Abstract
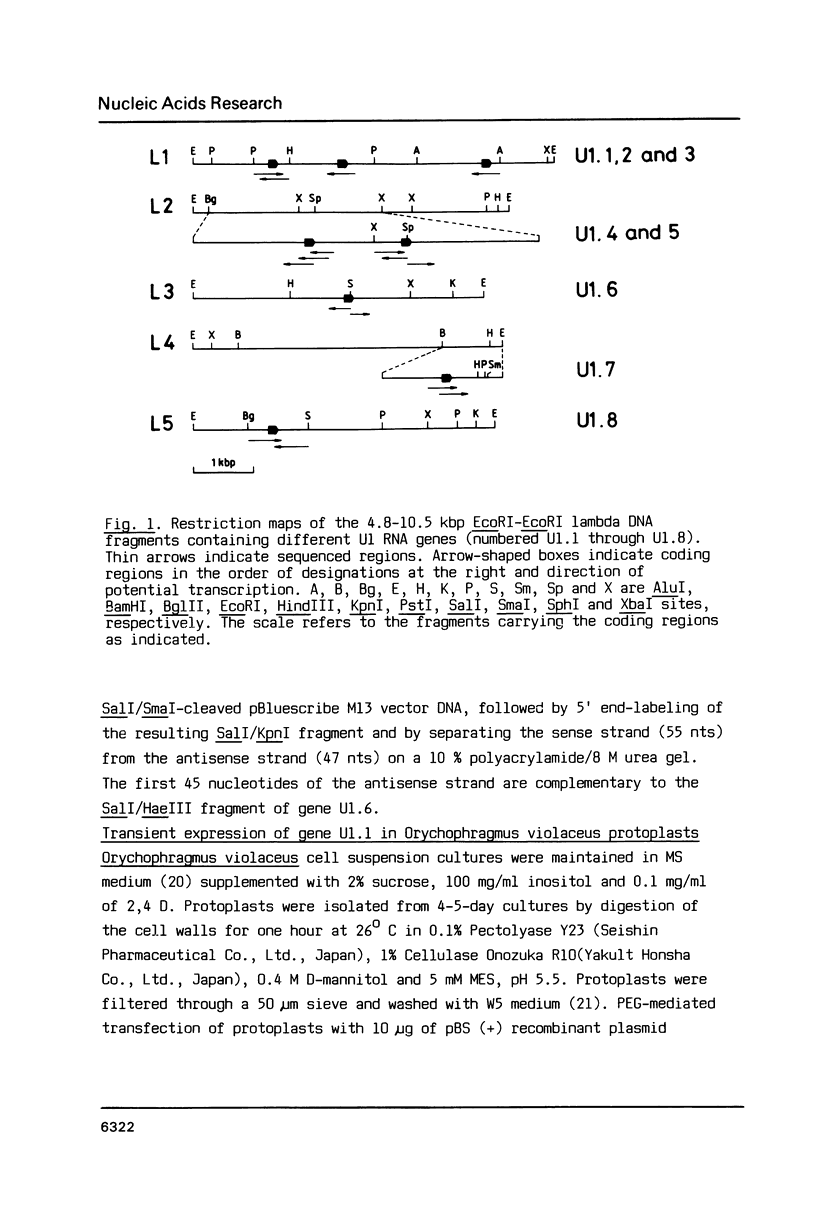
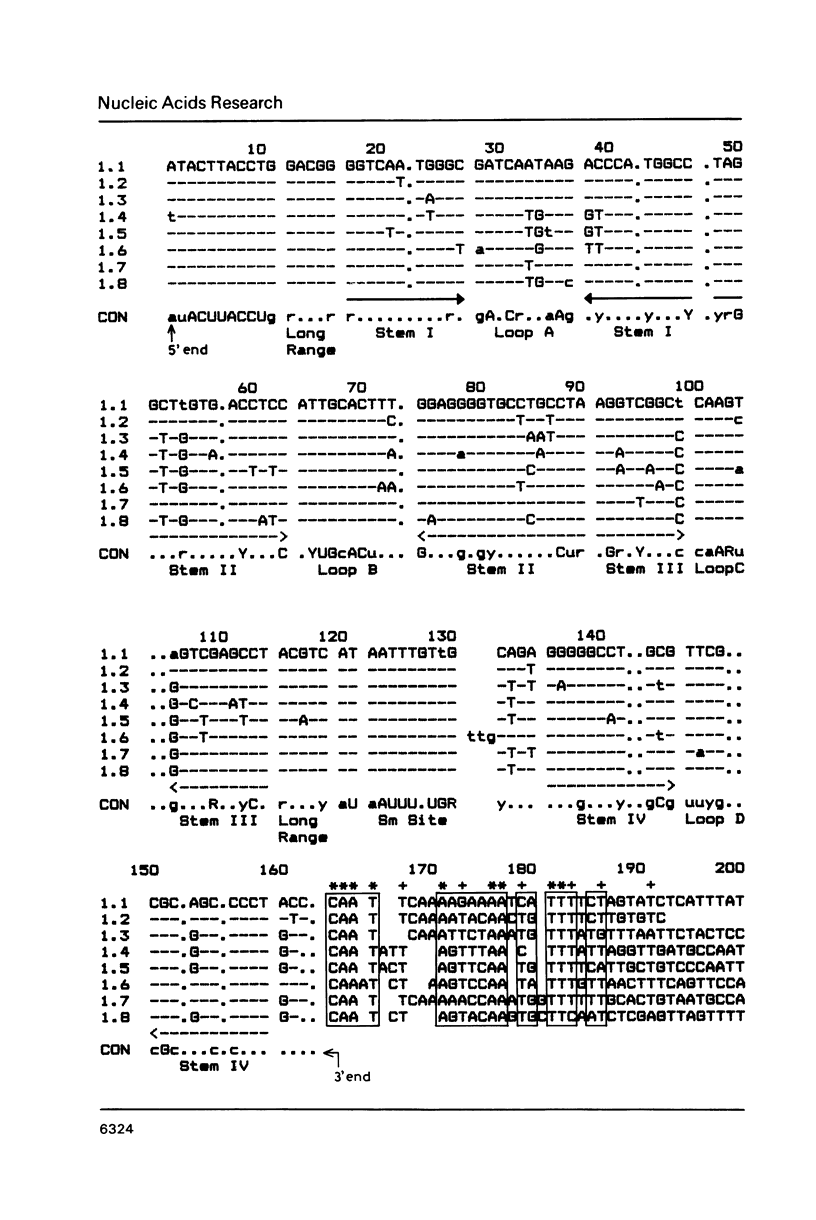
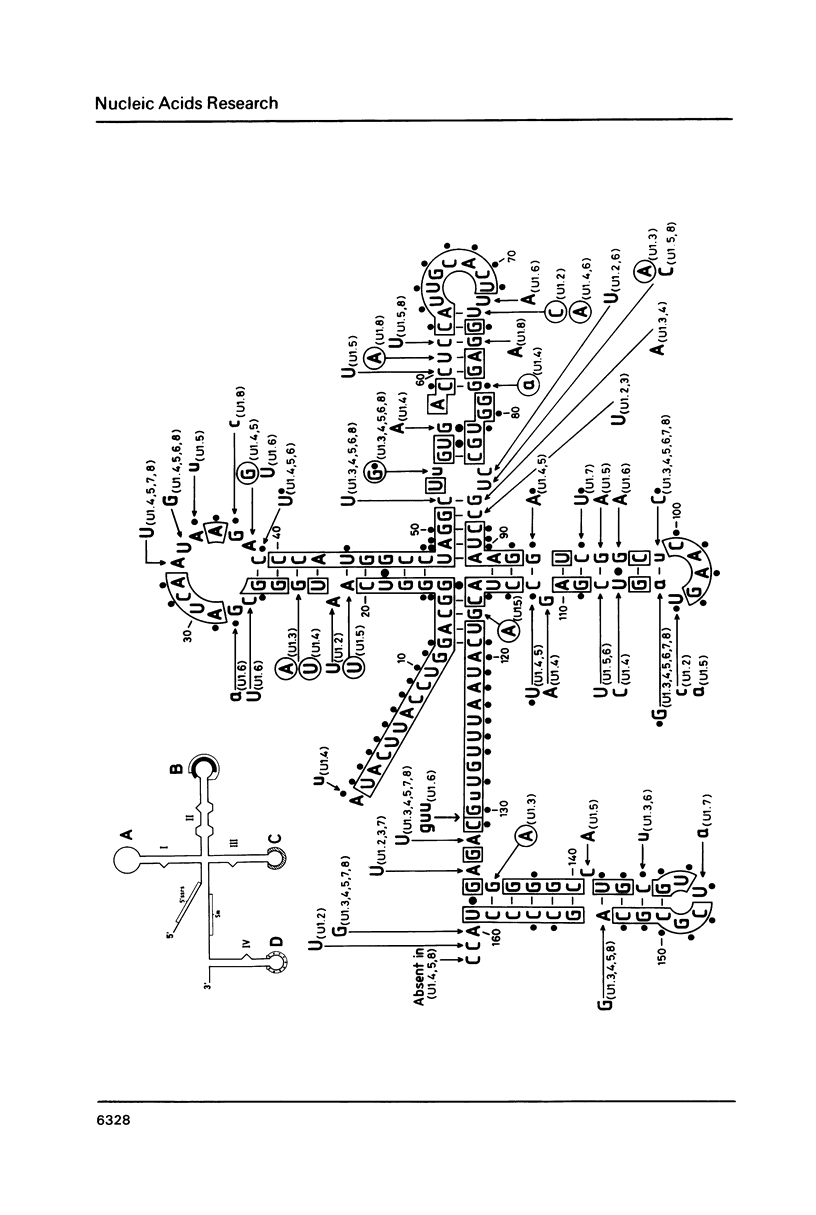
From a tomato genomic library we isolated and characterized eight U1 RNA gene candidates (U1.1 to U1.8) all of which possessed the canonical plant U-snRNA transcription signals in their 5' and 3' flanking regions and exhibited nucleotide sequence conservation in the 5' splice site recognition sequence, in the Sm antigen binding site and in Loops B, C, D as well as in Stems III and IV of their coding region. Deviations from the U1 RNA consensus sequence were mainly localized to Loop A and Stems I and II, suggesting that the putative transcripts of the tomato U1.1-U1.8 genes would differ from each other in their capacity of binding to the U1 RNA-specific snRNP proteins.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Denison R. A., Weiner A. M. Human U1 RNA pseudogenes may be generated by both DNA- and RNA-mediated mechanisms. Mol Cell Biol. 1982 Jul;2(7):815–828. doi: 10.1128/mcb.2.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hamm J., Kazmaier M., Mattaj I. W. In vitro assembly of U1 snRNPs. EMBO J. 1987 Nov;6(11):3479–3485. doi: 10.1002/j.1460-2075.1987.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., van Santen V. L., Spritz R. A., Mattaj I. W. Loop I of U1 small nuclear RNA is the only essential RNA sequence for binding of specific U1 small nuclear ribonucleoprotein particle proteins. Mol Cell Biol. 1988 Nov;8(11):4787–4791. doi: 10.1128/mcb.8.11.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C., Graham M. Y., Dutchik J. E., Olson M. V. A new method for purifying lambda DNA from phage lysates. DNA. 1985 Feb;4(1):39–49. doi: 10.1089/dna.1985.4.39. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Korf G. M., McNamara K. J., Stumph W. E. Structural and functional analysis of chicken U4 small nuclear RNA genes. Mol Cell Biol. 1986 Nov;6(11):3910–3919. doi: 10.1128/mcb.6.11.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Antal M., Hegyi H., Solymosy F. Plant small nuclear RNAs. IV. The structure of U1 RNA from Chlorella saccharophila: a phylogenetic support, in terms of RNA structure, for the probable interaction between U1 and U2 and RNPs during the splicing of pre-mRNA. Nucleic Acids Res. 1988 Mar 25;16(6):2734–2734. doi: 10.1093/nar/16.6.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Jakab G., Antal M., Pálfi Z., Hegyi H., Kis M., Solymosy F. Plant small nuclear RNAs. V. U4 RNA is present in broad bean plants in the form of sequence variants and is base-paired with U6 RNA. Nucleic Acids Res. 1988 Jun 24;16(12):5407–5426. doi: 10.1093/nar/16.12.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Tóth M., Solymosy F. Plant small nuclear RNAs. Nucleolar U3 snRNA is present in plants: partial characterization. Eur J Biochem. 1985 Oct 15;152(2):259–266. doi: 10.1111/j.1432-1033.1985.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Kretzner L., Rymond B. C., Rosbash M. S. cerevisiae U1 RNA is large and has limited primary sequence homology to metazoan U1 snRNA. Cell. 1987 Aug 14;50(4):593–602. doi: 10.1016/0092-8674(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Krol A., Ebel J. P., Rinke J., Luhrmann R. U1, U2 and U5 small nuclear RNAs are found in plants cells. Complete nucleotide sequence of the U5 RNA family from pea nuclei. Nucleic Acids Res. 1983 Dec 20;11(24):8583–8594. doi: 10.1093/nar/11.24.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E. Heterogeneity of human U1 snRNAs. Nucleic Acids Res. 1988 Jul 11;16(13):5813–5826. doi: 10.1093/nar/16.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Hamm J. Regulated splicing in early development and stage-specific U snRNPs. Development. 1989 Feb;105(2):183–189. doi: 10.1242/dev.105.2.183. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Pederson T. The Mr 70,000 protein of the U1 small nuclear ribonucleoprotein particle binds to the 5' stem-loop of U1 RNA and interacts with Sm domain proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(3):747–751. doi: 10.1073/pnas.85.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálfi Z., Bach M., Solymosy F., Lührmann R. Purification of the major UsnRNPs from broad bean nuclear extracts and characterization of their protein constituents. Nucleic Acids Res. 1989 Feb 25;17(4):1445–1458. doi: 10.1093/nar/17.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R. Compilation of small RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r71–r85. doi: 10.1093/nar/16.suppl.r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., Edoh D., Filipowicz W. Structure and expression of the U5 snRNA gene of Arabidopsis thaliana. Conserved upstream sequence elements in plant U-RNA genes. Nucleic Acids Res. 1988 Nov 25;16(22):10425–10440. doi: 10.1093/nar/16.22.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. Structure of U2 snRNA genes of Arabidopsis thaliana and their expression in electroporated plant protoplasts. EMBO J. 1988 Mar;7(3):791–799. doi: 10.1002/j.1460-2075.1988.tb02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- van Santen V. L., Spritz R. A. Nucleotide sequence of a bean (Phaseolus vulgaris) U1 small nuclear RNA gene: implications for plant pre-mRNA splicing. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9094–9098. doi: 10.1073/pnas.84.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen V. L., Swain W., Spritz R. A. Nucleotide sequences of two soybean U1 snRNA genes. Nucleic Acids Res. 1988 May 11;16(9):4176–4176. doi: 10.1093/nar/16.9.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]





