Abstract
Natural killer (NK) cells are recruited into the uterine stroma during establishment of the hemochorial placenta and are proposed regulators of uterine spiral artery remodeling. Failures in uterine spiral artery remodeling are linked to diseases of pregnancy. This prompted an investigation of the involvement of NK cells in placentation. NK cell depletion decreased the delivery of proangiogenic factors and delayed uterine spiral artery development, leading to decreased oxygen tension at the placentation site, stabilized hypoxia-inducible factor 1A protein, and redirected trophoblast differentiation to an invasive phenotype. Trophoblast cells replaced the endothelium of uterine spiral arteries extending the depth of the placental vascular bed and accelerating vessel remodeling. Hypoxia-regulated trophoblast lineage decisions, including expansion of invasive trophoblast, could be reproduced in vitro by using rat trophoblast stem cells and were dependent on hypoxia-inducible factor signaling. We conclude that NK cells guide hemochorial placentation through controlling a hypoxia-sensitive adaptive reflex regulating trophoblast lineage decisions.
Hemochorial placentation is shared by many mammalian species, including humans, some nonhuman primates, and rodents (1). The hemochorial placenta is organized into two distinct compartments. Each compartment provides a specialized function, which includes (i) delivery of maternal nutrients to the placenta and (ii) transfer of nutrients from the placenta to the developing fetus. These functionally distinct placental compartments are under different regulatory programs. The maternal–fetal interface is a dynamic site undergoing pregnancy-associated adaptations (2). A critical adaptation is the extensive vascular remodeling of the maternal uterine spiral arteries, which facilitates nutrient flow and gas exchange with the growing fetus (1–3). Failures in this process result in unmodified vessels and give rise to pregnancy-associated diseases such as preeclampsia, intrauterine growth restriction, and premature pregnancy termination (1–3). Mechanisms controlling uterine vascular remodeling are poorly understood. Putative regulators include natural killer (NK) cells and a specialized lineage of trophoblast cells referred to as invasive or extravillous trophoblast (2–4).
Uterine adaptations to pregnancy include highly regulated immune cell trafficking (4). Following embryo implantation in rodents and primates, most leukocytes are excluded from uterine tissue proximal to the placentation site except for NK cells (4, 5). Implantation-associated decidualization in the rat and mouse results in the accumulation of NK cells in the uterine mesometrial decidua (4, 5). NK cells have been implicated in pregnancy-associated uterine vascular development (4, 6, 7). Genetic deficiency of uterine NK cells results in a lack of remodeling of the uterine vasculature, resulting in hypertrophied vascular media, swollen endothelial cells, and narrow vessel lumens (8). NK cell-mediated effects on the uterine vasculature may be directed by NK cell production of an assortment of angiogenic and vasoactive factors (4–7, 9). Aberrant NK cell number, activation, and signaling have been implicated in pregnancy complications (4, 7).
The invasive trophoblast lineage arises from trophoblast stem (TS) cells through an as yet unknown differentiation process (10). This differentiated cell lineage possesses a unique phenotype and is specialized for adhesion, degradation, and migration through uterine stromal extracellular matrices and restructuring uterine spiral arteries (2, 3, 5). Invasive trophoblast cells take two routes as they penetrate into the uterus. They move between spiral arteries (interstitial) or, alternatively, they move within spiral arteries (endovascular), replacing the endothelium and in some instances acquiring a pseudoendothelial phenotype as they proceed (2, 3, 5, 11, 12). Rat and human placentation sites are characterized by deep trophoblast cell invasion into the uterine wall (2, 3, 5), a situation that contrasts with the mouse, where placentation is relatively superficial (5). Shallow trophoblast invasion in humans, however, is linked to poor spiral artery remodeling and diseases of pregnancy (2, 3).
A potential interaction between uterine NK cells and trophoblast cells is not clearly understood. A reciprocal relationship exists in rat and mouse regarding the presence of NK cells and invasive trophoblast cells (5, 13). NK cells direct the first wave of vascular modification. Their disappearance after midgestation marks the entry of invasive trophoblast cells and initiation of the second wave of vascular modification. The reciprocal relationship of NK cells and trophoblast cells is also supported by observations with an NK-deficient mouse model (5) and the brown Norway rat (13). NK cells may directly act on trophoblast cells (4, 6, 7) or, alternatively, they may have indirect effects on trophoblast cells via actions on other constituents of the uterine mesometrial compartment (4, 9). The latter may include uterine NK cell products targeting uterine spiral arteries and their delivery of nutrients, including oxygen, to the developing placenta.
Placental development is sensitive to oxygen availability. Oxygen tension at the maternal–fetal interface changes with gestation and is also affected by placentation (14–16). Oxygen tensions tend to be lower during early pregnancy and increase following the establishment of the hemochorial placenta (16). Insights about the role of oxygen as an intrinsic regulator of placentation have been derived from mutagenesis of genes in the mouse controlling cellular responses to hypoxia. Central to the cellular response to hypoxia is a transcription factor complex referred to as hypoxia-inducible factor (HIF) (17). Phenotypes of placentas with null mutations for several genes encoding components of the HIF signaling pathway exhibit failures in placentation (15, 18). Exposure of pregnant rats to a hypoxic environment leads to alterations in placentation, especially expansion of the invasive trophoblast lineage (19). This is a conserved adaptive response. Maternal anemia (human) and chronic constriction of the lower aorta (rhesus monkey), which lead to hypoxia at the maternal–fetal interface, are also triggers for increasing intrauterine trophoblast invasion (20, 21).
Results
Depletion of Uterine NK Cells.
NK cells were depleted in Holtzman Sprague-Dawley rats by treatment with anti-asialo GM1 antibodies (22, 23) on gestation day (E)4.5 or E4.5 and E9.5. Placentation sites were collected on E9.5 or E13.5 and examined for the presence of NK cells by using PRF1 immunostaining. A single injection of anti-asialo GM1 at E4.5 depleted NK cells from placentation sites at E9.5 (Fig. S1) and decreased uterine NK cell numbers on E13.5 (Fig. S1). Quantitative (q)RT-PCR of E9.5 uterine mesometrial decidua for Prf1 mRNA verified the depletion of NK cells (Fig. S1). A combination of two injections of anti-asialo GM1 (E4.5 and E9.5) was effective in depleting uterine NK cells at E13.5 (Fig. 1 B–F). Successful NK cell depletion was confirmed through the use of other strategies to detect NK cells (Fig. S2). The immunodepletion treatment was also effective in depleting systemic NK cells as assessed by flow cytometry (Fig. S1). Controls exhibited the expected full complement of systemic and uterine NK cells. Delaying anti-asialo GM1 antibody treatment until E6.5 was less effective in depleting NK cells (Fig. S1), suggesting that antibody access to the critical NK/NK precursor cell population homing to the uterus is developmentally restricted. Thus, we have established a protocol for preventing NK cell colonization of the placentation site and a tool for investigating the role of NK cells in hemochorial placentation, and gained some insight about NK cell homing to the uterus.
Fig. 1.
NK cell depletion leads to activation of endovascular trophoblast invasion on E13.5. (A) Rats were treated on E4.5 and E9.5 with normal rabbit serum (Control) or anti-asialo GM1 (NK cell depleted) and killed on E13.5. NK cells and invasive trophoblast were identified by PRF1 (B–E) and pan-cytokeratin (G–J) immunocytochemistry, respectively. (D, E, I, and J) High-magnification images of boxed regions in B, C, G, and H, respectively. (F) Quantification of depletion (n = 5 per group; P < 0.001, Student's t test). (K) Quantification of the depth of cytokeratin-positive cell penetration into the uterine mesometrial vasculature (n = 5 per group; *P < 0.001, Student's t test). (L and M) In situ hybridization of Prl7b1 mRNA in control and NK cell-depleted E13.5 placentation sites. (N and O) Representative images showing EGFP-expressing endovascular trophoblast cells at E13.5 placentation sites of control (N) and NK cell-depleted (O) wild-type female rats mated to EGFP transgenic male rats. (P) Prl7b1 transcript levels in E13.5 metrial gland tissues dissected from control and NK cell-depleted rats (n = 4 per group; *P < 0.001, Student's t test). (Scale bars, 0.25 mm.)
NK Cells, Endovascular Trophoblast Invasion, and Uterine Spiral Artery Remodeling.
The placentation site at E13.5 is well-defined but lacks penetration of invasive trophoblast cells beyond the decidual compartment (5). In contrast, E13.5 placentation sites from NK cell-depleted rats exhibited deep intrauterine endovascular trophoblast invasion (Fig. 1). Uterine spiral arteries were lined with cells expressing cytokeratin (Fig. 1 G–K and P) and Prl7b1 (Fig. 1 L and M). The latter is restricted to the invasive trophoblast cell lineage (13, 19). Similar effects of NK cell depletion on placentation were also observed in three additional inbred strains (F344, DSS, and BN; Fig. S3). We also verified the extraembryonic origin of the endovascular invasive trophoblast cells using a transgenic rat model constitutively expressing enhanced green fluorescent protein (EGFP) (19). NK cell depletion stimulated EGFP-expressing extraembryonic cells to invade the uterine vasculature of wild-type HSD females (Fig. 1 N and O). As indicated above, a single injection of anti-asialo GM1 on E4.5 depleted NK cells at E9.5 with some recovery by E13.5 (Fig. S1). This treatment was effective in stimulating endovascular trophoblast cell invasion on E13.5 (Fig. S4), suggesting that NK cell depletion was not required throughout the 8-d interval. Delaying anti-asialo GM1 treatment until E6.5 did not effectively deplete uterine NK cells (Fig. S1), nor did it promote endovascular trophoblast invasion (Fig. S4).
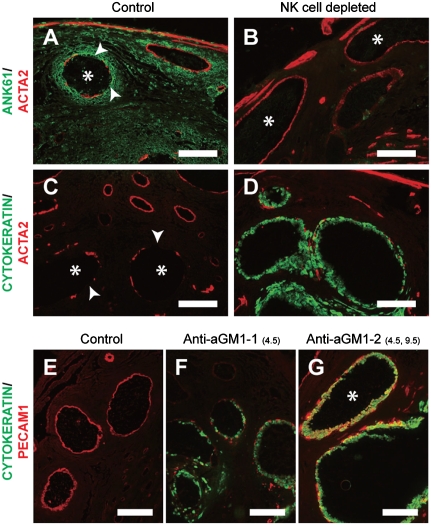
Three types of centrally located uterine spiral arteries were observed at E13.5: (i) vessels surrounded by NK cells (controls); (ii) vessels devoid of NK cells; and (iii) vessels devoid of NK cells but containing endovascular trophoblast cells. The presence of NK cells led to disruptions in the smooth muscle layer surrounding spiral arteries (Fig. 2 A and C). These modifications were not observed in spiral arteries devoid of NK cells (Fig. 2B). The trophoblast-bearing spiral arteries of NK cell-depleted rats exhibited diminished ACTA2 and in many instances complete loss of the smooth muscle layer (Fig. 2D). PECAM1 immunostaining was absent from some modified uterine spiral arteries, reflecting the disappearance of the endothelium (Fig. 2F), but present in others containing endovascular trophoblast (Fig. 2G), indicative of their acquisition of a pseudoendothelial phenotype (12). NK cells reappear in the uterine mesometrial compartment in rats receiving a single injection of anti-asialo GM1 on E4.5 but not in rats receiving injections on both E4.5 and E9.5 (Fig. S1 and Fig. 1). The pseudoendothelial trophoblast phenotype was much easier to identify following the latter treatment (Fig. 2G). Partial NK cell depletion versus complete NK cell depletion results in distinct endovascular trophoblast phenotypes on E13.5 and indicates that NK cells may modulate the appearance of the pseudoendothelial phenotype.
Fig. 2.
NK cells and endovascular trophoblast cells contribute to remodeling uterine spiral artery structure. (A–D) Rats were treated on E4.5 and E9.5 with normal rabbit serum (Control) or anti-asialo GM1 (NK cell depleted) and killed on E13.5. Double immunofluorescence staining for ANK61 and ACTA2 (A and B) and cytokeratin and ACTA2 (C and D). (A and C) Asterisks demarcate blood vessels possessing interruptions (arrowheads) in the tunica media. (B) Asterisks identify blood vessels with intact tunica media. (E–G) Rats were treated on E4.5 and E9.5 with normal rabbit serum (Control; E) or received a single injection of anti-asialo GM1 on E4.5 [Anti-aGM1-1(4.5); F] or injections on E4.5 and E9.5 [Anti-aGM1-2(4.5,9.5); G]. All animals were killed on E13.5. Double immunofluorescence staining for cytokeratin and PECAM1 (E–G). (G) The asterisk indicates the location of a blood vessel lined by cells doubly positive for cytokeratin and PECAM1 (yellow). (Scale bars, 0.25 mm.)
The results indicate that NK cells regulate uterine spiral artery remodeling through several mechanisms: (i) acting on the spiral artery tunica media; (ii) restraining endovascular trophoblast invasion; and (iii) modulating the endovascular trophoblast cell phenotype.
NK Cells Modulate Uterine Spiral Arteries and Oxygen Delivery.
The effects of NK cell depletion on placentation closely resembled our earlier observations on the effects of maternal hypoxia on placentation (19). A 24-h interval of sensitivity to maternal hypoxia was noted between E8.5 and E9.5 (19). This prompted an investigation of NK cell-depleted and control placentation sites at E9.5. A prominent difference in the distribution of uterine spiral arteries (ACTA2- and PECAM1-positive) was observed at E9.5 (Fig. 3 A–E). NK cell-depleted placentation sites showed significantly less development of uterine spiral arteries, especially their progression toward the primordial placenta (ectoplacental cone; Fig. 3E). Differences in vascular development between control and NK cell-depleted were not evident by E11.5 (Fig. S5), indicating that the absence of NK cells delayed but did not prevent uterine spiral artery development. Because uterine NK cells are potential sources of angiogenic factors, such as vascular/endothelial growth factors (VEGFs) (4, 9), we investigated Vegf transcript and VEGFA protein concentrations in the mesometrial compartment, where NK cells normally reside. Transcript levels for Vegfa, Vegfb, and Vegfc and VEGFA protein concentrations were all significantly lower in NK cell-depleted rats (Fig. 3 F–I). Decreases in Vegf transcripts and VEGFA protein are consistent with poor uterine spiral artery development.
Fig. 3.
NK cells, uterine spiral artery development, and oxygen delivery. Rats were treated on E4.5 with normal rabbit serum (Control) or anti-asialo GM1 (NK cell depleted) and killed on E9.5. Blood vessels were identified by PECAM1 (A and B) and ACTA2 (C and D) immunocytochemistry. (E) Numbers of PECAM1-positive vessels within the uterine mesometrial compartment were quantified. The asterisk indicates significant differences between control and NK cell-depleted (n = 5; P < 0.001, Student's t test). (F–H) Transcript levels for Vegfa (F), Vegfb (G), and Vegfc (H) were measured in E9.5 mesometrial decidual tissue dissected from control and NK cell-depleted rats. Asterisks indicate significant differences between control and NK cell-depleted (n = 5; P < 0.02 for each gene, Mann–Whitney rank-sum test). (I) VEGFA protein levels were measured in E9.5 mesometrial decidual tissue dissected from control and NK cell-depleted rats by ELISA and normalized to protein concentration. The asterisk indicates a significant difference between control and NK cell-depleted tissues (n = 10; P < 0.05, Mann–Whitney rank-sum test). (J–Q) NK cells regulate oxygen tension and stabilization of HIF1A at the E9.5 placentation site. Pimonidazole–protein adduct (PIM; J and K), cytokeratin (L and M), and HIF1A (N–Q) immunostaining within E9.5 placentation sites. Boxed areas in L and M are shown in N and P, and O and Q, respectively. (P and Q) DAPI counterstain. [Scale bars, 0.25 mm (A–D, J, and K) and 0.125 mm (L–Q).]
We next investigated whether the attenuated uterine spiral artery development impacted oxygen delivery to the placentation site. Pimonidazole hydrochloride forms adducts with proteins in tissues experiencing low-oxygen tensions (<10 mm of Hg). These adducts can be detected immunohistochemically (24). NK cell depletion at the placentation site was associated with an increase in the accumulation of pimonidazole–protein adduct formation, indicative of oxygen tensions below 10 mm Hg (Fig. 3 J and K). These results were suggestive of a local hypoxia but contrary to an earlier report that failed to observe increased pimonidazole–protein adduct formation at the maternal–fetal interface in mice possessing a combined genetic deficiency in NK, T, and B cells (25).
Cellular responses to low oxygen are regulated by activation of a hypoxia signaling pathway controlled by HIFs (17). HIFs consist of an oxygen-labile α-subunit (HIF1A) and HIF1B. Cellular responses to low oxygen are associated with stabilization of the HIF1A protein, formation of a heterodimer with HIF1B, and modulation of gene transcription. Consequently, we assessed HIF1A protein in NK cell-depleted and control placentation sites on E9.5 using immunocytochemistry. NK cell depletion resulted in stabilization of the HIF1A protein within cells of the ectoplacental cone (Fig. 3 L–Q). These results support the idea that NK cell depletion leads to activation of the hypoxia signaling pathway within trophoblast cells of the ectoplacental cone.
These experiments indicate that NK cells regulate development of uterine spiral arteries and oxygen delivery to the developing placenta.
NK Cells, Hypoxia, and Trophoblast Lineage Decisions Within the Placenta.
Maternal hypoxia during the labile period of placental morphogenesis activates invasive trophoblast cells, resulting in formation of a placenta that is adept at uterine vascular modification whereas later exposure to hypoxia is entirely ineffective (19). This implies a critical developmental window of sensitivity to low oxygen that impacts trophoblast cell lineage decisions. In the next series of experiments, we compared the effects of NK cell depletion and maternal hypoxia (exposure to 8.5% oxygen from E8.5 to E9.5) on the expression of trophoblast cell lineage markers within ectoplacental cone tissues isolated on E9.5 (Fig. S6). Transcripts for a series of junctional zone-specific trophoblast markers (Prl3d1: trophoblast giant cells; Tpbpa: junctional zone trophoblast; Prl5a1 and Prl7b1: invasive trophoblast) were significantly up-regulated, whereas a glycogen cell marker (Gjb3) and a labyrinthine trophoblast-specific transcript (Tfeb) were not affected (Fig. S6). The response was similar in NK cell-depleted and hypoxia-exposed tissues. Tpbpa is expressed in the earliest cell populations committed to junctional zone trophoblast lineages and many of their descendants (26). TPBPA protein-expressing cells in the ectoplacental cone were also up-regulated following NK cell depletion or exposure to maternal hypoxia (Fig. S7).
The hemochorial placenta is organized into two compartments that reflect trophoblast interactions with maternal (junctional zone) and fetal (labyrinth zone) vascular beds (27). Trophoblast cells connected to the maternal vasculature specialize in facilitating nutrient flow to the placenta. Invasive trophoblast cells of the rat arise from the junctional zone. Trophoblast cells developing in proximity to the fetal vasculature promote nutrient transfer to the fetus. We investigated the organization of E13.5 placentation sites in control and NK cell-depleted rats using vimentin immunostaining. This technique permits effective demarcation of junctional (negative) and labyrinth (positive) zones. NK cell depletion resulted in an expansion of the junctional zone (Fig. S6). A similar placental response occurs following maternal hypoxia exposure (19). Although the junctional zone expanded following NK cell depletion, junctional zone- and labyrinth zone-specific gene expression patterns were similar between controls and NK cell-depleted (Fig. S7).
We conclude from these experiments that NK cells and hypoxia modulate trophoblast cell lineage decisions and organization of the developing rat placenta.
Hypoxia Signaling Regulates Trophoblast Cell Lineage Decisions.
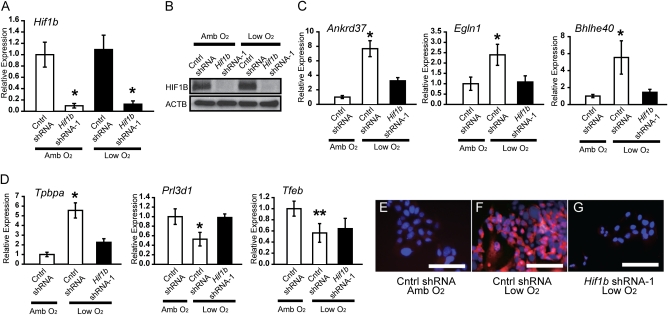
We next determined whether the impact of hypoxia on the development of junctional zone-specific trophoblast cell lineages was a direct action on the TS cell population and whether the response was dependent on HIF1B signaling. For these experiments, we used TS cells derived from rat blastocysts (28). We first evaluated the effects of a range of oxygen concentrations (0.5–2%) on Tpbpa gene expression by TS cells maintained in the stem cell state. A 24-h interval was selected because it is sufficient to elicit in vivo trophoblast responses (19) (Fig. S6). An oxygen concentration of 0.5% was a reliable activator of Tpbpa gene expression and TPBPA protein accumulation (Fig. S8) and was used in all subsequent experiments. HIF signaling was disrupted through knockdown of HIF1B, the binding partner for HIF1A, with specific short-hairpin RNAs (shRNAs). shRNAs were delivered to TS cells in lentiviral vectors. Hif1b shRNAs significantly decreased Hif1b mRNA and HIF1B protein levels, whereas control shRNAs showed no significant disruption of HIF1B expression (Fig. 4 A and B).
Fig. 4.
Oxygen tension, HIF1B signaling, and trophoblast cell lineage decisions. TS cells maintained under stem cell conditions were exposed to low oxygen (Low O2, 0.5%) for 24 h in the presence of control (Cntrl) shRNA or Hif1b shRNA-1. (A) qRT-PCR for Hif1b in TS cells exposed to ambient (Amb O2) or low O2 and control shRNA or Hif1b shRNA-1. (B) Western blotting for TS cells exposed to ambient O2 or low O2 and control shRNA or Hif1b shRNA-1. (C) qRT-PCR for known hypoxia-responsive genes in TS cells exposed to ambient O2 or low O2 and control shRNA or Hif1b shRNA-1. (D) qRT-PCR for trophoblast cell lineage markers in TS cells exposed to ambient O2 or low O2 and control shRNA or Hif1b shRNA-1. All experiments were performed in triplicate and evaluated by analysis of variance and Tukey's test (A, Cntrl shRNA vs. Hif1b shRNA, P < 0.01; C, Cntrl shRNA + Low O2 vs. other groups: Ankrd37, Egln1, *P < 0.01; Bhlhe40: *P < 0.05; D, Cntrl shRNA + Low O2 vs. other groups: Tpbpa and Prl3d1, *P < 0.01, Cntrl shRNA + Low O2 vs. Cntrl shRNA + Amb O2: Tfeb, **P < 0.01). (E–G) Immunofluorescence staining for TPBPA in TS cells exposed to ambient O2 or low O2 and control shRNA or Hif1b shRNA-1. (Scale bars, 0.125 mm.)
Transcript concentrations for known hypoxia-responsive genes (Ankrd37, Egln1, and Bhlhe40) (17, 29) were increased in response to low oxygen (Fig. 4C), as was Tpbpa (Fig. 4D and Fig. S8). In contrast, concentrations of Prl3d1 and Tfeb transcripts were significantly decreased (Fig. 4 and Fig. S8). All low oxygen-sensitive cellular measures were dependent upon HIF1B except for the Tfeb response (Fig. 4 C and D). Hypoxia-mediated inhibition of gene expression is more frequently associated with HIF independence (17). Concentrations of other trophoblast-associated transcripts (Gjb3, Prl5a1, and Prl7b1) were not reproducibly affected by low oxygen. The number of TS cells expressing TPBPA protein was increased by exposure to low oxygen and reversed by knockdown of HIF1B (Fig. 4 E–G and Fig. S8). Similar results were observed with a second independent Hif1b shRNA (Hif1b-2 shRNA; Fig. S9). Mouse TS cells exposed to low oxygen also show an up-regulation of Tpbpa gene expression (18, 30).
Oxygen tension also influenced the invasive phenotype of TS cells. Expression of mRNA for proteins implicated in trophoblast invasion and trophoblast-directed spiral artery remodeling (Cdh1, Mmp9, and Mmp12) (11, 31, 32) and trophoblast movement through Matrigel was evaluated. Cdh1 transcript levels were decreased in response to low oxygen (Fig. 5A), whereas Mmp9 and Mmp12 transcript levels were stimulated by low oxygen. Low oxygen stimulated trophoblast invasion through Matrigel (Fig. 5 B–E and Fig. S8). Each of these TS cell responses to low oxygen was dependent on HIF1B (Fig. 5 B–E and Fig. S9).
Fig. 5.
Oxygen tension, HIF1B signaling, and trophoblast cell invasion. TS cells maintained under stem cell conditions were exposed to low oxygen (Low O2, 0.5%) for 24 h in the presence of control (Cntrl) or Hif1b shRNA-1. (A) qRT-PCR analyses for genes associated with invasion (Cdh1, Mmp9, Mmp12) in TS cells exposed to ambient O2 or low O2 and control shRNA or Hif1b shRNA-1. All experiments were performed in triplicate and evaluated by analysis of variance and Tukey's test (*P < 0.05). (B–D) Assessment of the effects of oxygen tension and HIF1B signaling on TS cell invasion. TS cell images: (B) control shRNA + ambient O2; (C) control shRNA + low O2; (D) Hif1b shRNA-1 + low O2. Arrows indicate invaded cells. (E) Quantification of TS cell invasion. Experiments were performed in triplicate and evaluated with analysis of variance and Tukey's test (*P < 0.05). (F) Schematic representation of the effects of NK cells on uterine spiral artery development, oxygen delivery to TS cells, and HIF-dependent trophoblast differentiation.
The results indicate that trophoblast lineage decisions are sensitive to oxygen tension. Hypoxia-exposed TS cells are directed to differentiate toward a unique invasive and nongiant cell and nonlabyrinthine trophoblast phenotype. This process is regulated by HIF1B.
Discussion
The accumulation of NK cells in the uterus during pregnancy is a well-conserved biological event (4). In this report, we investigated the role of uterine NK cells in the rat, a species with hemochorial placentation and deep intrauterine trophoblast invasion (5). Our research demonstrates that NK cells act as regulators of hemochorial placentation (Fig. 5F). NK cells influence the development of uterine spiral arteries, at least in part, through their production of proangiogenic factors, such as VEGFs. Uterine spiral arteries deliver nutrients including oxygen to the developing placenta. Oxygen tension at the placentation site influences adaptive responses, which include trophoblast lineage decisions. Depending upon the internal milieu, trophoblast cells are differentially allocated to the uteroplacental vascular bed versus the placental–fetal interface, resulting in the creation of placentas with specialized properties for acclimation to specific environmental challenges. These experimental findings provide an important physiological link between the work of Croy and her colleagues demonstrating a role for NK cells in uterine spiral artery remodeling (4, 8) and the efforts of Simon and coworkers elucidating the involvement of HIF signaling in the regulation of placentation (15, 18).
There are temporal and quantitative features associated with the activities of NK cells and invasive trophoblast cells in uterine spiral artery maturation. NK cells promote uterine spiral artery growth toward the ectoplacental cone, a key event in the establishment of the hemochorial placenta, and cause at least a partial disruption of spiral artery tunica media integrity. All NK cells are not the same and differentially contribute to the establishment of the maternal–fetal interface (33). In the absence of NK cells, spiral artery extension is delayed and the tunica media initially remains intact. These observations are consistent with an angiogenic/vascular remodeling role for NK cells (4, 6, 7, 9). The actions of NK cells on uterine spiral artery and stromal cell compartments are not a prerequisite for endovascular trophoblast invasion. This process is actually accelerated and more robust in the absence of NK cells. Although NK cell and invasive trophoblast cell populations are each sufficient to facilitate establishment of the hemochorial placenta, there are quantitative differences in the extent of spiral artery remodeling achieved by each cell population. NK cell actions on spiral arteries can be viewed as modest, especially in comparison with the substantial actions of endovascular invasive trophoblast cells on uterine spiral artery structure and nutrient delivery to the placenta. The latter actions are exaggerated in the absence of NK cells. By promoting spiral artery development, oxygen delivery, and establishing the hemochorial placenta, NK cells effectively delay endovascular trophoblast invasion. Delaying trophoblast entry into spiral arteries and the concomitant enhanced flow of maternal resources to the placenta is protective to the mother and can be viewed as a maternal adaptive strategy.
How do NK cells communicate with the developing placenta? Previous in vitro experimentation indicated that NK cells produce factors that directly affect the behavior of trophoblast cells, including the invasive trophoblast lineage (4, 7, 9). Some reports suggested that NK cell secretory products stimulate trophoblast invasion, whereas others concluded the opposite action. Differences in the findings are probably related to methodological issues, such as the origin of NK cell and trophoblast cell preparations used in the analyses and/or other procedural considerations; however, they belie the most relevant mode of communication between NK cells and trophoblast cells, which is the delivery of oxygen. This view is supported by the similar placentation responses observed with two distinct in vivo manipulations: (i) NK cell depletion and (ii) maternal hypoxia (19). Our results indicate that the direct actions of NK cells on trophoblast cells are of secondary consideration to the fundamental role of NK cells in the development of the vasculature and oxygen delivery.
Oxygen tension is a regulator of trophoblast cell function (14–16, 30). A number of reports have demonstrated that trophoblast cells respond to oxygen restriction (reviewed in ref. 34). In vitro experimentation has yielded a range of responses, some of which are contradictory, but, again, methodological differences are the likely cause for the discrepancies. Our in vivo findings led us to a critical window during placental morphogenesis (E8.5–E9.5 in the rat) that is especially sensitive to oxygen tension (19). This is an interval where critical trophoblast lineage decisions are being finalized. These decisions impact morphogenesis of the placenta. Low oxygen favors TS cell and/or trophoblast precursor cell allocation to the junctional zone, a structure that establishes the placental interface with the uterus, including maternal vascular connectivity. Placentation sites are constructed to meet proximal challenges presented during the critical phase of their development, with the expectation that these challenges will continue throughout pregnancy and into postnatal extrauterine life. Problems and potentially disease arise when adaptations are ineffective and/or mismatches occur with the original environmental challenge directing placentation and subsequent prenatal and postnatal exposures. These postulates are consistent with central roles for the placenta in fetal programming and the development of adult health and disease (35).
In vivo placental adaptive responses can be simulated in vitro and include oxygen-sensitive lineage decisions. TS cell lineage responses are dependent upon HIF1B signaling pathways. HIF1B can interact with oxygen-sensitive HIF1A or HIF2A subunits or alternatively with other members of the bHLH-PAS family (17). The HIF1A–HIF1B heterodimer is a transcription factor complex controlling expression of genes that are vital to cell survival under conditions of oxygen deprivation (17). HIF1B binding partners and transcriptional targets in TS cells are unknown. Presumably, HIF1B is participating in transcriptional regulation of genes required for metabolic adaptations and also those controlling lineage decisions. The latter are keys to understanding how the invasive trophoblast cell lineage develops and potentially gaining insights about disorders that adversely affect placentation.
In conclusion, hypoxia-activated trophoblast invasion/uterine spiral artery remodeling is an adaptive response regulated by NK cells that ensures appropriate placentation and protects pregnancy.
Materials and Methods
Animals and Tissue Collection.
All rat strains used in the experimentation were acquired and maintained and timed pregnancies established as described (5, 13, 27). Sperm in the vaginal lavage was defined as E0.5. Intraperitoneal injections of anti-asialo GM1 (0.5 mL per injection; Wako Chemicals) were used to deplete NK cells (22, 23). Control pregnant rats received the same volume of normal rabbit serum. Some pregnant rats were placed in an oxygen-regulated chamber (BioSpherix) from E8.5 to E9.5. Oxygen tensions at placentation sites were estimated by pimonidazole–protein adduct formation (24) using the Hypoxyprobe-1 Kit (HPI). Placentation site dissections were performed as described (27). The University of Kansas Animal Care and Use Committee approved protocols for the care and use of animals.
Flow Cytometry.
Dissociated splenocytes were subjected to density-gradient centrifugation with Ficoll Hypaque solution (GE Healthcare). Enriched lymphocyte preparations (1 × 106) were labeled with phycoerythrin-conjugated mouse anti-CD161 (BD Pharmingen) and fluorescein (FITC)-conjugated mouse anti-CD3 (BD Pharmingen), and analyzed by flow cytometry with a BD LSRII (BD Biosciences).
Morphological Analyses.
Immunocytochemical analyses were used to detect trophoblast cells (pan-cytokeratin), NK cells (PRF1 and ANK61), endothelial cells (PECAM1), smooth muscle cells (ACTA2), and regions of the placentation site (vimentin) (13, 19). NK cells were also detected by in situ AP-PLP-A binding (36). Junctional zone trophoblast lineages were identified by TPBPA immunocytochemistry. HIF1A protein stabilization was monitored with an HIF1A antibody (BD Biosciences). Oxygen tension within placentation sites was estimated by detection of pimonidazole–protein adducts with the 4.3.11.3 mouse monoclonal antibody (HPI). Prl7b1 transcripts were localized at placentation sites using nonradioactive in situ hybridization (5, 13). Measurements of the depth of trophoblast invasion, the sizes of placental compartments, and blood vessel and uterine mesometrial compartment cross-sectional areas were performed with National Institutes of Health ImageJ software (13, 19).
Western Blot Analysis and ELISA.
HIF1B and beta actin (ACTB) levels were evaluated by Western blotting with antibodies to HIF1B (BD Pharmingen) and ACTB (Sigma-Aldrich). VEGFA was measured by ELISA (RayBiotech).
Rat TS Cells.
Blastocyst-derived rat TS cells were used to evaluate the effects of low oxygen on trophoblast lineage decisions (28). TS cells maintained in the stem cell state were exposed to low oxygen using a NAPCO Series 8000WJ incubator (Thermo Scientific). Invasiveness was determined by cell migration through Matrigel (32).
qRT-PCR, shRNA Constructs, and Production of Lentivirus.
Transcript levels were estimated by qRT-PCR (28). Primer sequences can be found in Table S1. Hif1b shRNA constructs in the pLKO.1 vector were used to disrupt HIF signaling. Control and Hif1b shRNA sequences are provided in Table S2. Lentiviral particles were produced as reported (37).
Supplementary Material
Acknowledgments
We thank Dr. Kunio Shiota (University of Tokyo) for the rat TPBPA antibodies and Dr. Masaru Okabe (University of Osaka) for the EGFP transgenic rats. We also thank Dr. Surendra Sharma (Brown University) for advice regarding the NK cell immunodepletion strategy. This research was supported by grants from the National Institutes of Health (HD20676 and HD048861) and a predoctoral fellowship from the American Heart Association (to D.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109478108/-/DCSupplemental.
References
- 1.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 2.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: Maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: Novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Guimond MJ, et al. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 9.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 10.Roberts RM, Fisher SJ. Trophoblast stem cells. Biol Reprod. 2011;84:412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris LK. Review: Trophoblast-vascular cell interactions in early pregnancy: How to remodel a vessel. Placenta. 2010;31(Suppl):S93–S98. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: Faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 13.Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the brown Norway rat: A model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- 14.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 15.Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 16.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 18.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, et al. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 21.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- 22.Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, Reynolds CW. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134:2783–2789. [PubMed] [Google Scholar]
- 23.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 24.Samoszuk MK, Walter J, Mechetner E. Improved immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. J Histochem Cytochem. 2004;52:837–839. doi: 10.1369/jhc.4B6248.2004. [DOI] [PubMed] [Google Scholar]
- 25.Leno-Durán E, et al. Fetal-placental hypoxia does not result from failure of spiral arterial modification in mice. Placenta. 2010;31:731–737. doi: 10.1016/j.placenta.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Iwatsuki K, et al. A novel secretory protein produced by rat spongiotrophoblast. Biol Reprod. 2000;62:1352–1359. doi: 10.1095/biolreprod62.5.1352. [DOI] [PubMed] [Google Scholar]
- 27.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- 28.Asanoma K, et al. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benita Y, et al. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32:475–481. doi: 10.1016/j.placenta.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vićovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 32.Peters TJ, et al. Differentiation-dependent expression of gelatinase B/matrix metalloproteinase-9 in trophoblast cells. Cell Tissue Res. 1999;295:287–296. doi: 10.1007/s004410051235. [DOI] [PubMed] [Google Scholar]
- 33.Madeja Z, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci USA. 2011;108:4012–4017. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuuli MG, Longtine MS, Nelson DM. Review: Oxygen and trophoblast biology—A source of controversy. Placenta. 2011;32(Suppl 2):S109–S118. doi: 10.1016/j.placenta.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003;204:65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 37.Lee D-S, Rumi MAK, Konno T, Soares MJ. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis. 2009;47:433–439. doi: 10.1002/dvg.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.