Abstract
Objective:
It has been speculated that amyotrophic lateral sclerosis (ALS) is characterized by a premanifest period during which neurodegeneration precedes the appearance of clinical manifestations. Magnetic resonance spectroscopy (MRS) was used to measure ratios of neurometabolites in the cervical spine of asymptomatic individuals with a mutation in the SOD1 gene (SOD1+) and compare their neurometabolic ratios to patients with ALS and healthy controls.
Methods:
A cross-sectional study of 1H-MRS of the cervical spine was performed on 24 presymptomatic SOD1+ volunteers, 29 healthy controls, and 23 patients with ALS. All presymptomatic subjects had no symptoms of disease, normal forced vital capacity, and normal electromyographic examination. Relative concentrations of choline (Cho), creatine (Cr), myo-inositol (Myo), and N-acetylaspartate (NAA) were determined.
Results:
NAA/Cr and NAA/Myo ratios are reduced in both SOD1+ subjects (39.7%, p = 0.001 and 18.0%, p = 0.02) and patients with ALS (41.2%, p < 0.001 and 24.0%, p = 0.01) compared to controls. Myo/Cr is reduced (10.3%, p = 0.02) in SOD1+ subjects compared to controls, but no difference was found between patients with ALS and controls. By contrast, NAA/Cho is reduced in patients with ALS (24.0%, p = 0.002), but not in presymptomatic SOD1+ subjects compared to controls.
Conclusions:
Changes in neurometabolite ratios in the cervical spinal cord are evident in presymptomatic SOD1+ individuals in advance of symptoms and clinical or electromyographic signs of disease. These changes reflect a reduction in NAA/Cr and NAA/Myo. Neurometabolic changes in this population resemble changes observed in patients with clinically apparent ALS. This suggests that neurometabolic changes occur early in the course of the disease process.
It has been speculated that amyotrophic lateral sclerosis (ALS) is characterized by a premanifest period during which neuronal degeneration precedes the appearance of clinical symptoms. Some evidence of this premanifest period has been derived from the superoxide dismutase (SOD1) mouse model of ALS in which there is a decline in the estimated motor unit number in advance of the appearance of symptoms.1 Limited data from a number of healthy individuals at risk for ALS by virtue of their carrying a mutation in the SOD1 gene (SOD1+) suggest a decline in motor unit numbers several months in advance of the appearance of symptoms.2 Similarly, limited threshold tracking transcranial magnetic stimulation data from 3 presymptomatic SOD1+ individuals suggests an increase in cortical excitability within 3 months prior to the onset of symptoms.3 Finally, a single prior study of 8 presymptomatic SOD1 mutation carriers reported decreased fractional anisotropy, increased tensor trace, and increased radial diffusivity in the posterior limb of the internal capsule.4
Magnetic resonance spectroscopy (MRS) is a noninvasive method for in vivo measurement of tissue metabolites that has been used to study neurometabolites in ALS,5–10 but there have been no prior studies of MRS in a presymptomatic population at risk for developing ALS. A number of studies have demonstrated a reduction in N-acetylaspartate (NAA), a marker of neuronal loss, in patients with ALS compared to controls expressed as absolute concentrations,11,12 or as ratios of other metabolites like choline (Cho) and creatine (Cr)10,13–24 in the primary motor cortex,13,17,19,23,25,26) centrum semiovale, internal capsule, corticospinal tract, cervical spine,10,12,23 medulla,5 and brainstem. Whether similar findings might be evident in people at risk for developing ALS, however, remains an unanswered question.
Here we present the results of an MRS study of presymptomatic SOD1+ individuals, contrasting the findings to those observed in patients with established ALS as well as age-matched healthy controls. The objective of our study was to determine whether there are changes in metabolite ratios in asymptomatic SOD1+ subjects in advance of the onset of disease. Magnetic resonance–detectable changes to metabolite concentration could potentially serve as a surrogate marker for disease progression.
METHODS
Participants.
This study was approved by the Emory University Institutional Review Board. All participants provided written informed consent.
Presymptomatic SOD1+ individuals were participants in the Pre-familial ALS (Pre-fALS) study, an ongoing prospective observational study of people who are at risk for developing familial ALS because they harbor a mutation in an ALS susceptibility gene. Participants for this study were recruited from among healthy relatives of patients with fALS due to mutations in known susceptibility genes (e.g., SOD1, TDP43, FUS). Study subjects undergo presymptomatic testing with the option of whether or not to learn the results of genetic tests. Those who elect to learn the results of genetic testing undergo both pre-test and post-test genetic counseling. Pre-fALS was initiated in 2007, and currently about 40 subjects have been enrolled in longitudinal follow-up. Pre-fALS participants are evaluated using multiple investigative modalities in an effort to identify presymptomatic evidence for disease. Included among these investigative modalities is MRS of the cervical spinal cord. All participants in Pre-fALS lack clinical symptoms and signs of upper and lower motor neuron dysfunction, and have no electrophysiologic abnormalities evident on routine nerve conduction studies and EMG, performed within 24 hours of the MRI. Participants in Pre-fALS are characterized as “presymptomatic” because: 1) ALS, as with other neurodegenerative diseases (Alzheimer disease, Parkinson disease, Huntington disease), is characterized by a presymptomatic phase of disease and 2) because those who harbor a mutation in the SOD1 gene are at an extremely high lifetime risk of developing ALS.
Cross-sectional data from Pre-fALS subjects were compared to subjects clinically diagnosed with ALS and age-matched healthy control subjects. Data from 14 patients with ALS and 16 healthy controls included in this manuscript have previously been reported10 but are presented here again (together with data from additional patients with ALS and healthy controls) for the purpose of comparison. Participant data were collected between November 2007 and March 2010. The study size represented all available data as of March 2010. As described previously,10 the subjects with ALS were recruited from the Emory University EMG laboratory, an ongoing clinical trial of arimoclomol in SOD1+ familial ALS as well as people with ALS who are registered with the Muscular Dystrophy Association. The healthy controls were recruited from several sources including spouses and other relatives of patients with ALS and a registry of healthy subjects maintained by one of the investigators (M.B.) as part of a prior study. After clinical examination, the revised ALS functional rating scale (ALSFRS-R)27 was administered in all patients with ALS, and forced vital capacity (FVC) was measured in all subjects.
Imaging and MRS data processing.
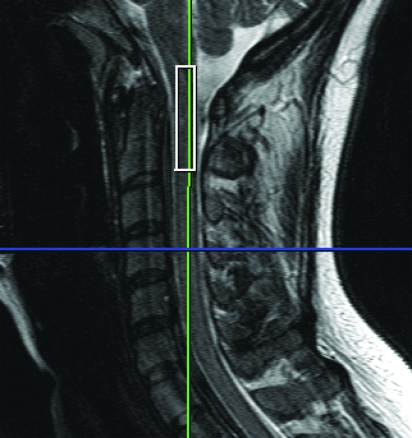
1H-MRS and data acquisition was performed on all subjects as described in detail previously.10 The participants were imaged on a 3-T whole-body system (TIM Trio, Siemens Medical Solutions, Malvern, PA) with head, neck, and spine matrix coils. A rectangular voxel (8 × 5 × 35 mm3) was placed along the main axis of the cord (figure 1) for MRS using point-resolved spectroscopy sequence after acquisition of localizers and shimming, with repetition time/echo time = 2,000/35 msec. Spectra were collected without (4 averages) and with water suppression (256 averages) using a chemical shift selective water suppression pulse. The total MRS acquisition time was approximately 12 minutes. The MRS data were fit with the LC model28 using LCModel version 6.1-A (S.W. Provencher) and relative concentrations of Cho, myo-inositol [Myo], and NAA determined as detailed previously.10
Figure 1.
Magnetic resonance spectroscopy voxels placed to cover the spinal cord at the level of the C1-C2 vertebral body.
Statistical analyses.
Metabolite peak estimates were rejected if the SD of the error was greater than 25%. Differences in age and metabolite ratios between patients with ALS, SOD1+ subjects, and controls were assessed with a 2-tailed Wilcoxon rank sum test. Multiple comparisons were addressed by controlling the false discovery rate procedure29 at 5%, which is the expected proportion of falsely rejected null hypothesis. All statistical analyses were performed with the R statistical environment (www.r-project.org).
RESULTS
We studied 24 Pre-fALS subjects (19 female, age 47 ± 11 years), 29 healthy controls (16 female, age 47 ± 13 years), and 23 patients with ALS (8 female, age 51 ± 13 years). Age was not significantly different between groups. All presymptomatic subjects have no symptoms or signs of disease, normal FVC, and normal electromyographic examination of the cranial, cervical, thoracic, and lumbosacral regions. The patients with ALS had El Escorial classifications: definite (7), probable (3), possible (5), and familial (8) (all SOD1-positive). Their average ALSFRS-R scores were 39 ± 9 and FVC 82 ± 26% predicted. Twenty-two patients had limb-onset disease and one patient had bulbar onset with affected limbs. Median disease duration was 567 days (interquartile range 262.5–1084). Among the subjects, we were unable to reliably estimate individual metabolite ratios for: Myo/Cr (1 Pre-fALS, 0 control, 1 ALS), NAA/Cho (0 Pre-fALS, 1 control, 0 ALS), NAA/Cr (2 Pre-fALS, 1 control, 2 ALS), and NAA/Myo (3 Pre-fALS, 1 control, 3 ALS). Statistical analyses were performed on available data.
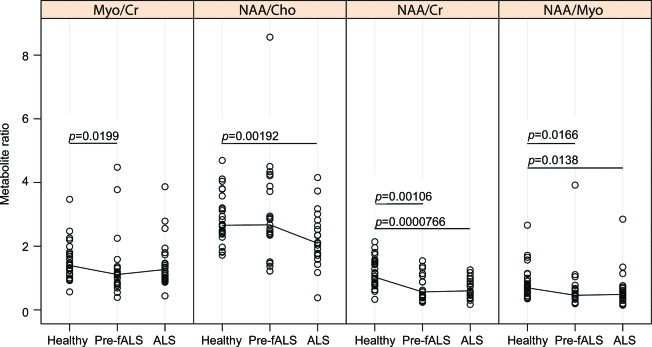
NAA/Cr and NAA/Myo ratios are reduced in presymptomatic SOD1+ subjects (39.7%, p = 0.001 and 18.0%, p = 0.02) and patients with ALS (41.2%, p < 0.001 and 24.0%, p = 0.01) compared to controls (figure 2). The Myo/Cr ratio is slightly reduced (10.3%, p = 0.02) in presymptomatic SOD1+ subjects compared to controls, but there is no difference in this ratio between patients with ALS and controls. By contrast, NAA/Cho is reduced in patients with ALS (24.0%, p = 0.002), but not in presymptomatic SOD1+ subjects compared to controls. After controlling the false discovery rate at 5%, our comparisons remain statistically significant.
Figure 2. Magnetic resonance spectroscopy metabolite ratios in the cervical cord from healthy controls, presymptomatic SOD1-positive subjects (Pre-fALS), and patients with amyotrophic lateral sclerosis (ALS).
The line segments connect the group medians. In Pre-fALS subjects, relative to healthy individuals, there are significant decreases in myo-inositol (Myo)/creatine (Cr) (10.3%), N-acetylaspartate (NAA)/Cr (39.7%), and NAA/Myo (18.0%). The metabolic patterns of NAA/Cho, NAA/Cr, and NAA/Myo among SOD1+ subjects fit between healthy controls and patients with ALS. Statistical significance of differences was assessed using Wilcoxon rank tests to mitigate outlier effects.
The 3 comparison groups were not well balanced by sex. To determine if sex is a predictor of metabolite ratios, we performed an additional analysis among the healthy controls. For each metabolite ratio, we performed a 2-sided Wilcoxon rank sum test to determine if the metabolite ratios are different between male and female subjects. We found no significant differences for any of the metabolite ratios (Myo/Cr p = 0.531, NAA/Cho p = 0.478, NAA/CR p = 0.507, and NAA/Myo p = 0.767).
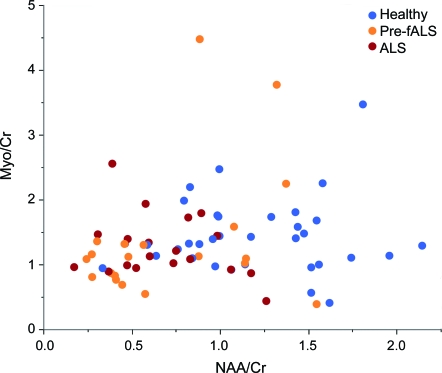
In general, the profile of metabolic changes in the presymptomatic SOD1+ group more closely resembles that found in patients with ALS than that observed in the control population. A scatterplot of NAA/Cr vs Myo/Cr (figure 3) shows a clustering pattern among the 3 subject groups. Clusters form around the patients with ALS (red) and around the healthy controls (blue). A majority of the Pre-fALS subjects (orange) tend to cluster near the patients with ALS, although a minority fall among the healthy controls.
Figure 3. N-acetylaspartate (NAA)/creatine (Cr) and myo-inositol (Myo)/Cr metabolite measurements showing a clustering pattern among the 3 subject types.
Despite some overlap, there is a clear distinction between healthy subjects and patients with amyotrophic lateral sclerosis (ALS). Pre-familial ALS (Pre-fALS) subjects tend to predominantly cluster near the patients with ALS, although a minority of Pre-fALS subjects tend to cluster among healthy controls.
DISCUSSION
There is increasing recognition that the disease process begins prior to the appearance of clinical symptoms and signs in many neurodegenerative diseases, including Parkinson disease,30 Huntington disease,31 and Alzheimer disease.32 It is similarly believed that the neurodegenerative process in ALS may begin prior to the onset of symptoms, but there are relatively little human data to support this hypothesis.2–4 An important reason for the paucity of human data is the practical difficulty of studying people at risk for ALS prior to the onset of symptoms. Pre-fALS is a prospective observational study of asymptomatic individuals from familial ALS pedigrees who harbor a mutation in an ALS susceptibility gene and are at high risk for developing ALS. This is the only known population that can realistically be studied prior to symptom onset.
In this article, we report the first MRS study of presymptomatic people who harbor a mutation in the SOD1 gene. We show that changes in neurometabolite ratios in the cervical spinal cord are evident in asymptomatic SOD1+ people in advance of the onset of symptoms and clinical or electromyographic signs of the disease. These changes primarily reflect a reduction in ratios of NAA to Cr and NAA to Myo. The profile of metabolite ratio changes in this population resembles that observed in patients with clinically apparent ALS, suggesting that the changes in metabolite ratios appear early in the course of the disease process. Of interest, similar observations have been made using transcranial magnetic stimulation to assess cortical excitability. Short-interval cortical inhibition is reduced together with an increase in intracortical facilitation in patients with established ALS as well as in 3 presymptomatic SOD1+ individuals who progressed to manifest disease within 3 months, but not in 14 asymptomatic SOD1+ individuals who did not develop symptoms or signs of disease during follow-up.3 A relevant difference between these electrophysiologic findings and our spectroscopic study is that our data are cross-sectional. As such, it is unclear when each presymptomatic individual will develop symptoms of ALS. It is likely that the population is heterogeneous in this regard, with some likely to develop disease earlier than others. Importantly, we have observed group differences between the presymptomatic SOD1+ population and the healthy controls despite this heterogeneity. Notwithstanding these group differences, there is also significant overlap between the groups such that MRS findings in the cervical cord of individual subjects cannot readily be used to predict, for example, whether a particular individual is presymptomatic or a healthy control.
Prior studies of MRS in ALS have not consistently shown a correlation between spectroscopic markers and clinical measures of disease progression. Some investigators have noted that reductions in NAA are only present, or at least more prominent, among subjects with more severe upper motor neuron involvement,14,15,17,21,26 or more prominent among those with El Escorial definite/probable compared to those with possible/suspected ALS,33 while others have suggested a correlation between spectroscopic findings and clinical metrics such as the Norris functional rating scale11 and finger tapping speeds.23 We have previously found that changes in metabolite ratios in the cervical spinal cord of patients with ALS have weak or no correlation with cross-sectional measures of disease severity such as ALSFRS-R and FVC.10 One possible explanation for this poor correlation is that spectroscopy permits detection of presymptomatic or subclinical manifestations of the disease. An alternative (but not mutually exclusive) explanation is that the spectroscopic findings we have observed are a manifestation of mutant SOD1 gene expression and reflect a stable rather than a dynamic state. Our finding of MRS changes in SOD1+ subjects prior to the onset of clinical symptoms provides support for these hypotheses as well as motivation for a longitudinal MRS study in the presymptomatic SOD1+ population, which is currently under way as part of Pre-fALS.
The next step is to unravel the temporal course of these changes in metabolite ratios in this population in order to determine whether their metabolite ratios change over time. Following SOD1+ people until the onset of disease will help to determine if MRS findings change over time in advance of the appearance of manifest disease and whether it might be possible to impact these changes with some form of preventative therapy. Since we currently have no ability to identify individuals at risk for developing sporadic ALS, there is limited potential to generalize our findings to people with sporadic ALS.
ACKNOWLEDGMENT
The authors thank the participants in this study; Christine Stanislaw for providing pre-test and post-test counseling to participants in the Pre-fALS study; and Debbie Lu and Sharon Usher for study coordination.
GLOSSARY
- %ALS
amyotrophic lateral sclerosis
- ALSFRS-R
revised ALS functional rating scale
- Cho
choline
- Cr
creatine
- FVC
forced vital capacity
- MRS
magnetic resonance spectroscopy
- Myo
myo-inositol
- NAA
N-acetylaspartate
- Pre-fALS
Pre-familial ALS
- SOD1
superoxide dismutase
AUTHOR CONTRIBUTIONS
Dr. Carew: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. Nair: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Dr. Andersen: analysis or interpretation of data, acquisition of data. Ms. Wuu: study concept or design, analysis or interpretation of data. Ms. Gronka: study concept or design, acquisition of data, study supervision. Dr. Hu: study concept or design, analysis or interpretation of data, study supervision. Dr. Benatar: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Carew receives research support from the NIH, AHRQ, the Cardiovascular Research Foundation, and the Carolinas HealthCare Foundation. Dr. Nair and Ms. Gronka report no disclosures. Dr. Andersen receives research support from the Swedish Science Council, the Swedish Hållsten's Brain Research Foundation and Swedish Brain Power; Dr. Andersen has served as a paid consultant for Hoffman La Roche. Ms. Wuu receives research support from NIH, FDA, CDC, MDA, ALS Association, Consolidated Anti-Aging Foundation and the Woodruff Health Sciences Center (Emory University). Dr. Hu has received research support from the NIH/NHLBI. Dr. Benatar is funded by FDA grants FD003517 and FD003710, ALS Association grants 1491, 1712, and 1862, and Muscular Dystrophy Association grant 172123; and is a paid consultant for Cytokinetics and Bayhill Therapeutics. Dr. Benatar receives publishing royalties for Neuromuscular Disease: Evidence and Analysis in Clinical Neurology (Humana Press, 2006), BluePrints in Neurology (Lippincott Williams & Wilkins, 2002), and Field of Vision: A Manual and Atlas of Perimetry (Humana Press, 2010); and has received research support from CytRx Corporation, the Centers for Disease Control and Prevention, the Woodruff Health Sciences Center (Emory University), and the NIH; he has participated in medico-legal cases.
REFERENCES
- 1. Shefner JM. MUNE studies in mouse models of motor neuron disease. Clin Neurophysiol Suppl 2003;55:301–307 [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal A, Nicholson G. Detection of preclinical motor neurone loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry 2002;73:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 2008;131:1540–1550 [DOI] [PubMed] [Google Scholar]
- 4. Ng MC, Ho JT, Ho SL, et al. Abnormal diffusion tensor in nonsymptomatic familial amyotrophic lateral sclerosis with a causative superoxide dismutase 1 mutation. J Magn Reson Imaging 2008;27:8–13 [DOI] [PubMed] [Google Scholar]
- 5. Pioro EP, Majors AW, Mitsumoto H, Nelson DR, Ng TC. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology 1999;53:71–79 [DOI] [PubMed] [Google Scholar]
- 6. Pioro E. Proton magnetic resonance spectroscopy (1H-MRS) in ALS. Amyotroph Lateral Scler 2000;5:S7–S16 [DOI] [PubMed] [Google Scholar]
- 7. Kalra S, Arnold DL. ALS surrogate markers. MRS Amyotroph Lateral Scler 2004;5:S111–S114 [DOI] [PubMed] [Google Scholar]
- 8. Grehl T, Fischer S, Muller K, Malin J, Zange J. A prospective study to evaluate the impact of 31P-MRS to determine mitochondrial dysfunction in skeletal muscle of ALS patients. Amyotroph Lateral Scler 2007;8:4–8 [DOI] [PubMed] [Google Scholar]
- 9. Khiat A, D'Amour M, Souchon F, Boulanger Y. MRS study of the effects of minocycline on markers of neuronal and microglial integrity in ALS. Magn Reson Med 2010;28:1456–1460 [DOI] [PubMed] [Google Scholar]
- 10. Carew JD, Nair G, Pineda-Alonso N, Usher S, Hu X, Benatar M. Magnetic resonance spectroscopy of the cervical cord in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2011;12:185–191 [DOI] [PubMed] [Google Scholar]
- 11. Sarchielli P, Pelliccioli GP, Tarducci R, et al. Magnetic resonance imaging and 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. Neuroradiology 2001;43:189–197 [DOI] [PubMed] [Google Scholar]
- 12. Schuff N, Rooney WD, Miller R, et al. Reanalysis of multislice (1)H MRSI in amyotrophic lateral sclerosis. Magn Reson Med 2001;45:513–516 [DOI] [PubMed] [Google Scholar]
- 13. Abe K, Takanashi M, Watanabe Y, et al. Decrease in N-acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology 2001;43:537–541 [DOI] [PubMed] [Google Scholar]
- 14. Chan S, Shungu DC, Douglas-Akinwande A, Lange DJ, Rowland LP. Motor neuron diseases: comparison of single-voxel proton MR spectroscopy of the motor cortex with MR imaging of the brain. Radiology 1999;212:763–769 [DOI] [PubMed] [Google Scholar]
- 15. Cwik VA, Hanstock CC, Allen PS, Martin WR. Estimation of brainstem neuronal loss in amyotrophic lateral sclerosis with in vivo proton magnetic resonance spectroscopy. Neurology 1998;50:72–77 [DOI] [PubMed] [Google Scholar]
- 16. Kalra S, Cashman NR, Caramanos Z, Genge A, Arnold DL. Gabapentin therapy for amyotrophic lateral sclerosis: lack of improvement in neuronal integrity shown by MR spectroscopy. AJNR Am J Neuroradiol 2003;24:476–480 [PMC free article] [PubMed] [Google Scholar]
- 17. Pioro EP, Antel JP, Cashman NR, Arnold DL. Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology 1994;44:1933–1938 [DOI] [PubMed] [Google Scholar]
- 18. Rule RR, Suhy J, Schuff N, Gelinas DF, Miller RG, Weiner MW. Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord 2004;5:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suhy J, Miller RG, Rule R, et al. Early detection and longitudinal changes in amyotrophic lateral sclerosis by (1)H MRSI. Neurology 2002;58:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalra S, Cashman NR, Genge A, Arnold DL. Recovery of N-acetylaspartate in corticomotor neurons of patients with ALS after riluzole therapy. Neuroreport 1998;9:1757–1761 [DOI] [PubMed] [Google Scholar]
- 21. Block W, Karitzky J, Traber F, et al. Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: subgroup analysis and follow-up measurements. Arch Neurol 1998;55:931–936 [DOI] [PubMed] [Google Scholar]
- 22. Jones AP, Gunawardena WJ, Coutinho CM, Gatt JA, Shaw IC, Mitchell JD. Preliminary results of proton magnetic resonance spectroscopy in motor neurone disease (amyotrophic lateral sclerosis). J Neurol Sci 1995;129(suppl):85–89 [DOI] [PubMed] [Google Scholar]
- 23. Rooney WD, Miller RG, Gelinas D, Schuff N, Maudsley AA, Weiner MW. Decreased N-acetylaspartate in motor cortex and corticospinal tract in ALS. Neurology 1998;50:1800–1805 [DOI] [PubMed] [Google Scholar]
- 24. Kalra S, Hanstock CC, Martin WR, Allen PS, Johnston WS. Detection of cerebral degeneration in amyotrophic lateral sclerosis using high-field magnetic resonance spectroscopy. Arch Neurol 2006;63:1144–1148 [DOI] [PubMed] [Google Scholar]
- 25. Bradley WG, Bowen BC, Pattany PM, Rotta F. 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. J Neurol Sci 1999;169:84–86 [DOI] [PubMed] [Google Scholar]
- 26. Gredal O, Rosenbaum S, Topp S, Karlsborg M, Strange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology 1997; 48:878–881 [DOI] [PubMed] [Google Scholar]
- 27. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299 [DOI] [PubMed] [Google Scholar]
- 28. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–264 [DOI] [PubMed] [Google Scholar]
- 29. Banjamini Y, Hochberg Y. Controlling the false discovery rate: a piratical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300 [Google Scholar]
- 30. Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 1998;64:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology 2004;63:66–72 [DOI] [PubMed] [Google Scholar]
- 32. Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellis CM, Simmons A, Andrews C, Dawson JM, Williams SC, Leigh PN. A proton magnetic resonance spectroscopic study in ALS: correlation with clinical findings. Neurology 1998;51:1104–1109 [DOI] [PubMed] [Google Scholar]