the gel-shift assay. (B) Determination of rAvaR1 binding site in the upstream region of aco by DNase I footprinting analysis. (Upper) The upstream region of aco was labeled in the antisense (Left) and sense (Right) strands. 32P-labeled fragment was incubated with DNase I (40–5 μg/mL) in the absence (−) or presence (+) of purified rAvaR1 (0.6 μg). The sequences protected by the presence of rAvaR1 are shown to the right of each image. Lower shows nucleotide sequence of the upstream region of aco. The rAvaR1 binding site is highlighted by a gray box. The putative start codon of aco gene is shown in an open box. (C) Avenolide activity in the aco mutant. Avenolide activity at 48 h of cultivation was detected by using the gel-shift assay. One unit of avenolide activity was defined as described in Materials and Methods.
To determine how such specific DNA binding activity is influenced or inhibited, an AcOEt extract of culture broth was assayed for the ability to prevent rAvaR binding to the aco-ARE sequence. AcOEt extract of the wild-type culture clearly inhibited AvaR1 from forming the AvaR1–aco-ARE complex (Fig. 3A), leading to 560 inhibitory units/mL medium (1 inhibitory unit is defined as the activity of releasing half of DNA-bound rAvaR1 from the aco-ARE sequence) (Fig. 3C). In contrast, AcOEt extract of the aco mutant showed almost no activity (<4% of the wild-type level), whereas the aco-complemented strain showed a similar level of inhibitory activity to the wild-type strain, implying that the factor inhibiting the DNA binding activity of AvaR1 seems to be synthesized by enzymatic function of Aco and that the factor is responsible for inducing avermectin production as a signaling molecule like γ-butyrolactone autoregulators. γ-Butyrolactone autoregulators are known to be resistant to proteases, heat, and acid treatments but sensitive to alkaline treatment that breaks an ester bond of the lactone ring (16, 17). Interestingly, the AvaR1 ligand was resistant to alkaline treatment (pH 11, 10 min) as well as proteases, heat, and acid treatments (Fig. S1). It therefore appears that the AvaR1 ligand is structurally different from γ-butyrolactone autoregulators, which implies that the AvaR1 ligand belongs to another class of signaling molecules controlling antibiotic production.
Isolation and Structure Elucidation of AvaR1-Interactive Ligand.
To isolate the AvaR1 ligand, we prepared 2,000 L of wild-type culture broth, which was adsorbed on the synthetic adsorbent HP-20. Active compounds were recovered as a 100% methanol eluate. Half of the methanol–eluate concentrate, showing specific activity of 1.6 × 103 units/mg residue to dissociate the rAvaR1–DNA complex, was extracted with chloroform and chromatographed on a silica gel column, followed by further purification by HPLC on a preparative C18 reverse-phase column. The purified compound (1.2 mg, colorless oil) possessed a specific activity of 1.0 × 107 units per mg, indicating 6.3 × 103-fold purification with a yield of 67%. No other fractions showed any DNA dissociation activity, implying that the compound purified is the sole avermectin-eliciting factor.
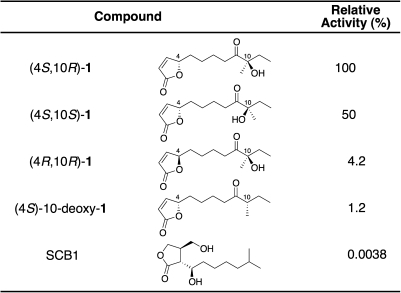
The structure of the active compound (1) was assigned as a 2(5H)-furanone derivative on the basis of spectroscopic evidence (olefinic proton signals at δ7.45 and δ6.12 and quaternary carbon at δ173.0). The high-resolution fast atom bombardment mass spectrometry (HR-FAB-MS; positive mode) showed a molecular ion peak at m/z 263.1261 [M+Na]+, corresponding to a molecular formula of C13H20O4. The proton signals at δ5.05 indicated a characteristic methine signal of butenolides from marine actinomycetes (18, 19). The 1H-1H-COSY spectrum of 1 showed the connections from C-2 to C-8 and between C-11 and C-12. Furthermore, the connection from C-8 to C-11 via a ketone (C-9, δ212.2) and quaternary carbon with a hydroxyl and a methyl group (C10, δ79.9) was demonstrated on the basis of heteronuclear multiple bond correlation (HMBC) spectrum of 1 (Fig. 1B). From these data, we elucidated the planner structure of 1 as 10-hydroxy-10-methyl-9-oxo-dodec-2-en-1,4-olide (Fig. 1A), which has not been isolated from any natural sources. The absolute configuration at the C-4 position was determined as 4S by comparison with a circular dichroism (CD) spectrum of stereochemically known butenolide (20). To further establish the absolute configuration of 1, we synthesized two candidates (4S,10R and 4S,10S forms of 1) (21) and purified by chiral chromatography, together with the 4R,10R form of 1 (Fig. 4). The identical elution time of the natural compound with the 4S,10R form on chiral HPLC (Fig. S2) together with the highest activity of the 4S,10R form (Fig. 4) confirmed that the natural compound has the absolute configuration of 4S,10R. The 4S,10S isomer possessed half the activity of the 4S,10R isomer, whereas the 4R,10R-isomer and 10-deoxy form (21) showed much reduced activity, suggesting that the 4S-absolute configuration and the presence of a hydroxyl group at C-10 are important for the binding to or signal perception by AvaR1.
Fig. 4.
Comparison of dissociation activity against the rAvaR1–DNA complex among avenolide and synthetic avenolide analogs.
Induction of Avermectin Production by AvaR1-Interactive Ligand Avenolide.
To confirm that compound 1 was the missing factor in the aco mutant and is required to elicit avermectin production, synthetic 4S,10R-1 was added to the aco mutant. Avermectin production was restored to wild-type levels by the addition of 4S,10R-1, mimicking the addition of wild-type AcOEt extract to the aco mutant (Fig. 5A). Thus, the compound was named “avenolide” (for S. avermitilis butenolide).
Fig. 5.
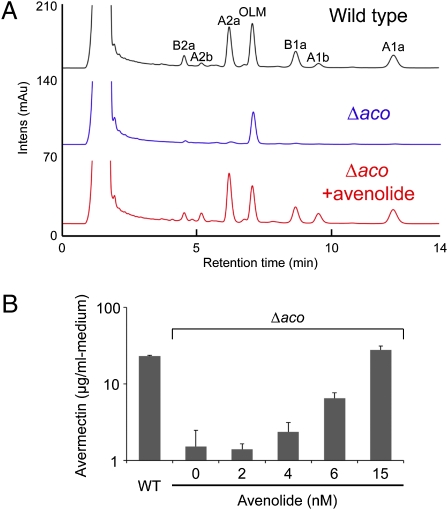
Effect of avenolide on avermectin (AVM) production. Synthetic (4S,10R)-1 was added into the culture of the aco mutant at 48 h of cultivation. (A) HPLC chromatogram of AVM production in the aco mutant by addition of synthetic (4S,10R)-1. Synthetic (4S,10R)-1 was added at a final concentration of 15 nM. Peaks eluted at 4.5, 5.2, 6.2, 8.7, 9.5, and 12.3 min are AVM derivatives, such as AVM B2a, AVM A2b, AVM A2a, AVM B1a, AVM A1b, and AVM A1a, respectively. A peak eluted at 7.1 min is oligomycin A (OLM). (B) AVM production with increasing concentrations of synthetic (4S,10R)-1. Error bars represent SDs from triplicate experiments.
Finally, to determine whether the identified avenolide elicits avermectin production at nanomolar concentrations, we tested it on the S. avermitilis aco mutant at final concentrations ranging from 2 to 15 nM (Fig. 5B). Avenolide (1) triggered avermectin production at a minimum effective concentration of 4 nM, together with saturation at 50–150 nM, resulting in avermectin production up to 1.8-fold of that of the wild-type strain (wild-type strain, 15 μg/mL; aco mutant with 150 nM avenolide, 27 μg/mL). Thus, avenolide (1) is a hormone-like signaling molecule effective at nanomolar concentrations in eliciting avermectin production.
Discussion
Intercellular communication by low-MW signaling molecules has been reported in a variety of microorganisms, including Gram-negative bacteria (such as Vibrio fischeri) (22) and Gram-positive bacteria (such as Bacillus subtilis and Streptomyces species) (23). The discovery of signaling molecules involved in the regulation of secondary metabolism, typically in antibiotic production, has played a key role in developing our understanding of the molecular mechanisms of microbial intercellular communication and opens many possibilities for the manipulation of antibiotic production. We have shown here that avenolide is an essential signaling molecule for avermectin production in S. avermitilis effective at nanomolar concentrations and is therefore another class of Streptomyces autoregulators distinct from γ-butyrolactone and furan autoregulators. Four nanomolar avenolide is sufficient to induce avermectin production in the aco mutant. This result is well in line with the concentrations and signaling activities of other microbial hormones, which range from micromolar (homoserine lactones) (24) and submicromolar (0.05 μM PI factor) (25) to nanomolar (AI-2 and γ-butyrolactone autoregulators) (26). The activity of avenolide (1) is clearly dependent on the butenolide structure; 4R-1 had only 4% of the activity of 4S-1. SCB1, a typical butanolide that possessed the highest binding activity toward rAvaR1 of all of the γ-butyrolactones tested (Fig. S3), had negligible elicitor activity compared with the avenolide analogs (Fig. 4). Hence, the addition of the γ-butyrolactone autoregulator did not influence avermectin production. The presence of the hydroxyl group at C-10 is also crucial for recognition of avenolide by its receptor protein AvaR1, although its configuration appears less important.
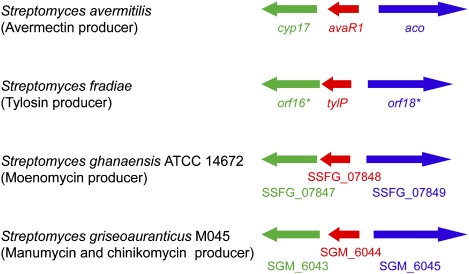
Elucidation of avenolide (1) as a signaling molecule now allows us to explore the distribution of this chemical signal in streptomycetes. Our previous findings based on screening of autoregulator producers suggested that ∼60% of Streptomyces species have the ability to produce a γ-butyrolactone–type autoregulator (7), but little was known about molecules used as the signaling molecules for antibiotic production by other species. The furan-type autoregulator (typically MMF) is one such example. However, the sparse occurrence of homologs of the MMF biosynthetic genes (only one example among sequenced Streptomyces genomes) suggests that the furans might be a minor group of Streptomyces autoregulator. In contrast, gene arrangements similar to the aco/cyp17/avaR cluster are found frequently in the genomes of Streptomyces strains (Fig. 6). Aco presumably introduces a double bond into the C-2–C-3 positions of avenolide (1). Our preliminary data demonstrated that Cyp17, encoding the cytochrome P450 hydroxylase CYP105B2, is also involved in the production of avenolide, generating avenolide (1) from (4S)-10-deoxy-1. In contrast, avaA (sav2269; 1.8 Mb away from the aco/cyp17/avaR cluster and 1.6 Mb away in the opposite side from the avermectin cluster), which encodes a homolog of well-studied AfsA family protein required for the formation of phosphorylated butenolides for γ-butyrolactone autoregulator (27) and MMF (8), does not participate in avenolide (1) production (Fig. S4). These results support our hypothesis that other streptomycetes may produce avenolide-type autoregulators and that they are acting as a general class of signaling molecule in regulating antibiotic production in Streptomyces. It will be interesting to determine the mechanism by which this unique class of signaling molecules initiates antibiotic production. The identification of avenolide as a unique Streptomyces autoregulator should allow the construction of high-level producers of antibiotics, including the important therapeutic agent avermectin, as well as facilitate genetic understanding of regulatory mechanism for microbial antibiotic production. The result could also lead to the discovery of clinically useful natural compounds by activating the numerous silent natural product gene clusters prevalent in actinomycete genomes.
Fig. 6.
The biosynthetic gene clusters of the avenolide family of autoregulators. Genes are color-coded by the predicted function of their products: avaR1 homologs (red), aco homologs (blue), and cyp17 homologs (green).
Materials and Methods
Analysis of Avermectin Production.
The derivative strains of S. avermitilis KA320 were cultivated in production medium (28). The culture broth was withdrawn and mixed with an equal volume of methanol. After centrifugation, the supernatant was analyzed by an HPLC system as described (29). The amounts of four avermectin components, A1a, A2a, B1a, and B2a, were quantified by using an authentic sample of avermectins.
Preparation of rAvaR1.
An avaR1 gene amplified by the primer pair avaR1-Fw/avaR1-Re was digested by NcoI and BamHI and then inserted into the NcoI–BamHI sites of pET-3d (Merck), resulting in pLT118. Escherichia coli BL21(DE3) pLysS harboring pLT118 was inoculated into LB medium and the cultivation was continued at 37 °C until the OD600 reached 0.4. Isopropyl β-d-thiogalactopyranoside was then added to a final concentration of 0.5 mM, and the cultivation was continued for a further 4 h. After the collected cells were washed with 0.9% NaCl solution, they were resuspended in buffer A [20 mM triethanolamine-HCl (pH 7.0) containing 20% vol/vol glycerol, 1 mM 1,4-DTT, and 0.1 mM (p-amidinophenyl)methanesulfonyl fluoride] containing 50 mM KCl. After sonication and centrifugation, the supernatant was absorbed on a DEAE-Sephacel column preequilibrated with buffer A containing 50 mM KCl, and a fraction showing SCB1 binding activity was eluted with buffer A containing 150 mM KCl. The SCB1 binding assay was performed as described (30). The autoregulator binding activity of rAvaR1 was measured by observing the difference between the binding of the 3H-labeled autoregulator (86 nM [3H]SCB1 [1.73 TBq/mmol]) in the absence and presence of 1,450-fold excess nonlabeled SCB1. The radioactivity was quantified by an LS-6000 liquid scintillation counter (Beckman Coulter). After the fraction was concentrated by ultrafiltration (UP-20; Advantec Toyo Kaisha), the concentrate was applied to a gel filtration column (Superrose 12 PC 3.2/30; GE Healthcare Bio-Sciences) and eluted with buffer A containing 200 mM KCl. Protein concentration was measured with Bio-Rad protein assay kit using BSA as a standard. Purity of rAvaR1 was analyzed by SDS/PAGE.
Avenolide Assays.
Two bioassays were used to detect avenolide activity: a DNA dissociation activity on the rAvaR1–DNA complex in the gel-shift experiment and an avermectin-inducing activity with S. avermitilis aco mutant. Synthetic avenolide and the chiral analogs were dissolved in methanol and added at the specified concentrations for these bioassays. One unit of avenolide activity in the gel-shift experiment was defined as the potency for releasing half of DNA-bound rAvaR1 from the specific target DNA, which was measured by image analysis on Typhoon 9210 Variable Mode Imager.
Gel-Shift Assay.
An upstream region of aco gene was amplified by PCR with the primer pair aco-gel-Fw/aco-gel-Re (Table S1). The amplified fragment was digested by BamHI and then cloned into the BamHI site of pUC19 (Takara Bio), resulting in pLT119. The DNA probe was labeled by PCR using pLT119 containing an aco-upstream region as a template with FITC-labeled M13-47 primer and RV primer. After FITC-labeled probe (7.5 ng) and rAvaR1 (0.2 μg) were mixed and incubated at 25 °C for 10 min, the reaction mixture was separated at 4 °C by electrophoresis. The gel-shift condition to detect DNA retardation was followed as described (31). To investigate effect of an ethyl-acetate extract as described below on DNA binding activity of rAvaR1, 2 μL of the extract was added into the reaction mixture, and the mixture was incubated for a further 5 min. For preparation of an ethyl-acetate extract, the culture supernatant, adjusted under pH 5.0 with acetate, was extracted with ethyl acetate. The ethyl-acetate broth was evaporated and dissolved in 20% ethanol.
DNase I Footprinting.
DNase I footprinting was performed as described (32). DNA fragment was prepared by PCR using pLT119 as a template and the 32P-labeled aco-gel-Fw or aco-gel-Re primers. Each 32P-labeled primer was end-labeled with T4 polynucleotide kinase and ATP.
Isolation of Avenolide (1).
The vegetative culture of S. avermitilis KA320 grown in the medium containing 5 g of glucose, 15 g of soy flour, and 5 g of yeast extract per liter of tap water (adjusted to pH 7.2) at 30 °C for 2 d was inoculated to the fermentation medium containing 60 g of glucose, 2 g of NaCl, 0.5 g of K2HPO4, 0.1 g of MgSO4·7H2O, 2 g of (NH4)2SO4, 2 g of yeast extract, 0.05 g of FeSO4·7H2O, 0.05 g of MnSO4·4H2O, 0.05 g of ZnSO4·7H2O, and 5 g of CaCO3 per liter of tap water (adjusted to pH 7.0) and grown at 28 °C for 24 h. The level of avenolide in all purification steps was evaluated by using the gel-shift assay. About 2,000 L of the culture filtrate was mixed with 200 L of a synthetic adsorbent Diaion HP-20 (Mitusbishi Chemical Co. Ltd.). The adsorbent was washed with 2 volumes of water, and avenolide was successively eluted with 2 volumes of 100% methanol. After methanol eluate was concentrated to 1 L, half of the concentrate was extracted with chloroform (200 mL × 5). The organic layers were combined and concentrated under the reduced pressure. The brownish oily residue (2.2 g) was subjected to silica gel column chromatography (70∼230 mesh, 30 × 150 mm in chloroform), the active fractions were eluted with chloroform/methanol (99:1), and the fractions were combined and concentrated to dryness (107.5 mg). The yellowish oily material was subjected to preparative HPLC (PEGASIL ODS; 20 × 250 mm, 5 μm, developing with 20% aqueous acetonitrile, flow rate 9.0 mL/min, and detection at 210 nm). Because avenolide was eluted at 45–60 min, the fractions were combined and concentrated to obtain as an oily material (4.7 mg). Final purification of the active fraction (4.2 mg) by preparative HPLC (PEGASIL ODS; 10 × 250 mm, 5 μm, developing with 15% aqueous acetonitrile, flow rate 4.0 mL/min, and detection at 210 nm) gave 1.2 mg of avenolide (1) eluted at 73.0 min. Fractions were assayed for avenolide activity as described above.
Physicochemical Properties of Avenolide (1).
Avenolide (1): colorless oil; [α]D23 +22.0° (c 0.01, methanol); HR-FAB-MS (positive mode) C13H20O4Na [M+Na]+ (obsd. 263.1261, calcd. 263.1259); UV (methanol) λmax (log ε) 208.0 nm (4.13); CD (methanol) λmax (Δε): 208 (+4.4) nm. 13C NMR (125 MHz, δ ppm in CDCl3): 212.2 (C-9, s), 173.0 (C-1, s), 156.1 (C-3, d), 121.8 (C-2, d), 83.0 (C-4, d), 79.0 (C-10, s), 35.5 (C-8, t), 33.1 (C-5, t), 32.5 (C-11, t), 25.3 (C-13, q), 24.8 (C-6, t), 23.3 (C-7, t), 7.8 (C-12, q). 1H NMR (500 MHz, δ ppm in CDCl3): 7.45 (1H, dd, J = 5.9, 1.4 Hz, H-3), 6.12 (1H, dd, J = 5.9, 2.1 Hz, H-2), 5.05 (1H, m, H-4), 2.57 (1H, dddd, J =14.5, 8.2, 6.4, 3.2 Hz, H-8), 2.48 (1H, dddd, J = 14.5, 7.8, 6.4, 3.7 Hz, H-8´), 1.83 (1H, m, H-5), 1.72 (1H, m, H-5′), 1.72 (2H, quint., J = 7.3 Hz, H-11), 1.69 (2H, m, H-7), 1.46 (2H, m, H-6), 1.34 (3H, s, CH3-13), 0.80 (3H, t, J = 7.3 Hz, CH3-12).
Chemical and Analytical Methods.
NMR (1H, 500 MHz; 13C, 125 MHz) spectra were obtained on JEOL JNM-ECP 500 FT NMR system. Chemical shifts are reported on the δ scale, using tetramethylsilane as an internal reference. Molecular mass spectra were measured on a JEOL JMS-700 Mstation spectrometer. CD spectra and optical rotations were recorded on JASCO J-720 and JASCO DIP-1000 polarimeter, respectively.
Supplementary Material
Acknowledgments
This work is a part of the Ph.D. dissertation of K.T.M. We thank E. Cundliffe for providing genetic information of S. fradiae orf16* and orf18*; A. Arisawa, R. Kaneto, K. Matsuzaki, K. Takeda, H. Tsunekawa, and A. Watanabe (Mercian Corp.; now MicroBiopharm Japan Co., Ltd.) for providing culture extract from large-scale fermentation of S. avermitilis. This work was supported by a Noda Institute for Scientific Research research grant (to S.K.), a Japan Society for the Promotion of Science (JSPS) Joint Program in the Field of Biotechnology grant, the National Research Council of Thailand and National Science and Technology Development Agency of Thailand (to T. Nihira), a JSPS Grant-in-Aid for Scientific Research (B) (to T. Nihira and S.K.), a Ministry of Education, Culture, Sports, Science and Technology of Japan Grant-in-Aid for Scientific Research on Innovative Areas (to H. Ikeda), and an Institute for Fermentation, Osaka research grant (to H. Ikeda).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113908108/-/DCSupplemental.
References
- 1.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Yim G, Wang HH, Davies J. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takano E. γ-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol. 2006;9:287–294. doi: 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Khokhlov AS, et al. Effect of A-factor on the growth of asporogenous mutants of Streptomyces griseus, not producing this factor. Z Allg Mikrobiol. 1973;13:647–655. doi: 10.1002/jobm.3630130803. [DOI] [PubMed] [Google Scholar]
- 5.Khokhlov AS, et al. [The A-factor, responsible for streptomycin biosynthesis by mutant strains of Actinomyces streptomycini.] Dokl Akad Nauk SSSR. 1967;177:232–235. [PubMed] [Google Scholar]
- 6.Hsiao NH, et al. Analysis of two additional signaling molecules in Streptomyces coelicolor and the development of a butyrolactone-specific reporter system. Chem Biol. 2009;16:951–960. doi: 10.1016/j.chembiol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y. Butyrolactone autoregulators, inducers of secondary metabolites, in Streptomyces. Actinomycetologica. 1995;9:57–65. [Google Scholar]
- 8.Corre C, Song L, O'Rourke S, Chater KF, Challis GL. 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc Natl Acad Sci USA. 2008;105:17510–17515. doi: 10.1073/pnas.0805530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabala JC, et al. Ivermectin, a new broad-spectrum antiparasitic agent. J Med Chem. 1980;23:1134–1136. doi: 10.1021/jm00184a014. [DOI] [PubMed] [Google Scholar]
- 10.Aziz MA, Diallo S, Diop IM, Lariviere M, Porta M. Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet. 1982;2:171–173. doi: 10.1016/s0140-6736(82)91026-1. [DOI] [PubMed] [Google Scholar]
- 11.Shikiya K, et al. Efficacy of ivermectin against Strongyloides stercoralis in humans. Intern Med. 1992;31:310–312. doi: 10.2169/internalmedicine.31.310. [DOI] [PubMed] [Google Scholar]
- 12.Nishida H, Ohnishi Y, Beppu T, Horinouchi S. Evolution of γ-butyrolactone synthases and receptors in Streptomyces. Environ Microbiol. 2007;9:1986–1994. doi: 10.1111/j.1462-2920.2007.01314.x. [DOI] [PubMed] [Google Scholar]
- 13.Bignell DR, Bate N, Cundliffe E. Regulation of tylosin production: Role of a TylP-interactive ligand. Mol Microbiol. 2007;63:838–847. doi: 10.1111/j.1365-2958.2006.05541.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto KT, Kitani S, Komatsu M, Ikeda H, Nihira T. The autoregulator receptor homologue AvaR3 plays a regulatory role in antibiotic production, mycelial aggregation and colony development of Streptomyces avermitilis. Microbiology. 2011;157:2266–2275. doi: 10.1099/mic.0.048371-0. [DOI] [PubMed] [Google Scholar]
- 15.Folcher M, et al. Pleiotropic functions of a Streptomyces pristinaespiralis autoregulator receptor in development, antibiotic biosynthesis, and expression of a superoxide dismutase. J Biol Chem. 2001;276:44297–44306. doi: 10.1074/jbc.M101109200. [DOI] [PubMed] [Google Scholar]
- 16.Waki M, Nihira T, Yamada Y. Cloning and characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. strain FRI-5. J Bacteriol. 1997;179:5131–5137. doi: 10.1128/jb.179.16.5131-5137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takano E, et al. Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2) J Biol Chem. 2000;275:11010–11016. doi: 10.1074/jbc.275.15.11010. [DOI] [PubMed] [Google Scholar]
- 18.Mukku VJ, Speitling M, Laatsch H, Helmke E. New butenolides from two marine streptomycetes. J Nat Prod. 2000;63:1570–1572. doi: 10.1021/np0001676. [DOI] [PubMed] [Google Scholar]
- 19.Cho KW, et al. New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod. 2001;64:664–667. doi: 10.1021/np000599g. [DOI] [PubMed] [Google Scholar]
- 20.Gawronski JK, Oeveren AV, van der Deen H, Leung CW, Feringa BL. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J Org Chem. 1996;61:1513–1515. [Google Scholar]
- 21.Uchida M, et al. Total synthesis and absolute configuration of Avenolide, extracellular factor in Streptomyces avermitilis. 2011 doi: 10.1038/ja.2011.90. , in press. [DOI] [PubMed] [Google Scholar]
- 22.Robson ND, Cox AR, McGowan SJ, Bycroft BW, Salmond GP. Bacterial N-acyl-homoserine-lactone-dependent signalling and its potential biotechnological applications. Trends Biotechnol. 1997;15:458–464. doi: 10.1016/S0167-7799(97)01102-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser D, Losick R. How and why bacteria talk to each other. Cell. 1993;73:873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard A, et al. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 25.Recio E, Colinas A, Rumbero A, Aparicio JF, Martín JF. PI factor, a novel type quorum-sensing inducer elicits pimaricin production in Streptomyces natalensis. J Biol Chem. 2004;279:41586–41593. doi: 10.1074/jbc.M402340200. [DOI] [PubMed] [Google Scholar]
- 26.Neiditch MB, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato JY, Funa N, Watanabe H, Ohnishi Y, Horinouchi S. Biosynthesis of γ-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proc Natl Acad Sci USA. 2007;104:2378–2383. doi: 10.1073/pnas.0607472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda H, Kotaki H, Ōmura S. Genetic studies of avermectin biosynthesis in Streptomyces avermitilis. J Bacteriol. 1987;169:5615–5621. doi: 10.1128/jb.169.12.5615-5621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitani S, Ikeda H, Sakamoto T, Noguchi S, Nihira T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl Microbiol Biotechnol. 2009;82:1089–1096. doi: 10.1007/s00253-008-1850-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Nihira T, Tada H, Yanagimoto M, Yamada Y. Identification of binding protein of virginiae butanolide C, an autoregulator in virginiamycin production, from Streptomyces virginiae. J Antibiot. 1989;42:769–778. doi: 10.7164/antibiotics.42.769. [DOI] [PubMed] [Google Scholar]
- 31.Kitani S, et al. Identification of genes involved in the butyrolactone autoregulator cascade that modulates secondary metabolism in Streptomyces lavendulae FRI-5. Gene. 2008;425:9–16. doi: 10.1016/j.gene.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 32.Kitani S, Kinoshita H, Nihira T, Yamada Y. In vitro analysis of the butyrolactone autoregulator receptor protein (FarA) of Streptomyces lavendulae FRI-5 reveals that FarA acts as a DNA-binding transcriptional regulator that controls its own synthesis. J Bacteriol. 1999;181:5081–5084. doi: 10.1128/jb.181.16.5081-5084.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.