Abstract
Oxycodone, a popularly used opioid for treating pain, is widely abused. Other drugs of abuse have been shown to affect time perception, which in turn may affect sensitivity to future consequences. This may contribute to continued use. The current study evaluated the effect of oxycodone on time perception in normal healthy volunteers. For this within-subject, double-blind design study, participants performed a temporal reproduction task before and after receiving placebo or oxycodone (15 mg, po) over 6 outpatient sessions. Participants were first trained with feedback to reproduce three standard intervals (1.1, 2.2, and 3.3 s) in separate blocks by matching response latency from a start signal to the duration of that block’s standard interval. During testing participants were instructed to reproduce the three intervals from memory without feedback before and after drug administration . Oxycodone significantly lengthened time estimations for the two longer intervals relative to placebo. These results suggest that opioids alter temporal processing for intervals greater than one second, raising questions about the effect of these drugs on valuation of future consequences.
Keywords: time perception, interval timing, opioid, oxycodone, human
Oxycodone, an opioid agonist, is used clinically to treat patients with moderate to severe persistent pain (Riley, et al. 2008). It is also emerging as a substance of abuse (Cicero, et al. 2007). Addicts of opiates such as heroin are said to have shortened time horizons, leading to devaluation of future consequences, poor decision making and continued drug use (Petry, et al. 1998). Devaluation of future outcomes has been suggested to be related to altered time perception in drug users (Takahashi 2005, 2006, Wittmann, et al. 2007, Wittmann and Paulus 2008). After all, one of the most salient factors in the choice between receiving a small immediate reward (e.g., intoxication after drug administration) and a larger delayed reward (e.g., health benefits associated with abstinence) is the distance in time between the two rewards. The notion that delay discounting is related to interval timing is supported by functional imaging findings showing that brain areas associated with intertemporal choice with delays of less than one year are some of those also involved in interval timing, or time perception in the seconds-to-minutes range (Wittmann, et al. 2007). Acute administration of d-amphetamine, an indirect dopamine agonist which is thought to shorten time estimations in rats (Meck 1996), has also been shown to decrease impulsivity on a delay discounting task when modest doses (10 and 20 mg) are administered orally to normal, healthy volunteers (de Wit, et al. 2002). This decrease in impulsivity on delay discounting was accompanied by a non-significant trend for underestimations of time intervals. If a drug of abuse such as oxycodone alters the experience of time, for example by making a given period of time seem longer, this may cause future rewards to seem even further in the distance than they really are, and the impulsive choice that much more attractive.
The aim of the current study was to examine the effects of oxycodone on the accuracy of interval timing in human volunteers. Interval timing research over the past few decades has been dominated by Scalar Expectancy Theory (SET; Gibbon 1977). In this model, a pacemaker produces signals that count off the time as it passes. These signals are collected by an accumulator which keeps track of how many signals have been produced during the interval being timed. Between the pacemaker and the accumulator is an attention mechanism, such that attention “closes the switch” between the pacemaker and the accumulator and allows signals to reach the accumulator. The number of signals in the accumulator is transferred to working memory and compared with a reference memory for pacemaker signals for the relevant time. When the number of signals is close to the reference memory value, within some threshold, the appropriate response is made. Thus there are four stages that can be affected by a given manipulation such as drug administration: the pacemaker/accumulator stage, the attentional switch, the reference memory stage, or the decision (threshold) stage. Drugs that affect cognitive functions, such as attention or memory, can have specific effects on various stages of the SET model. Some reports suggest that oxycodone may affect some of these cognitive functions under specific circumstances (Cherrier, et al. 2009, Friswell, et al. 2008), but others suggest that these effects are unreliable at best (for review, see Zacny 1995, Zacny and Gutierrez 2003), leaving open the question of whether oxycodone can affect interval timing.
The effect of opioid agonists other than oxycodone, such as morphine and heroin, on time perception has been studied with mixed results. Rhesus monkeys were trained to perform a temporal response differentiation (TRD) task, in which they were required to hold a lever down for no less than 10 s but no more than 14 s in order to gain a reward. Morphine disrupted TRD performance, such that animals released the lever earlier than they would under placebo conditions (Schulze and Paule 1991). In the scalar timing framework this suggests either a speeded clock, which would lengthen estimations of the current time or a shortened memory for the criterion duration, or a lowered threshold for responding. Similarly, heroin-dependent volunteers in the first few days of withdrawal made short reproductions of a 12-s interval compared to normal, healthy volunteers (Aleksandrov 2005), suggesting a shortening of the perception of time when heroin is withheld. Some studies with pigeons suggest that time may be overestimated in response to morphine administration, but alternative accounts based on the ability of morphine to change response rates and discrimination accuracy complicate the interpretation of these studies (Knealing and Schaal 2002, Odum and Ward 2004, Ward and Odum 2005). Thus more work needs be done to clarify the impact, if any, that opiates have in interval timing.
Oxycodone may have an effect on interval timing, but at which range? An emerging theme in interval timing research is the distinction between automatic and cognitively controlled timing (for a review, see Lewis and Miall 2003). The suggestion is that timing of about a second or less is automatic, or mainly under the control of the motor system. On the other hand, cognitively-controlled timing involves intervals of greater than about one second, and engages cognitive mechanisms such as working memory and attention. The differences in putative timing mechanisms and results of perturbations to these systems, depending on the stimulus duration, have been termed range effects (Gibbon, et al. 1997). One line of evidence for these range effects is the difference in the effect of drugs upon timing of various intervals. For example, the benzodiazepine midazolam (Rammsayer 1999) and the NMDA receptor antagonist memantine (Rammsayer 2006) both disrupt timing of intervals of around 1 s, but not intervals in the millisecond range. Benzodiazepines (Cole and Hillman 1994) and NMDA receptor antagonists (Adler, et al. 1998, Morgan, et al. 2004) have been shown to interfere with working memory function. In part because of this, it has been argued that drugs that affect cognitive processes such as attention and working memory consequently affect cognitively-controlled timing, but not automatic timing (Lewis and Miall 2003, Madison 2001, Poppel 1997, Rammsayer 1999). Oxycodone has been shown to have detrimental effects on attention and working memory in healthy subjects generally (digit symbol and number sequencing, Zacny and Lichtor 2008), over the age of 35 (digit symbol and number letter sequencing, Cherrier, et al. 2009) and women but not men at a dose of 5 mg (reverse digit span, Friswell, et al. 2008). Therefore, these studies suggest that oxycodone could affect timing in the suprasecond range (> 1 s, also characterized as cognitively-controlled timing) via one or more of these processes.
The current study evaluated how oxycodone affects time perception in humans by administering a temporal reproduction task before and after administration of placebo or active oxycodone. The choice of tasks was guided by previous work showing the efficacy of single interval production tasks in characterizing timing deficits in impaired (Malapani, et al. 1998, Malapani et al., 2002) and unimpaired (Rakitin 2005, Rakitin, et al. 1998, 2005, 2006) human populations. Participants learned the time intervals to be reproduced, and then were tested on their memory for those intervals. Drug or placebo was given, and then participants were tested again on their memory for the interval. The results of this study shed light on the role of opiates in interval timing.
Method
Participants
Volunteers were recruited through local newspaper advertisements and were invited to the laboratory for screening after meeting preliminary inclusion/exclusion criteria in a telephone screen. During screening, participants received psychiatric and medical evaluations, and provided detailed drug use and medical histories. Participants were required to be healthy as determined by a physical examination, which included urinalysis, blood chemistries, and an electrocardiogram; to be between 21–55 years of age; normal body weight as determined within 20% of the body weight for appropriate frame according to 1983 Metropolitan Weight tables; have been prescribed and used opioids for medical purposes at least twice with no serious adverse effects; and females had to be using an effective form of birth control. Exclusion criteria included a recent conviction of a crime of violence; current Axis I psychopathology or significant Axis II disorder; currently pregnant, pregnant within the past 6 months, or nursing as determined by self-report, and plasma and urine pregnancy tests; current use of psychotropic medications; history or current opioid dependence or recreational opioid use; chronic pain or use of over-the-counter analgesics more than four times each month; high blood pressure; sensitivity, allergy or contraindication to opioids; meeting the Diagnostic and Statistical Manual (of Mental Disorders), fourth edition, revised criteria for substance abuse or dependence in the past two years . Once eligibility was verified, participants were enrolled in the study only after written informed consent was obtained. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki. Participants were compensated for their time.
Task
A single interval production (SIP) training block included 20 identical synchronization trials that presented pairs of tones separated by one of three target intervals, either 1.1 (e.g., “short”), 2.2 (e.g., “medium”), or 3.3 (e.g., “long”) s. A yellow square on the screen cued the beginning of the synchronization trials, and the first of the tones occurred after an average of 0.675 s (0.450 – 0.900 s, in equally probable 0.150 s increments). Participants were instructed to skip as many trials as necessary to get a feel for the target interval, and then respond in synchrony to the second tone. Trials in which participants did not respond terminated after four-times the target interval. The cue turned red for 0.500 s with each response or following a time-out, signaling the end of a trial, and then cleared. SIP testing blocks included 20 trials. Each testing trial presented a single tone, and participants attempted to respond so their response latency following the tone matched the target interval established by the tone pairs. A green square cued the start of testing trials. The square turned red following a response or a time-out. A 0.500 s inter-trial interval separated all trials. Counting all delays, tone cues followed the preceding trials’ response by an average of 1.675 s.
Instructions presented on the screen preceding each block indicated the cueing and response contingencies for that task. The instructions did not indicate the duration of the target interval. The instructions cleared following a key press. The task block began 0.500 seconds after the instructions cleared.
Procedure
Prior to each testing session, participants provided a urine sample, which was tested for morphine, methadone, benzoylecgonine, amphetamine, methamphetamine, phencyclidine, barbiturates, THC, and benzodiazepines and a breathalyzer test was administered. Also, a urine pregnancy test was administered to female participants prior to each session. Cigarette smokers were allowed to smoke one cigarette 15 min prior to physiological monitoring and task participation. Prior to the session, a baseline balancing task was administered, a standardized breakfast was provided, and physiological monitoring began and continued throughout the session (oxygen saturation measured continuously and recorded every 1 min, blood pressure measured and recorded every 15 min). On each testing day participants were trained and tested on reproduction of all 3 target intervals. Participants completed one training block for each target interval, then 2 testing blocks for each target interval (pretest). Then, 75 minutes after capsule administration, 2 more testing blocks for each target interval were completed (posttest). Previous results (Zacny and Gutierrez 2003) suggest that the optimal effect of oxycodone on cognitive measures occurs at around this time. Order of target interval testing with immediate and delayed testing phases was the same, and randomized for each participant. All participants received a field sobriety test prior to discharge from each laboratory session, and if there was any indication of intoxication, a car service was called to take the participant home.
Study Design
During one week, effects of oxycodone on task performance were assessed on three consecutive days, and during a second week, placebo capsules were administered and performance was assessed on three consecutive days. Each testing week was separated by a full week during which time no medications were administered. The order of the oxycodone and placebo weeks was randomized across participants. At the end of each session, participants received one oxycodone (oxycodone week), or placebo capsule (placebo week) in a child-proof bottle. Participants were instructed to take the capsule in the evening. The purpose of evaluating effects of oxycodone over three consecutive days, and the administration of the evening doses was to assess tolerance development to effects of oxycodone the primary aim of the larger study. As part of the general study, participants received increasing doses of oxycodone at 45 minute intervals (3 participants received 0, 10, and 30 mg/70 kg oxycodone and 8 received 0, 5, and 15 mg/70 kg oxycodone) on the day before and the day after the three consecutive dosing days described for the current study. On these days, analgesic effects of oxycodone were assessed using the cold pressor test (CPT) and measurements of the drug’s subjective and cognitive effects were obtained. Results of these tests will be published separately.
Drug
Oxycodone capsules (placebo or 15 mg) were packaged into size 00 opaque capsules with lactose filler by the New York State Psychiatric Institute Research Pharmacy. Participants were given capsules to be taken at home in a child-proof bottle. On Mondays and Fridays, Oxycodone HCl [Oxyfast® Immediate-Release Oral Concentrate Solution (20 mg/ml), Purdue Pharma] was prepared at doses of 0, 5 and 15 mg per 70 kg. The solution was mixed in orange-flavored Gatorade with 1 ml peppermint oil floated on top to mask the taste of the drug. A total volume of 200 ml was administered at each dosing, and was consumed within 5 min. The oxycodone doses used for this study were within the recommended dose range of orally administered oxycodone for treating pain (5–30 mg every 6 hr; Physician’s Desk Reference, 2009), and were previously tested safely in normal, healthy volunteers (Zacny and Gutierrez 2003).
Data Analysis
The dependent variables were the intra-condition, intra-individual proportional error of mean response latencies ((latency – target)/target), to allow direct comparison among time intervals, and the coefficient of variation (CV; standard deviation/mean) of response latencies. Standard data treatment excluded the first two responses in each block, as well as the fastest and slowest latency in each condition, to remove spurious variability in response. Hypotheses were tested for each variable using separate analyses of variance (ANOVAs). The following were within-subjects factors: target duration (1.1, 2.2, and 3.3 s); task phase (pre-dose testing or pretest, post-dose testing or posttest); dose (0 or 15 mg) and test day (Day 1, Day 2, and Day 3). Planned contrasts on the duration and test day factors were chosen to look for differences between the shortest duration and the other two durations (range effects), or between Day 1 and later days (learning or tolerance effects).
Results
Participants
Twenty-one individuals enrolled in the study and 13 participants completed it. Two were excluded because pre-drug administration training performance indicated they did not learn the intervals to be timed. Thus 11 participants (8 men; 3 women; 9 white; 2 Hispanic; average age of 30 years with a range of 22–51) contributed to the reported data. Nine of the included participants reported previous marijuana use (last use ranged from 6 weeks to 25 years prior to screening, with maximum lifetime use ranging from 3 times ever used to once per week). Past cocaine use was reported by two of the included participants (last use ranged from 4 years to 25 years prior to the study screening process, with maximum use ranging from twice ever used to once per week). One included participant reported past recreational use of Ecstasy and Ritalin® (each drug was used once: Ecstasy 8 years prior to study screening and Ritalin® 2 months before study screening). Daily tobacco use was reported by six of the included participants (range: 1/week – 10/day). Of the 8 volunteers who discontinued study participation, 2 dropped due to medication side effects (excessive sedation, nausea, vomiting, intense headache), 1 was discontinued due to elevated blood pressure during the CPT, and 5 discontinued for unknown reasons.
Effects of oxycodone on interval reproduction
Differences in proportional errors between 15 mg oxycodone and placebo, for each of the 3 target intervals, collapsed over dosing day, are shown in Figure 1. There was a significant interaction between duration, testing phase and dose of drug administration, such that the pretest was different from the posttest, depending on whether participants received oxycodone or placebo (F(2,20) = 3.9, p < 0.05), for the 2.2 and 3.3 s intervals only (contrast, 1.1 s vs others x phase x dose: F(1,10) = 7.2, p < 0.05). For the 2.2 and 3.3 s intervals, temporal reproductions were longer after 15 mg oxycodone administration than after placebo. A significant main effect for duration was found (F(2,20) = 4.7, p < 0.05), and the contrast testing whether the 1.1 s interval was different from the others was marginally significant (F(1,10) = 5.0, p = 0.05). This indicates that reproductions of the 1.1 s interval had smaller proportional errors than those for the other 2 intervals, overall. The duration x phase interaction was also significant (F(2,20) = 9.3, p < 0.05): the 1.1-s interval showed a different pattern between pretest and posttest than the other times (contrast 1.1 s vs other intervals x phase: F(1,10) = 12.9, p < 0.05). Specifically, reproductions of the 1.1 s interval were proportionally shorter in the posttest condition compared to those of the other 2 intervals. There were no significant effects for the test day x dose interaction, so results presented in Figure 1 are collapsed over test day. A complex significant interaction arose among the four factors, duration, phase, test day and dose (F(4,40) = 2.6, p < 0.05). Planned contrasts did not reveal a pattern.
Figure 1.
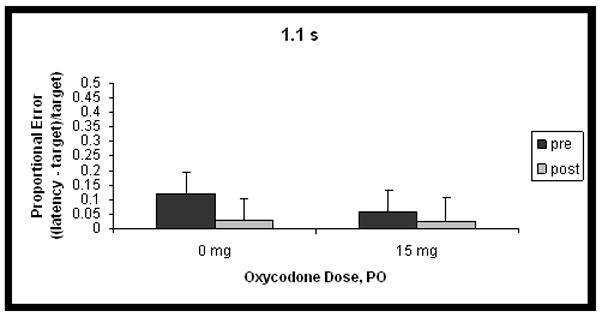
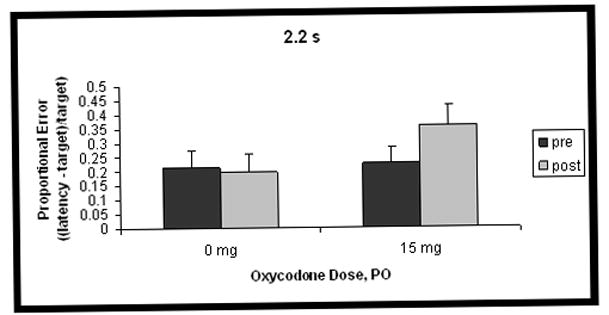
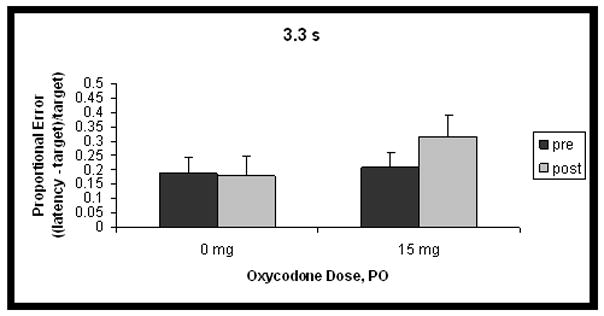
Mean proportional error ((latency-target)/target; accurate responses = 0) of temporal reproductions before (pretest) and after (posttest) administration of 0 or 15 mg oxycodone for the three time intervals. Panel A shows data for the 1.1 s interval, Panel B for the 2.2 s interval, and Panel C for the 3.3 s interval. Error bars represent standard error of the mean. Response latencies are longer after administration of 15 mg oxycodone PO, for the 2.2 and 3.3 s intervals only.
CV results are shown in Table 1. There was a significant main effect for dose (F(1,10) = 5.1, p < 0.05), indicating that intra-individual variability was greater on days when oxycodone was administered as opposed to placebo. There was also a significant main effect for duration (F(2,20) = 9.4, p < 0.05), such that reproductions of the 1.1 s interval were more variable than those for other times (contrast 1.1 s vs other intervals: F(1,10) = 11.6, p < 0.05).
Table 1.
CV data.
| Pretest | Posttest | ||
|---|---|---|---|
| 1.1 s | 0 mg | 14.0 ± 2.3 | 14.6 ± 2.2 |
| 15 mg | 18.4 ± 3.4 | 19.4 ± 2.7 | |
| 2.2 s | 0 mg | 11.5 ± 1.4 | 12.9 ± 1.8 |
| 15 mg | 14.7 ± 2.3 | 15.1 ± 1.4 | |
| 3.3 s | 0 mg | 9.6 ± 1.3 | 9.8 ± 1.4 |
| 15 mg | 14.0 ± 2.5 | 13.4 ± 2.0 |
Discussion
Reproductions of previously learned time intervals greater than about one second are lengthened after oral administration of 15-mg oxycodone, as compared to placebo. This is in line with previous findings in animals, which suggest that other opioid agonists, such as morphine (Schulze and Paule 1991, Knealing and Schaal 2002, Odum and Ward 2004, Ward and Odum 2005), lengthen time estimations. Conversely, heroin withdrawal shortens time estimations in humans (Aleksandrov 2005).
Our findings lend further support to range theories that suggest different mechanisms for timing intervals of about a second or lower and those longer than approximately 1 s. We found that 15 mg oxycodone selectively lengthens temporal reproductions in intervals greater than about 2 s while leaving the approximately 1 s estimations unaffected. This is similar to findings for benzodiazepines (Rammsayer 1999) and NMDA receptor antagonists (Rammsayer 2006), which also affect working memory function. The picture emerges that drugs that influence working memory also affect time perception of intervals greater than 1 s, or what is termed cognitive timing.
One can speculate regarding the possible mechanisms of the effect of oxycodone on supra-second timing. Mu-opioid antagonists enhance working memory performance in rats (Canli et al. 1990) and evidence in rats also shows that morphine administration interferes with working memory on an 8-arm maze task (Braida, et al. 1994). Further, working memory function has been shown to be compromised in middle-aged and older adults (ages 35 and up) in response to 10-mg oxycodone administration (Cherrier, et al. 2009) and in women in response to 5-mg oxycodone administration (Friswell et al. 2008), although results with oxycodone and other opioids in younger adults have been mixed (for review, see Zacny 1995, Zacny and Gutierrez 2003). Not only has working memory been suggested to be a part of the machinery supporting time perception (Baudouin, et al. 2006a,b), it has also been proposed to rely upon the same neural mechanism (Lewis and Miall 2006). In a neural model based upon the Multiple Time Scales (MTS) cognitive model of timing (Staddon 2005), Lewis & Miall suggest that the same DA-modulated neurons in dorsolateral prefrontal cortex (DLPFC) that show changes in activity during working memory tasks are those that represent duration during time perception tasks. This model provides the framework to hypothesize that the negative effects of oxycodone on working memory (Cherrier, et al. 2009, Friswell, et al. 2008) and time perception may be influenced by the same process in humans.
It is well-documented that interval timing is influenced by dopamine (DA), a neurotransmitter that is modulated by opioid activity (Mansour, et al. 1995). Indirect DA agonists such as methamphetamine produce shortened temporal reproduction, perhaps due to the speeding up of the internal clock (Buhusi and Meck 2002, Maricq, et al. 1981, Maricq and Church 1983, Matell, et al. 2006, Meck 1983), while dopamine antagonists such as haloperidol produce longer reproductions (Buhusi and Meck 2002, Drew, et al. 2003, Meck 1983, 1986). In humans with Parkinson’s disease, a disorder characterized by depletion of DA in the nigrostriatal pathway, timing of two intervals “migrate” toward each other, pointing to an effect on retrieval of temporal memories (Malapani, et al. 1998, 2002, Malapani and Rakitin 2003).
Opioids have the ability to inhibit or stimulate DA release, depending on the dopamine and opioid receptor subtypes and neuroanatomical location (Mansour, et al. 1995). For instance, in the nigrostriatal and mesolimbic DA systems, mu-opioid receptor activation stimulates DA release while kappa-opioid receptor activation inhibits DA release. Indeed, a recent microdialysis study showed that oxycodone increases striatal dopamine in mice (Zhang et al., 2009). Furthermore, D2 receptors, which have been suggested to be responsible for some of dopamine’s effects on timing in rats (Drew, et al. 2003, Meck 1986), are up- or down-regulated depending on whether the injection protocol and time frame resulted in behavioral tolerance or sensitization to a morphine challenge (Le Marec, et al. 2011). Anatomical studies showing co-localization of D2 receptors and mu-opioid receptors in dorsolateral striatum (Ambrose, et al. 2004), which has been suggested to be a critical structure for interval timing (Hinton and Meck 2004, Matell and Meck 2004, Shea-Brown, et al. 2006), suggest that mu opioid-modulation of dopaminergic activity may have profound effects on timing. Oxycodone has an affinity for both mu (Yoburn, et al. 1995) and kappa (Ross and Smith 1997) receptor subtypes. Thus the possibility exists that oxycodone, through its effect on mu opioid receptors, could have an effect similar to DA agonists on interval timing, or that some as-yet unknown action through kappa receptors on timing may be discovered.
In the SET model, effects can broadly be divided into those that are scalar, such that variability increases proportionally with mean estimates of time, and those that are not. A finding of scalar changes with oxycodone administration is indicated by the lack of phase x dose effects on CV in the current experiment: CV did not change after drug administration. A number of mechanisms for this effect are suggested by the scalar property of the changes seen.
Oxycodone may dampen attention to time, especially since performance on the Digit Symbol Substitution Test, a measure dependent upon attention, among other faculties, has been shown to be disrupted by oxycodone in healthy volunteers (Zacny and Lichtor 2008), and more specifically in middle aged and older adults (Cherrier, et al. 2009). In attentional-gating models, continual disruptions in attention to time would be indistinguishable from a clock-speed effect, because a loss of pacemaker “ticks” would occur in both cases, presumably throughout the interval timed, leading to overproductions of time intervals (Fortin 1999, Zakay and Block 1997). DA activity, which is modulated by opiates, also has been shown to influence clock processes and as well as attention to time (Buhusi and Meck 2002).
Alternatively, oxycodone could have an effect on the reference, or long-term, memory for time, such that the remembered accumulator value for each time is warped to seem longer than it actually was. Such an effect would also lengthen reproductions of time intervals and thus is also consistent with the current results. One study found effects of oxycodone at a lower dose (5 mg) on memory (source identification) in women but not men (Friswell, et al., 2008), although this effect is described as “marginal”, and a report in humans using similar doses of oxycodone to that used in the current study (10–30 mg) does not support it (Zacny and Gutierrez 2003).
Given the scalar quality of the increases in response latency produced by oxycodone, one mechanism for this effect can be ruled out. As described briefly in the introduction, in the SET model the pacemaker, which produces timing signals, and the accumulator, which collects these signals, are separated by an attentional “switch” that closes when the cue to begin timing is presented, allowing pacemaker signals to make their way to the accumulator (Meck 1983, Rakitin 2005). When the cue to end timing is perceived, the switch opens (or attention to time stops), stopping the flow of pacemaker ticks. In dual-task conditions, mean time productions are lengthened because the switch opens so executive functions can contend with the secondary task. This leads to fewer pacemaker pulses accumulated over the same time period, thus lengthening time productions. However, this opening of the switch also contributes non-scalar variability, because the variability of switch-open time is not related to the interval being timed (Rakitin 2005). It has been shown that opioid agonists cause reduced motor activity (e.g., Di Chiara and Imperato 1988). This could lead to a general slowing of motor responses, which could manifest as longer latencies to reproduce the time intervals learned. However, this would lead to proportionally greater errors in reproduction at the shorter time interval, whereas we found the opposite. Reduced error at the 1-s interval compared to the longer time intervals such as those that we found argue against a motor slowing account of our findings.
The current findings show that 15 mg oral oxycodone has a lengthening effect on reproductions of times greater than about one second. More studies are required to determine the mechanism by which the drug exerts this effect, and any discussion of how this occurs is speculative given the current results. It has been postulated that distorted time perception could impact delay discounting, or the perception of values of future outcomes, in such a way that the future seems more remote and future consequences seem less important (Takahashi 2005, 2006). This can lead to decision processes that give greater weight to immediate gains, producing impulsive behavior. Although administration of naltrexone (an endogenous opioid antagonist) has been shown to attenuate impulsive behavior in humans in a variety of domains (Kim, et al. 2001, Marrazzi, et al. 1995, Raymond, et al. 2002), a recent finding by Zacny and de Wit (2009) showed little effect on impulsive behavior, including delay discounting, by doses of oxycodone similar to that used in the current study in healthy adult volunteers. This finding, in conjunction with the current results, suggests a complicated relationship between delay discounting and time perception in the seconds range, indicating that they may not as closely tied as has been proposed (Takahashi 2005, 2006); instead, increased rates of discounting in addicted populations may be due to some other cause.
Acknowledgments
This study was supported by NIDA Grant DA016759, NIMH Grant MH068073, NIDA Grant DA015210, and NIA Grant AGO00261. The authors would like to acknowledge the contribution made to this paper by Susanna Stephens.
References
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43:811–6. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Aleksandrov S. Dynamics of Assessments of Time Intervals by Patients with Heroin Addiction. Neuroscience and Behavioral Physiology. 2005;35:371–4. doi: 10.1007/s11055-005-0034-0. [DOI] [PubMed] [Google Scholar]
- Ambrose LM, Unterwald EM, van Bockstaele EJ. Ultrastructural evidence for co-localization of dopamine D2 and mu-opioid receptors in the rat dorsolateral striatum. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2004;279A:583–91. doi: 10.1002/ar.a.20054. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Isingrini M, Pouthas V. Differential involvement of internal clock and working memory in the production and reproduction of duration: A study on older adults. Acta Psychol (Amst) 2006a;121:285–96. doi: 10.1016/j.actpsy.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: Involvement of working memory processes. Brain Cogn. 2006b;62:17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Braida D, Gori E, Sala M. Relationship between morphine and etonitazene-induced working memory impairment and analgesia. Eur J Pharmacol. 1994;271:497–504. doi: 10.1016/0014-2999(94)90811-7. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–7. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Canli T, Cook RG, Miczek KA. Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacol Biochem Behav. 1990;36:521–5. doi: 10.1016/0091-3057(90)90250-l. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Amory JK, Ersek M, Risler L, Shen DD. Comparative cognitive and subjective side effects of immediate-release oxycodone in healthy middle-aged and older adults. J Pain. 2009;10:1038–50. doi: 10.1016/j.jpain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Surratt H. Trends in the use and abuse of branded and generic extended release oxycodone and fentanyl products in the United States. Drug Alcohol Depend. 2007;91:115–20. doi: 10.1016/j.drugalcdep.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Hillman M. Effects of benzodiazepine receptor ligands on the performance of an operant delayed matching to position task in rats: opposite effects of FG 7142 and lorazepam. Psychopharmacology. 1994;115:350–7. doi: 10.1007/BF02245076. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Therapeutics. 1988;244:1067–80. [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav. 2003;75:9–15. doi: 10.1016/s0091-3057(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Fortin C. Short-term memory in time interval production. Int J Psychol. 1999;34:308–16. [Google Scholar]
- Friswell J, Phillips C, Holding J, Morgan CJA, Brandner B, Curran HV. Acute effects of opioids on memory functions of healthy men and women. Psychopharmacology. 2008;198:243–50. doi: 10.1007/s00213-008-1123-x. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev. 1977;84:279–325. [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7:170–84. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Cognitive Brain Research. 2004;21:171–82. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–21. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Knealing TW, Schaal DW. Disruption of temporally organized behavior by morphine. J Exp Anal Behav. 2002;77:157–69. doi: 10.1901/jeab.2002.77-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence for neuroimaging. Current Opinion in Neurobiology. 2003;13:250–5. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: a continuous clock. Trends Cog Sci. 2006;10:401–6. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Madison G. Variability in isochronous tapping: Higher order dependencies as a function of intertap interval. J Exp Psychol Hum Percept Perform. 2001;27:411–22. doi: 10.1037//0096-1523.27.2.411. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin BC. Interval timing in the dopamine-depleted basal ganglia: From empirical data to timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 485–514. [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J Cogn Neurosci. 1998;10:316–31. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cogn Neurosci. 2002;14:311–22. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–9. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Roberts S, Church RM. Methamphetamine and time estimation. J Exp Psychol Anim Behav Process. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology. 1983;79:10–5. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Markham KM, Kinzie J, Luby ED. Binge eating disorder: response to naltrexone. Int J Obes Relat Metab Disord. 1995;19:143–5. [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cog Brain Res. 2004;21:139–70. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WM. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology. 2006;188:201–12. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D-sub-2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav. 1986;25:1185–9. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms on healthy volunteers. Neuropsychopharmacology. 2004;29:208–18. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Odum AL, Ward RD. The effects of morphine on the production and discrimination of interresponse times. J Exp Anal Behav. 2004;82:197–212. doi: 10.1901/jeab.2004.82-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–38. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Physicians’ Desk Reference. 63. Montvale, NJ: Thompson PDR; 2009. [Google Scholar]
- Poppel E. A hierarchical model of temporal perception. Trends Cog Sci. 1997;1:56–61. doi: 10.1016/S1364-6613(97)01008-5. [DOI] [PubMed] [Google Scholar]
- Rakitin BC. The effects of spatial stimulus-response compatibility on choice time production accuracy and variability. J Exp Psychol Hum Percept Perform. 2005;31:685–702. doi: 10.1037/0096-1523.31.4.685. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. J Exp Psychol Anim Behav Process. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Stern Y, Malapani C. The effects of aging on time reproduction in delayed free-recall. Brain Cogn. 2005;58:17–34. doi: 10.1016/j.bandc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Scarmeas N, Li T, Malapani C, Stern Y. Single-dose levodopa administration and aging independently disrupt time production. J Cogn Neurosci. 2006;18:376–87. doi: 10.1162/089892906775990615. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52B:273–86. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Effects of pharmacologically induced changes in NMDA receptor activity on human timing and sensorimotor performance. Brain Res. 2006;1073–1074:407–16. doi: 10.1016/j.brainres.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Raymond NC, Grant JE, Kim SW, Coleman E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharmacol. 2002;17:201–205. doi: 10.1097/00004850-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Riley J, Eisenberg E, Muller-Schwefe G, Drewes AM, Arendt-Nielsen L. Oxycodone: a review of its use in the management of pain. Curr Med Res Opin. 2008;24:175–92. doi: 10.1185/030079908x253708. [DOI] [PubMed] [Google Scholar]
- Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain. 1997;73:151–7. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Schulze GE, Paule MG. Effects of morphine sulfate on operant behavior in rhesus monkeys. Pharmacol Biochem Behav. 1991;38:77–83. doi: 10.1016/0091-3057(91)90592-p. [DOI] [PubMed] [Google Scholar]
- Shea-Brown ET, Rinzel J, Rakitin BC, Malapani C. A firing-rate model of Parkinson’s disease deficits in interval timing. Brain Res. 2006;1070:189–201. doi: 10.1016/j.brainres.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg T. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–50. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M, Nevin S, Hosztafi SRAZ, Toth G, Borsodi A. Affinity profiles of novel delta-receptor selective benzofuran derivatives of non-peptide opioids. Neurochem Res. 1998;23:1211–6. doi: 10.1023/a:1020738304036. [DOI] [PubMed] [Google Scholar]
- Staddon JER. Interval timing: memory, not a clock. Trends Cog Sci. 2005;9:312–4. doi: 10.1016/j.tics.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Loss of self-control in intertemporal choice may be attributable to logarithmic time perception. Med Hypotheses. 2005;65:691–3. doi: 10.1016/j.mehy.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Time-estimation error following Weber-Fechner law may explain subadditive time-discounting. Med Hypotheses. 2006;67:1372–4. doi: 10.1016/j.mehy.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Effects of morphine on temporal discrimination and color matching: general disruption of stimulus control or selective effects on timing? J Exp Anal Behav. 2005;84:401–15. doi: 10.1901/jeab.2005.94-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cog Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–53. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Shah S, Chan K, Duttaroy A, Davis T. Supersensitivity to opioid analgesics following chronic opioid agonist treatment: relationship to receptor selectivity. Pharmacol Biochem Behav. 1995;51:535–9. doi: 10.1016/0091-3057(94)00375-s. [DOI] [PubMed] [Google Scholar]
- Zacny JP. A review of the effects of opioids on psychomotor and cognitive functioning in humans. Exp Clin Psychopharmacol. 1995;3:432–66. [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacol. 2003;170:242–54. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacol. 2008;196:105–16. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, De Wit H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav. 2009;94:108–13. doi: 10.1016/j.pbb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Curr Dir Psychol Sci. 1997;6:12–6. [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacol. 2009;34:912–22. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]


