Abstract
Objective:
Several studies report that diabetes increases risk of cognitive impairment; some have hypothesized that advanced glycation end products (AGEs) underlie this association. AGEs are cross-linked products that result from reactions between glucose and proteins. Little is known about the association between peripheral AGE concentration and cognitive aging.
Methods:
We prospectively studied 920 elders without dementia, 495 with diabetes and 425 with normal glucose (mean age 74.0 years). Using mixed models, we examined baseline AGE concentration, measured with urine pentosidine and analyzed as tertile, and performance on the Modified Mini-Mental State Examination (3MS) and Digit Symbol Substitution Test (DSST) at baseline and repeatedly over 9 years. Incident cognitive impairment (a decline of >1.0 SD on each test) was analyzed with logistic regression.
Results:
Older adults with high pentosidine level had worse baseline DSST score (p=0.05) but not different 3MS score (p=0.32). On both tests, there was a more pronounced 9-year decline in those with high and mid pentosidine level compared to those in the lowest tertile (3MS 7.0, 5.4, and 2.5 point decline, p overall <0.001; DSST 5.9, 7.4, and 4.5 point decline, p=0.03). Incident cognitive impairment was higher in those with high or mid pentosidine level than those in the lowest tertile (3MS: 24% vs 17%, odds ratio=1.55; 95% confidence interval 1.07–2.26; DSST: 31% vs 22%, odds ratio=1.62; 95% confidence interval 1.13–2.33). There was no interaction between pentosidine level, diabetes status, and cognitive decline. Multivariate adjustment for age, sex, race, education, hypertension, cardiovascular disease, estimated glomerular filtration rate, and diabetes diminished results somewhat but overall patterns remained similar.
Conclusion:
High peripheral AGE level is associated with greater cognitive decline in older adults with and without diabetes.
Mounting evidence suggests that diabetes increases risk for cognitive impairment and dementia, including Alzheimer disease (AD),1 although the pathogenesis is unknown. Accumulation of advanced glycation end products (AGEs) in the brain is one possible mechanism linking diabetes to cognitive impairment. AGEs are a group of highly stable crosslinked products that form through a series of reactions between glucose and proteins. While AGEs form during normal aging, formation accelerates in diabetes in the setting of hyperglycemia and oxidative stress.2 Although all proteins are prone to AGE formation, deleterious AGE accumulation occurs in tissues with low turnover, including the CNS.3
In brains of patients with AD, AGEs, including pentosidine, colocalize with senile plaques and neurofibrillary tangles (NFT).4–10 One study showed evidence of more severe AD pathology with greater AGE levels in brains of patients with comorbid diabetes and AD compared to those with AD alone.5 In a case-control study serum level of the AGE pentosidine was elevated in patients with AD compared to controls.11 Circulating levels of AGE may provide a marker for risk of cognitive impairment. However, no studies have prospectively analyzed the association of circulating AGE levels and cognitive decline in elders without dementia.
We determined if elders with elevated levels of the AGE pentosidine, as measured by urine, had greater decline in cognitive function in a prospective study of diabetic and nondiabetic elderly men and women without dementia. Our hypothesis was that elders with higher pentosidine levels would experience greater 9-year decline on cognitive testing than those with lower levels.
METHODS
Study population.
Participants were part of the Health, Aging and Body Composition (Health ABC) study, a prospective cohort study beginning in 1997–1999 of 3,075 community-dwelling elders then aged 70–79 years and living in Memphis, TN, or Pittsburgh, PA. To identify potential participants, a random sample of white and all black Medicare-eligible elders within designated zip code areas were contacted. To be eligible, participants had to report no difficulty with activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. They could not have life-threatening cancer diagnoses and could not be intending to move out of the study area for at least 3 years.
Urine pentosidine was assayed for 920 of the 3,075 Health ABC participants, including almost all (495) of the 527 participants with baseline diabetes. Previous studies indicate urine pentosidine is highly correlated with serum levels.12 Diabetes mellitus was defined by use of hypoglycemic medication or a fasting glucose of ≥126 mg/dL, in accordance with the American Diabetes Association (ADA) criteria in place near the start of the Health ABC study (ADA 2002). Pentosidine was also measured in a random sample of 425 participants with normal glucose level (fasting glucose <110 mg/dL and oral glucose tolerance test <140 mg/dL) matched to diabetic patients on race, sex, and clinic site.
Standard protocol approvals, registrations, and patient consents.
All participants in the study signed a written informed consent. The study was approved by the institutional review boards of the 2 field centers (University of Pittsburgh and University of Tennessee, Memphis) and by that of the coordinating center at the University of California, San Francisco.
Measurements.
Two cognitive tests were administered at baseline and at follow-up visits over the next 9 years. The Modified Mini-Mental State Examination (3MS) was administered 5 times, at baseline and at 2, 4, 7, and 9 years after baseline. The 3MS is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory.13 The maximum (best) score is 100. The Digit Symbol Substitution Test (DSST) measures attention, psychomotor speed, and executive function,14 and was administered 4 times, at baseline and at 4, 7, and 9 years after baseline. The DSST score was calculated as the total number of test items correctly coded in 90 seconds, with a higher score indicating better cognition. The maximum (best) score is 90. Clinically significant cognitive impairment over 9 years was defined as a decline of greater than 1 SD of change scores between baseline and the last visit. On the 3MS, this corresponded to a decline of 9 points or more over 9 years; on the DSST, this corresponded to a decline of 10 points or more.
Urine samples were obtained at the baseline visit after an overnight fast and specimens were frozen at −70°C at McKesson Bioservices (Rockville, MD). Pentosidine assays were performed by Dr. Evelyne Gineyts (Institut National de la Santè et de la Recherche Mèdicale Research Unit 831, Lyon, France). Pentosidine was measured on hydrosylated sample by high-performance liquid chromatography using purified bone pentosidine as a standard.15 The pentosidine recovery rate was 93 ± 4%, with a detection limit below 0.02 pmol. The intra-assay and interassay coefficients of variation (CV) were less than 8% and 15%, respectively. We analyzed pentosidine level by tertile range as low (1.56–9.25 pmol/mmol creatinine [Cr]), mid (9.26–13.60 pmol/mmol Cr), and high (13.6–98.66 pmol/mmol Cr) tertile.
We considered several measures which, based on previous studies, could confound the relationship between pentosidine and cognitive scores. These included the baseline characteristics of self-reported age, race, sex, education level (categorized as less than high school vs high school or more education), and number of alcoholic drinks per day (categorized as less than one vs one or more drinks per day). Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies–Depression Scale (CES-D).16 Body mass index (BMI) (kg/m2) was calculated from direct height and weight measurements. Current hypertension was determined using self-report, medication use, and clinical measurements taken at the baseline examination. Baseline prevalent cardiovascular disease (CVD) was defined by self-report, medications, and medical chart review and included stroke and coronary artery disease. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula.17APOE genotype was determined by the 5′-nuclease assay18 in the human genetics laboratory at the University of Pittsburgh and participants were coded as ɛ4 carriers or noncarriers.
Statistical analyses.
We first performed bivariable tests for associations between each of the baseline characteristics and tertile of pentosidine using χ2 tests for categorical variables and analysis of variance for continuous characteristics.
Since 3MS scores were negatively skewed, we used the Box-Cox method to find a transformation that would satisfy normal distribution assumptions for analyses. DSST scores did not require transformation. To test for associations between pentosidine tertile and continuous cognitive scores, we used mixed effects linear regression models with random subject-specific intercepts and slopes and fixed effects for pentosidine tertile and all other variables. Best linear unbiased predictions of the latent baseline values and 9-year changes were estimated based on these models. Predicted 3MS means were back-transformed to the original scale. Standard errors (SE) were estimated for each of the predicted mean scores via bootstrapping.
To test for associations between pentosidine tertile and clinically significant cognitive impairment over 9 years, we used logistic regression. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for the mid and high tertiles combined vs the low pentosidine tertile (reference).
We tested the association between pentosidine tertile and each outcome in models with and without adjusting for baseline characteristics significantly (p < 0.05) associated with tertile of pentosidine in bivariable analyses. These included age, sex, hypertension, CVD and eGFR. In addition, we adjusted for education and baseline diabetes status. We also tested for interactions between pentosidine tertile and diabetes status in all multivariable models. All analyses were conducted using Stata 11.0 (Stata Corporation, College Station, TX).
RESULTS
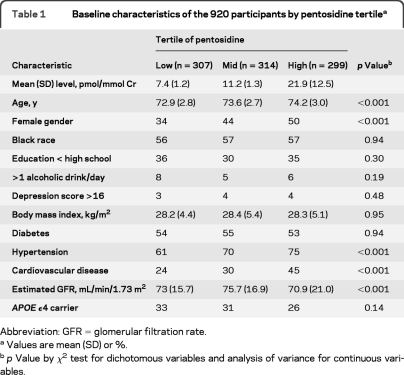
The mean (SD) age at baseline of the 920 participants was 73.6 (2.9) years. Forty-four percent were white and 43% were female. The mean (SD) pentosidine level was 13.4 (9.4) pmol/mmol Cr. Pentosidine level was similar among diabetic and nondiabetic patients (13.2 and 13.6 pmol/mmol Cr, respectively, p=0.51). Higher tertile of pentosidine was associated with older age, being female, having a history of hypertension or CVD, and having lower eGFR (table 1).
Table 1.
Baseline characteristics of the 920 participants by pentosidine tertilea
Abbreviation: GFR=glomerular filtration rate.
Values are mean (SD) or %.
p Value by χ2 test for dichotomous variables and analysis of variance for continuous variables.
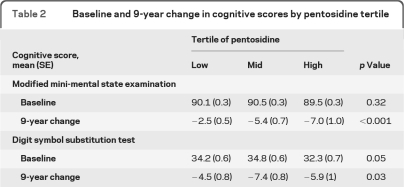
In unadjusted mixed effects linear regression models, baseline 3MS scores did not differ by pentosidine tertile (table 2). However, higher tertile of pentosidine was significantly associated with greater 3MS decline (p < 0.001). Higher tertile was also associated with lower baseline DSST scores (p=0.05) and greater decline (p=0.03). After adjusting for age, sex, education, hypertension, CVD, eGFR, and diabetes, 9-year 3MS decline remained significantly greater for those in the higher pentosidine tertile (p < 0.001). Those in the lowest pentosidine tertile had a mean (SE) adjusted 3MS change score of −2.6 (0.5), vs −5.4 (0.7) and −6.7 (0.9) in mid and high tertile, respectively. Differences in baseline DSST score were no longer significant in the adjusted model (p=0.20), but differences in 9-year DSST decline scores were of borderline significance across pentosidine tertile (p=0.08). Those in the lowest tertile had mean (SE) adjusted DSST change scores of −4.8 (0.5), vs −7.2 (0.6) and −6.0 (0.7) in mid and high tertile, respectively.
Table 2.
Baseline and 9-year change in cognitive scores by pentosidine tertile
In unadjusted logistic regression models, those in the mid and high tertile had a greater likelihood of decline than those in the low tertile on both the 3MS (24% vs 17%, respectively, OR=1.55, 95% CI 1.07–2.26) and DSST (31% vs 22%, respectively, OR=1.62, 95% CI 1.13–2.33). After adjusting for age, sex, education, hypertension, CVD, and eGFR, there was a trend level association with odds of 3MS decline (OR=1.42, 95% CI 0.96–2.12); odds of decline on the DSST remained significantly greater for elders in the mid and high vs those in the low pentosidine tertile (OR=1.52, 95% CI 1.04–2.22).
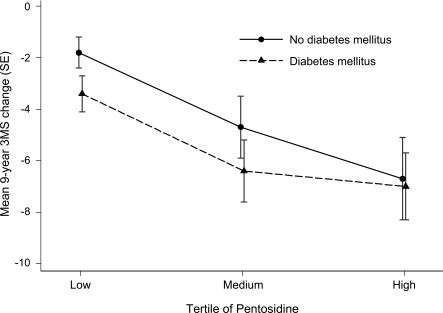
There were no significant interactions between pentosidine tertile and diabetes status on baseline cognitive score (p=0.27 for 3MS and p=0.66 for DSST), or on 9-year change score (p=0.84 for 3MS and p=0.65 for DSST) in the multivariable mixed effects models. However, cognitive decline scores were greater for those with diabetes on the 3MS (figure) in each of the pentosidine tertiles. Similarly, mean (SE) DSST decline scores in the low, mid, and high tertile among those with diabetes (−5.9 [1.1], −10.2 [1.2], and −7.7 [1.6], respectively) were greater than scores among those without diabetes (−3.2 [1.2], −4.7 [1.1], and −4.5 [1.2]).
Figure. Multivariable mixed effects model with adjusted mean Modified Mini-Mental State Examination (3MS) 9-year change score by diabetes status and pentosidine tertile.
Models are adjusted for age, sex, education, hypertension, cardiovascular disease, and estimated glomerular filtration rate.
DISCUSSION
Among older well-functioning adults, those with higher urine pentosidine levels exhibited greater decline in cognitive function over 9 years, independent of demographic factors and comorbidities, including diabetes, although the results were slightly diminished after adjusting for covariates. This study presents novel findings for an association between a peripheral AGE and cognitive decline among elderly without dementia.
These results are supported by a few small cross-sectional studies suggesting an association between elevated AGE level and dementia. In one case-control study, Meli et al.11 reported high pentosidine levels in serum of patients with AD and diabetic patients compared to controls. Another study found CSF and blood serum concentrations of pentosidine cross-sectionally related to vascular dementia.19
AGEs colocalize with several AD-related proteins, including tau proteins, β-amyloid (Aβ),5–8,10,20 and APOE.9 As a result, pentosidine and other AGEs are prominent neuropathic features of plaques and NFTs and correlate with the progression of senile plaques in AD brains.8,20 Greater AGE accumulation associated with diabetes21 may also contribute to more severe dementia. Brains from patients with comorbid diabetes and AD show higher AGE levels and a greater amount of Aβ dense plaques and receptor for AGE (RAGE)–positive and tau-positive cells compared to those with only AD.5 This finding was particularly apparent in the hippocampus. This is of interest as RAGE is able to bind to Aβ and is involved in transport of Aβ across the blood–brain barrier.6,22 In our study, those with diabetes exhibited greater decline in cognitive scores; however, the association between higher peripheral AGE level and decline in cognitive scores also occurred in those without diabetes. It was surprising that in this study pentosidine level did not differ by diabetes status. This may be due to the relatively old age of our population as AGE levels also accumulate with age,3 or possibly due to a survivor bias in those with diabetes enrolled in the Health ABC Study.
Increased AGE levels are also linked to age-related conditions including inflammation, vascular disease, and chronic kidney disease.2,23,24 Each of these conditions may play a role in cognitive impairment, especially in aggregate. For example, previous studies have shown cognitive decline associated with markers of inflammation25,26 and chronic kidney disease.27,28 Vascular disease also is known to contribute to cognitive impairment.29,30 We did not have MRI brain scans that might have detected subclinical cerebral infarcts. Other possible mechanisms include direct toxic effects of AGEs in the brain or the upregulation of RAGE-mediated proinflammatory processes.31 However, a recent RAGE inhibitor trial ended early due to the futility of the agent to improve or prevent dementia.
Strengths of the present study include a prospective design conducted in a large diverse sample of adults without dementia. Pentosidine level was measured in urine obtained at baseline before the onset of cognitive impairment and cognitive testing was done longitudinally, whereas prior studies were cross-sectional,11,19 limiting interpretation of casual pathways. In addition, the study allowed for long follow-up and adjustment for demographics and comorbidities. However, our study has several limitations. We could not test all cognitive domains and are limited in the interpretation of our findings because of the lack of etiology of cognitive impairment. In addition, we do not know for certain how peripheral pentosidine level correlates to those in CSF or brain tissue, although urine pentosidine has been shown to correlate with pentosidine levels in serum.12
Our findings suggest that higher peripheral pentosidine is associated with risk of cognitive decline in elder adults. Future studies should determine the value of peripheral AGEs as a marker for cognitive function and the neuropathologic etiology underlying this association.
GLOSSARY
- 3MS
Modified Mini-Mental State Examination
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADA
American Diabetes Association
- AGE
advanced glycation end product
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies–Depression Scale
- CI
confidence interval
- CV
coefficient of variation
- DSST
Digit Symbol Substitution Test
- eGFR
estimated glomerular filtration rate
- Health ABC
Health, Aging and Body Composition
- MDRD
Modification of Diet in Renal Disease
- NFT
neurofibrillary tangle
- OR
odds ratio
- RAGE
receptor for advanced glycation end product
- SE
standard error
Footnotes
Editorial, page 1326
AUTHOR CONTRIBUTIONS
Dr. Yaffe: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, obtaining funding. K. Lindquist: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Dr. Schwartz: drafting/revising the manuscript, analysis or interpretation of data. C. Vitartas: drafting/revising the manuscript. Dr. Vittinghoff: analysis or interpretation of data, statistical analysis. Dr. Satterfield: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Simonsick: analysis or interpretation of data, acquisition of data. Dr. Launer: drafting/revising the manuscript. Dr. Rosano: drafting/revising the manuscript. Dr. Cauley: drafting/revising the manuscript, acquisition of data. Dr. Harris: drafting/revising the manuscript, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Yaffe has served on data safety monitoring boards for Pfizer Inc, Medivation, Inc. and the NIH (NIMH and NIA trials); and has received research support from the NIH (NIA, NIDDK, NIMH), the Department of Defense, American Health Assistance Foundation, Anonymous Foundation, and the Alzheimer Association. K. Lindquist reports no disclosures. Dr. Schwartz serves on a scientific advisory board for GlaxoSmithKline; has received speaker honoraria from Amgen and Merck Serono; has received funding for travel from Amgen; and receives research support from Merck Serono, GlaxoSmithKline, the NIH (NIDDK, NIA). C. Vitartas reports no disclosures. Dr. Vittinghoff receives publishing royalties for Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models (Springer Verlag, 2005); and receives research support from Medtronic, Inc., Zoll Medical Corporation, Amgen, and the NIH (NIA, NIDDK, NHLBI, NIAID, NIMH). Dr. Satterfield receives research support from the NIH/NIA. Dr. Simonsick serves as an Associate Editor for the Journal of Gerontology Medical Sciences and on the editorial board of the Journal of Aging and Health. Dr. Launer receives research support from the NIH/NIA Intramural Research Program. Dr. Rosano reports no disclosures. Dr. Cauley receives research support from Novartis. Dr. Harris receives research support from the NIH.
REFERENCES
- 1. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia 2005;12:2460–2469 [DOI] [PubMed] [Google Scholar]
- 2. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001;44:129–146 [DOI] [PubMed] [Google Scholar]
- 3. Krautwald M, Münch G. Advanced glycation end products as biomarkers and gerontotoxins-A basis to explore methylglyoxal-lowering agents for Alzheimer's disease? Exp Gerontol 2010;45:744–751 [DOI] [PubMed] [Google Scholar]
- 4. Horie K, Miyata T, Yasuda T, et al. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer's disease and aged neurons. Biochem Biophys Res Commun 1997;236:327–332 [DOI] [PubMed] [Google Scholar]
- 5. Valente T, Gella A, Fernàndez-Busquets X, Unzeta M, Durany N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer's disease and diabetes mellitus. Neurobiol Dis 2010;37:67–76 [DOI] [PubMed] [Google Scholar]
- 6. Srikanth V, Maczurek A, Phan T, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging Epub 2009 May 21 [DOI] [PubMed] [Google Scholar]
- 7. Cruz-Sanchez FF, Gironès X, Ortega A, Alameda F, Lafuente JV. Oxidative stress in Alzheimer's disease hippocampus: a topographical study. J Neurol Sci 2010;299:163–167 [DOI] [PubMed] [Google Scholar]
- 8. Sasaki N, Fukatsu R, Tsuzuki K, et al. Advanced glycation end products in Alzheimer's disease and other neurodegenerative diseases. Am J Pathol 1998;153:1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickson DW, Sinicropi S, Yen SH, et al. Glycation and microglial reaction in lesions of Alzheimer's disease. Neurobiol Aging 1996;17:733–743 [DOI] [PubMed] [Google Scholar]
- 10. Smith MA, Taneda S, Richey PL, et al. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA 1994;91:5710–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meli M, Perier C, Ferron C, et al. Serum pentosidine as an indicator of Alzheimer's disease. J Alzheimers Dis 2002;4:93–96 [DOI] [PubMed] [Google Scholar]
- 12. Takahashi M, Hoshino H, Kushida K, Kawana K, Inoue T. Direct quantification of pentosidine in urine and serum by HPLC with column switching. Clin Chem 1996;42:1439–1444 [PubMed] [Google Scholar]
- 13. Teng E, Chui H. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318 [PubMed] [Google Scholar]
- 14. Wechsler D. Wechsler Adult Intelligence Scale–Third Edition (WAIS-III), Third ed. San Antonio: The Psychological Corporation; 1997 [Google Scholar]
- 15. Tsukahara H, Shibata R, Ohta N, et al. High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: relationship with oxidative stress. Metabolism 2003;52:1601–1605 [DOI] [PubMed] [Google Scholar]
- 16. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 18. Livak K. SNP genotyping by the 5′-nuclease reaction. Methods Mol Biol 2003;212:129–147 [DOI] [PubMed] [Google Scholar]
- 19. Bär KJ, Franke S, Wenda B, et al. Pentosidine and N(epsilon)-(carboxymethyl)-lysine in Alzheimer's disease and vascular dementia. Neurobiol Aging 2003;24:333–338 [DOI] [PubMed] [Google Scholar]
- 20. Lüth HJ, Ogunlade V, Kuhla B, et al. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer's disease brains. Cereb Cortex 2005;15:211–220 [DOI] [PubMed] [Google Scholar]
- 21. Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care 2006;29:1420–1432 [DOI] [PubMed] [Google Scholar]
- 22. Deane R, Du Yan S, Submamaryan R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003;9:907–913 [DOI] [PubMed] [Google Scholar]
- 23. Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med 2002;251:87–101 [DOI] [PubMed] [Google Scholar]
- 24. Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci 2010;65:963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yaffe K, Lindquist K, Penninx B, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003;61:76–80 [DOI] [PubMed] [Google Scholar]
- 26. Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004;292:2237–2242 [DOI] [PubMed] [Google Scholar]
- 27. Yaffe K, Ackerson L, Tamura M, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2010;58:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin C as a marker of cognitive function in elders: findings from the Health ABC Study. Ann Neurol 2008;63:798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease Stroke 2010;41:s144–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warsch JR, Wright CB. The aging mind: vascular health in normal cognitive aging. J Am Geriatr Soc 2010;58:s319–s324 [DOI] [PubMed] [Google Scholar]
- 31. Münch G, Westcott B, Menini T, Gugliucci A. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids Epub 2010 Oct 14 [DOI] [PubMed] [Google Scholar]