Abstract
INTRODUCTION
The aims of this study were to investigate the practice of axillary lymph node management within different units throughout the UK, and to assess changes in practice since our previous survey in 2004.
SUBJECTS AND METHODS
A structured questionnaire was sent to 350 members of the British Association of Surgical Oncology.
RESULTS
There were 177 replies from respondents who managed more than 100 patients a year with breast cancer. Of these: 12 did not perform axillary ultrasound at all in their centre; 17 (10%) employed axillary node clearance (ANC) on all patients; 122(69%) performed sentinel node biopsy (SNB) with dual localisation; and 111 respondents had attended the New Start Course. Radioisotope was most frequently injected 2 h or more before operation. Just 13 surgeons were convinced of the value of dissecting internal mammary nodes visualised on a scan. Reasons for not using dual localisation included lack of nuclear medicine facilities, no local ARSAC licence holder, no probe, and no funding. Sixty-six surgeons stated that, if they had an ARSAC licence and could inject the radioactivity in theatre, this would be a major improvement. In addition, 83 (47%) did not perform SLNB in patients receiving neo-adjuvant chemotherapy.
CONCLUSIONS
Despite significant changes since 2004, substantial variation remains in management of the axilla. A number of surgeons are practicing outwith current guidelines.
Keywords: Breast neoplasms, Sentinel node biopsy, Axilla, Ultrasound, Neo-adjuvant therapy
Breast cancer has the highest incidence of any cancer in the UK with over 45,000 new cases diagnosed each year.1 Axillary node status is recognised as the single most important prognostic indicator of survival in these patients. Since the advent of effective surgical management of breast cancer by Halsted at the end of the 19th century,2 and his use of radical mastectomy with axillary lymph node dissection, there has been a continual effort to optimise locoregional surgery, both in terms of morbidity and mortality for breast cancer patients. A key component to this effort has been the development of an appropriate method of assessing the status of the axillary lymph nodes following on from the introduction of four-node sampling (ANS) over 30 years ago.3,4 The incorporation of sentinel lymph node biopsy (SLNB), originally pioneered in penile cancer in the late 1970s5 and then adapted for use with melanoma patients,6 into breast cancer management began in 1993.7 This first report documented the identification of SLNB with radioisotopes; the following year, SLNB localisation using blue dye alone was reported by Giuliano et al.8 Finally, Albertini et al.9 introduced the concept of combining radioactive isotope and blue dye (‘dual localisation’) in 1996. A recent meta-analysis of over 8000 patients concluded that dual localisation is the method for optimising identification of the SLNB;10 indeed, this is the method being introduced across the UK as part of the NEW START training programme.
Surgeons today are faced with a variety of options when considering how best to assess and manage the axilla including: observation only; SLNB with dye only; SLNB with isotope only; SLNB with dual localisation; blind ANS; blue-dye-assisted ANS; and/or an axillary lymph node dissection (ALND). Even within these options, there are further decisions to be made, including the optimal site of injection of radioisotope or dye, and which is the best dye or radioisotope to use. Added to this, there are issues in relation to the value of internal mammary node dissection, the role and value of scintigraphy, and pre-operative axillary ultrasound and percutaneous axillary node core biopsy and fine-needle aspiration (FNA). Furthermore, once the status of the axilla is established, there then arises the question of how to manage a patient with involved sentinel nodes and whether to proceed to ALND, consider axillary radiotherapy or to perform no further axillary surgery in the presence of low volume axillary disease.
A previous survey by us in 2004 demonstrated a lack of consensus across the UK in relation to the use of SLNB in breast cancer patients.11 Further work, published recently by Mansfield et al.,12 concluded that just 52% of surgeons use SLNB in patients who have a clinically node-negative breast cancer. The principal objective of this study was to build on previous work, and to assess the conformity with which surgeons in the UK are approaching issues related to the axilla, including SLNB, internal mammary biopsy, axillary ultrasound, and scintigraphy. A second aim of the study was to establish satisfaction levels with the current NEW START programme, and was to identify those factors which are making it difficult for surgeons to incorporate the use of sentinel node biopsy.
Subjects and Methods
A structured questionnaire was distributed to 350 Association of Breast Surgery (ABS) associates, at the British Association of Surgical Oncology (BASO), throughout the UK, in the summer of 2008. The questionnaire was divided into three sections. The first section collected information related to the post in which the surgeon was working, including the type and size of breast unit, the number of breast cancer patients treated per year, and whether they performed other types of surgery. The second series of questions focused on axillary investigation and surgery, including the use of pre-operative axillary ultrasound scans, and the use of SLNB. Further details of procedures were also sought. The final series of questions related to the use of radioisotope and blue dye and attendence at the NEW START programme.
Results
Demographic data
A total of 177 questionnaires (50.1%) were returned completed. Of these, 168 (95%) were consultants, with the remainder being staff grades and registrars working in breast units around the UK. Data on the surgeon's background are presented in Table 1. Of respondents, 117 (66.1%) treated greater than 100 patients per year, whilst just less than 2% treated less than 50. Overall, 174 (98%) practiced in either a specialist breast unit or a breast unit within a district general hospital and 152 (86%) worked in hospitals with nuclear medicine facilities. Those surgeons treating greater than 100 breast cancer patients per year were less likely to perform other types of surgery (P = 0.041). That said, 60% of surgeons (107 of 177) had an on-call commitment for general surgical emergencies.
Table 1.
Demographic Data
| Question | Option | Result (%) |
|---|---|---|
| Which grade of surgeon applies to you? | Consultant | 95 |
| SpR | 3 | |
| Staff Grade/Ass Specialist | 2 | |
| How many breast cancer patients do you treat in one year? | <50 | 2 |
| 50-100 | 32 | |
| >100 | 66 | |
| Which of the following best applies to the setting in which you work? | Specialist Breast Unit | 39 |
| Breast Unit with in DGH | 59 | |
| General Surgical | 2 | |
| What other types of surgery do you perform? | General | 61 |
| Endocrine | 27 | |
| GI | 7 | |
| Other | 17 | |
| Nil | 20 | |
| Do you have an on-call commitment for general surgical emergencies? | Yes | 60 |
| No | 40 | |
| Does you hospital have nuclear medicine facilities? | Yes | 86 |
| No | 14 |
ASS, Associate; DGH, District General Hospital; GI, Gastrointestinal; SpR, Specialist Registrar
Axillary ultrasound
A total of 114 (65%) surgeons organised or performed axillary ultrasound on all patients with proven invasive cancer, 50 performed it on selected patients and 12 never used this investigation. Actions taken as a result of an abnormal or suspicious ultrasound scan are shown in Figure 1.
Figure 1.
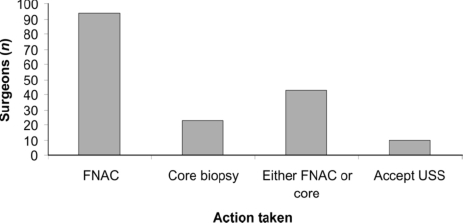
Actions taken following abnormal or suspicious axillary ultrasound. FNAC, fine needle aspiration cytology; USS, ultrasound scan.
Axillary node clearance (ANC)
Of surgeons, 146 (82%) performed ANC regularly and almost 10% (n = 17) performed it on all patients with breast cancer. Of those respondents who maintained a selective policy in relation to ANC (n = 154), 13 performed ANC for all patients undergoing breast conserving surgery whereas 116 (75%) performed ANC only for patients with proven lymph node involvement undergoing breast conserving surgery. Thirty-four (22%) performed ANC for all patents with invasive cancer undergoing mastectomy, with 96 (62%) performing ANC only for patients with proven lymph node involvement undergoing mastectomy.
Axillary node sampling
Fifty-four (30.5%) surgeons performed ANS on a regular basis with the majority performing a blue-dye directed ANS. Over 95% (102 of 107) of respondents used patent blue V with the remainder using methylene blue. Of 111 surgeons who answered the question, 17 (15%) injected dye at more than one anatomical site. The most common sites for injection of dye were subareolar (n = 44) and peri-areolar (n = 44) Less common sites included peritumoural and intradermal. Some surgeons did perform an axillary sampling procedure without blue dye, with the majority performing a level 1 axillary dissection (n = 15).
Sentinel lymph node biopsy (SLNB)
Overall, 122 (69%) surgeons performed SLNB regularly with dual localisation; one surgeon used SLNB with radioisotope only. However, 133 (75%) performed SLNB with radioisotope, at least occasionally. SLNB was employed most commonly for patients who were clinically and ultrasound node negative, as demonstrated in Figure 2. When the lesion was palpable, radioisotope was injected in the nuclear medicine department in the vast majority (123 of 137; 90%) of cases. A similar situation applied for cases of impalpable cancer (111 of 133; 83%); 13 surgeons injected isotope in the X-ray department during localisation.
Figure 2.
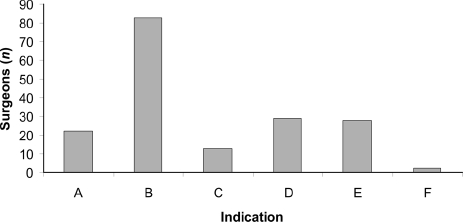
Indications for sentinel node biopsy. (A) Clinically node-negative patients (n = 22); (B) clinically and ultrasound node-negative patients (n = 83); (C) all patients, irrespective of imaging or node status (n = 13); (D) clinically node-negative and clinically and ultrasound node-negative patients (n = 29); (E) no answer (n = 28); and (F) other (n = 2).
Radioisotope was rarely injected in the ward and operating theatre. Of 136 surgeons who responded to the question, 80 (59%) injected the blue dye and radioisotope at the same anatomical site. The most common sites for injection of blue dye were subareolar (n = 67) and peri-areolar (n = 67). The most popular sites for injection of radioisotope were peri-areolar (n = 61), intradermal (n = 36), and subareolar (n = 34). A significant number of respondents (22 of 136; 16%) chose to inject radioisotope at more than one anatomical location. The vast majority (105 of 135; 78%) of surgeons using radioisotope injected it more than 2 h prior to the operation. Factors most frequently identified as inhibiting the use of SLNB with radioactivity included a lack of nuclear medicine facilities, no local ARSAC licence holder, and a lack of funding. Sixty-six surgeons stated that, if they had an ARSAC licence and could inject the radioactivity in theatre, this would be a major improvement. Forty-three thought this would be of no improvement and 23 thought this would not be acceptable because no scintigraphy would be possible.
Sentinel lymph node biopsy and neo-adjuvant chemotherapy
Eighty-three surgeons did not perform SLNB in patients receiving neo-adjuvant chemotherapy; 30 performed it prior to chemotherapy, and a further 25 performed it post-chemotherapy if the patient was clinically node-negative at diagnosis.
NEW START Programme
A total of 111 (62%) respondents had attended a NEW START course; these represented 83% of those surgeons working with radioisotope.
Scintiscan
Of those who responded to the questions on scintiscan (n = 137), 104 (75%) surgeons regularly performed the investigation. Of 122 respondents, just 21 (17%) routinely removed internal mammary nodes if visualised on scintiscan, and only 13 (9%) of respondents were convinced that there was value in dissecting internal mammary nodes visualised on scintiscan; 86 (63%) were not convinced and 38 (28%) were unsure of the value of excising internal mammary nodes.
Discussion
In this study, one questionnaire was sent to each centre, and hence only 350 members out of the total ABS at BASO membership were surveyed. The surgeons involved in this study had previously been surveyed in a similar questionnaire distributed by us in 2004.11 A limitation of this study is the relatively low rate of survey completion (50%), though examination of the literature reveals this to be in line with, and indeed exceed, the completion rate of similar studies in the breast cancer arena. Two-thirds of the surgeons completing this current survey managed over 100 patients with breast cancer per year. Since 2004,11 there have been changes with a reduction from 70% to 60% of breast surgeons having an on-call commitment. There has been an overall reduction in the number of breast surgeons doing general surgery from 81% to 60%. There have also been falls in the number doing endocrine surgery from 34% to 27% and GI surgery from 24% to 7%. This is in keeping with the continued trend to breast cancer being looked after by specialist breast surgeons rather than general surgeons who have a specialist breast interest.
One change since the last survey has been the introduction of axillary ultrasound. There is now clear evidence that this is of value;17 however, 7% of units do not use this at all and 28% only use it selectively. This is a cost-effective method of assessing the axilla and the evidence is that it has considerable benefits and no harms. Further studies are needed to identify the barriers as to why it is not used routinely in patients with invasive breast cancer. The particular value of ultrasound is that it identifies up to half of patients who have involved nodes18,19 and these women can be spared an unnecessary sentinel node biopsy (SNB). In those units who utilise axillary ultrasound, the majority investigate suspicious nodes with FNA or core biopsy with smaller numbers utilising a combination of the two. A minority (10 surgeons) accept the ultrasound result and do not perform FNAC or core biopsy. Ultrasound is neither sufficiently sensitive nor specific to be used alone; in patients with indeterminate cortical thickening in the range of 2–4 mm, it is essential to assess whether such patients have involved nodes by FNAC or core biopsy.20–23
The majority of surgeons continue to perform ANC which is an appropriate treatment for involved axillary nodes. Axillary radiotherapy is another option for involved nodes, and this is used in some centres and has been shown to produce good long-term disease control rate.24 A randomised study of ANC versus axillary radiotherapy is ongoing. Approximately 10% of surgeons continue to perform ANC on all patients with invasive breast cancer; this includes 8.4% of surgeons who perform it routinely in patients undergoing breast conserving surgery and 22% who perform ANC on all patients with invasive cancer undergoing mastectomy. The majority restrict ANC to patients with involved axilla on cytology or follow a SNB. ANC has significant morbidity25 and it is difficult to support the routine use of ANC in patients without definite proof of axillary node involvement.
Almost a third of surgeons perform axillary node sample (ANS) on a regular basis. The majority perform blue dye directed sampling. There has been a considerable change where in the breast the blue dye is injected. Previously, 82% of surgeons injected blue dye around the tumour,11 whereas now 80% inject it in the subareolar or peri-areolar regions. There remain some surgeons who perform a level I axillary dissection rather than an ANS or SNB. With the considerable literature demonstrating the efficacy of SNB,26–28 the on-going use of lower axillary dissection as a method of assessing node status of the axilla is difficult to support. Studies with reverse axillary mapping where blue dye is injected into the upper arm have shown that there are lymphatics in the lower axilla which drain the arm and these will be potentially damaged by a level I dissection where they are much more likely to be preserved by a SNB.29
Increasing numbers of surgeons are performing SNB regularly. Only 36% in our previous survey were performing SNB outside of trials whereas 69% of surgeons now perform dual localisation SNB. SNB is appropriately employed by most surgeons in patients who are clinically and ultrasound node-negative. Radioisotope is, in the majority, injected in the nuclear medicine department although radioisotope is sometimes injected in the ward and in the operating theatre. The majority of surgeons inject blue dye and radioisotope in the same anatomical location with the most common site for injection being subareolar or peri-areolar.
The injection of blue dye and radioactivity at the same site is in keeping with the literature which suggests that drainage to the axilla is the same regardless of the site of injection.30–32 Nonetheless, 40% of surgeons inject blue dye and radioactivity at different sites. Radioisotope is most frequently injected more than 2 h prior to surgery, although recent data have shown that localisation rates are identical regardless of the time the radioisotope is injected.29,34 This includes injecting the radioisotope in the anaesthetic room after induction of anaesthesia. Factors identified as inhibiting the use of SNB include a lack of nuclear medicine facilities in the hospital, no local ARSAC licence holder and a lack of funding. When asked what would be the one change in practice which would have the benefit, almost one half of surgeons stated that the acquisition of an ARSAC licence and the ability to inject the radioactivity when the patient has been anaesthetised would be a major improvement. A third thought this would not be of any advantage and one in six thought this would not be a disadvantage because scintigraphy could not be performed prior to surgery.
The results of this analysis have demonstrated the lack of consensus regarding the use of SNB in the setting of neo-adjuvant chemotherapy. There have been numerous discussions about the value of performing SNB both prior to, and following, neo-adjuvant treatment,36–39 but it appears there remains a divergence of views amongst surgeons as to the most appropriate practice in this context.
There continues to be uncertainty about the value of scintigraphy and the value of removing internal mammary nodes. Only 17% of surgeons remove internal mammary nodes routinely if visualised on the scan and only 9% of surgeons are convinced that there is value in dissecting out internal mammary nodes. Almost two-thirds are not convinced of the value of removing internal mammary nodes. The number of patients with isolated internal mammary nodes is small.40 Although the morbidity associated with removal of the internal mammary nodes has been reported by some to be small,40 the procedure can involve a second skin incision and mobilisation of large amount of tissue. There also remains controversy as to whether once internal mammary node metastases are identified it is possible to give adequate doses of radiotherapy to these areas without including significant amounts of underlying heart in the radiation fields,41 particularly on the left side.
Conclusions
There have been significant changes in the management of the axilla since the previous survey. SNB is now much more widely used but there remain units where SNB cannot be performed. Barriers have been identified and these need to be overcome if equal access to the same standard of care is available throughout the UK. Wide variations in practice have been identified which are not evidence based. Consensus and standards of care need to be defined to ensure treatment is based on stage of disease rather than which surgeon manages a patient.
Acknowledgments
This study was presented, in part, at the Association of Surgeons of Great Britain and Ireland (ASGBI) Annual Conference, Glasgow, May 2009.
References
- 1.Cancer Research UK. About Cancer [Breast]: Statistics – Incidence and Mortality. 2005 < http://info.cancerresearchuk.org/cancerstats/types/breast/_.
- 2.Halsted W. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospitals from June 1889 to January 1894. Johns Hopkins Hosp Bull. 1895;4:297–302. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest APM, Stewart HJ, Roberts MM, Steele RJ. Simple mastectomy and axillary node sampling (pectoral node biopsy) in the management of primary breast cancer. Ann Surg. 1982;196:371–8. doi: 10.1097/00000658-198209000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele RJC, Forrest APM, Gibson T, Stewart HJ, Chetty U. The efficacy of lower axillary sampling in obtaining lymph node status in breast cancer: a controlled randomized trial. Br J Surg. 1985;72:368–9. doi: 10.1002/bjs.1800720512. [DOI] [PubMed] [Google Scholar]
- 5.Cabanas RM. An approach for the treatment of penile cancer. Cancer. 1977;39:456–66. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Morton DL, Wen DR, Wong J, Economou JS, Cagle LA, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 7.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalisation of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–40. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–22. [PubMed] [Google Scholar]
- 10.Kim T, Giuliano AE, Lyman G. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 11.Gaston MS, Dixon JM. A survey of surgical management of the axilla in UK breast cancer patients. Eur J Cancer. 2004;40:1738–42. doi: 10.1016/j.ejca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Mansfield L, Sosa I, Dionello R, Subramanian A, Devalia H, Mokbel K. Current management of the axilla in patients with clinically node-negative breast cancer: a nationwide survey of United Kingdom breast surgeons. Int Semin Surg Oncol. 2007;4:4. doi: 10.1186/1477-7800-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair SL, Thompson K, Rococco J, Malcarne V, Beitsch PD, Ollila DW. Attaining negative margins in breast-conservation operations: is there a consensus among breast surgeons? J Am Coll Surg. 2009;209:608–13. doi: 10.1016/j.jamcollsurg.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Aristei C, Amichetti M, Ciocca M, Nardone L, Bertoni F, et al. Radiotherapy in Italy after conservative treatment of early breast cancer. A survey by the Italian Society of Radiation Oncology (AIRO) Tumori. 2008;94:333–41. doi: 10.1177/030089160809400308. [DOI] [PubMed] [Google Scholar]
- 15.Garreau JR, Nelson J, Cook D, Vetto J, Walts D, et al. Geographic variation in sentinel node adaptation by practicing surgeons in Oregon. Am J Surg. 2005;189:616–9. doi: 10.1016/j.amjsurg.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Schroen AT, Brenin DR. Clinical trial priorities among surgeons caring for breast cancer patients. Am J Surg. 2008;195:474–80. doi: 10.1016/j.amjsurg.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Nathanson SD, Burke M, Slater R, Kapke A. 3rd UK e-Science All Hands Meeting (AHM 2004) Springer: 2004. Preoperative identification of the sentinel lymph node in breast cancer; pp. 3102–10. 2004 Aug 31-Sep 02; Nottingham. [DOI] [PubMed] [Google Scholar]
- 18.Gilissen F, Oostenbroek R, Storm R, Westenend P, Plaisier P. Prevention of futile sentinel node procedures in breast cancer: Ultrasonography of the axilla and fine-needle aspiration cytology are obligatory. Eur J Surg Oncol. 2008;34:497–500. doi: 10.1016/j.ejso.2007.07.198. [DOI] [PubMed] [Google Scholar]
- 19.Podkrajsek M, Music MM, Kadivec M, Zgajnar J, Besic N, et al. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur Radiol. 2005;15:1044–50. doi: 10.1007/s00330-004-2545-4. [DOI] [PubMed] [Google Scholar]
- 20.Moore A, Hester M, Nam MW, Brill YM, McGrath P, et al. Distinct lymph nodal sonographic characteristics in breast cancer patients at high risk for axillary metastases correlate with the final axillary stage. Br J Radiol. 2008;81:630–6. doi: 10.1259/bjr/21933846. [DOI] [PubMed] [Google Scholar]
- 21.Abe H, Schmidt RA, Kulkarni K, Sennett CA, Mueller JS, Newstead GM. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-guage core-needle biopsy – clinical experience in 100 patients. Radiology. 2009;250:41–9. doi: 10.1148/radiol.2493071483. [DOI] [PubMed] [Google Scholar]
- 22.Swinson C, Ravichandran D, Navagam M, Allen S. Ultrasound and fine needle aspiration cytology of the axilla in the pre-operative identification of axillary nodal involvement in breast cancer. Eur J Surg Oncol. 2009;35:1152–7. doi: 10.1016/j.ejso.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Deurloo EE, Tanis PJ, Gilhuijs KGA, Muller SH, Kröger R, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–73. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radio-therapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 25.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Ann Surg Oncol. 2008;15:262–7. doi: 10.1245/s10434-007-9593-3. [DOI] [PubMed] [Google Scholar]
- 26.Kidd SA, Keto JL, Tran H, Fitzgerald TL. First three sentinel lymph nodes accurately stage the axilla in breast cancer. Am Surg. 2009;75:253–6. [PubMed] [Google Scholar]
- 27.Amersi F, Hansen NM. The benefits and limitations of sentinel lymph node biopsy. Curr Treat Options Oncol. 2006;7:141–51. doi: 10.1007/s11864-006-0049-y. [DOI] [PubMed] [Google Scholar]
- 28.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary- lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 29.Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, et al. Intraoperative radioisotope injection for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:3216–21. doi: 10.1245/s10434-008-0010-3. [DOI] [PubMed] [Google Scholar]
- 30.Bauer TW, Spitz FR, et al. 54th Annual Meeting of the Society-of-Surgical-Oncology; Washington, DC: 2001. Subareolar and peritumoral injection identify similar sentinel nodes for breast cancer; pp. 169–76. 2001 Mar 15-18. [DOI] [PubMed] [Google Scholar]
- 31.Kesmodel SB, Canter RJ, Terhune KP, Bauer TW, Mick R, et al. Use of radiotracer for sentinel lymph node mapping in breast cancer optimizes staging independent of site of administration. Clin Nucl Med. 2006;31:527–33. doi: 10.1097/01.rlu.0000233070.06956.69. [DOI] [PubMed] [Google Scholar]
- 32.Mateos JJ, Vidal-Sicart S, Zanon G, Pahisa J, Fuster D, et al. Sentinel lymph node biopsy in breast cancer patients: subdermal versus peritumoural radiocolloid injection. Nucl Med Commun. 2001;22:17–24. doi: 10.1097/00006231-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–3. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 34.Dixon JM, Mak C, Radhakrishna S, Kehoe T, Millar AM, et al. Effectiveness of immediate preoperative injection of radiopharmaceutical and blue dye for sentinel node biopsy in patients with breast cancer. Eur J Cancer. 2009;45:795–9. doi: 10.1016/j.ejca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, et al. Intraoperative radioisotope injection for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:3216–21. doi: 10.1245/s10434-008-0010-3. [DOI] [PubMed] [Google Scholar]
- 36.Jones JL, Zabicki K, et al. 6th Annual Meeting of the American-Society-of-Breast-Surgeons; Los Angeles, CA: 2005. A comparison of sentinel node biopsy before and after neoadjuvant chemotherapy: timing is important; pp. 517–20. 2005 Mar 16–20. [DOI] [PubMed] [Google Scholar]
- 37.Mamounas, Soran A. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 38.Papa MZ, Zippel D, Kaufman B, Shimon-Paluch S, Yosepovich A, et al. Timing of sentinel lymph node biopsy in patients receiving neoadjuvant chemotherapy for breast cancer. J Surg Oncol. 2008;98:403–6. doi: 10.1002/jso.21128. [DOI] [PubMed] [Google Scholar]
- 39.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 40.Paredes P, Vidal-Sicart S, Zanon G, Pahisa J, Fernández PL, Velasco M. Clinical relevance of sentinel lymph nodes in the internal mammary chain in breast cancer patients. Eur J Nucl Med Mol Imaging. 2005;32:1283–7. doi: 10.1007/s00259-005-1867-z. [DOI] [PubMed] [Google Scholar]
- 41.Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management – a systematic review. J Clin Oncol. 2008;26:4981–9. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]


